Abstract
BACKGROUND
Exposure to nicotine via tobacco smoking may influence leptin release and decrease food intake among smokers. However, the effect of nicotine exposure on leptin and food intake among different nicotine dependent groups is unclear.
OBJECTIVE
We aimed to measure leptin and calorie intake among different nicotine dependent groups.
DESIGN
Cross-sectional study.
SETTING
Research department in school of medical sciences.
PATIENTS AND METHODS
Subjects were selected by purposive (non-probability) sampling and categorized as having low, moderate and high nicotine dependency based on the Fagerstrom Test for Nicotine Dependence (FTND) score. Diet was recorded by interview. Anthropometry, blood pressure, body composition, lipid profile, and physical activity level were measured accordingly. Fasting serum leptin was measured using a commercial ELISA kit.
MAIN OUTCOME MEASURE(S)
Nicotine dependency, 24-hour diet, clinical anthropometric and clinical measurements.
RESULTS
In 107 Malay male smokers leptin concentration was inversely correlated with nicotine dependence. However, body weight, smoking period, blood pressure, body composition, lipid profile and physical activity level were not significantly different among low, moderately and highly dependent smoking groups. Leptin concentration and total calorie intake were also not significantly different among these groups.
CONCLUSION
Leptin concentration was inversely correlated with nicotine dependence, but leptin concentration and total calorie intake status were not significantly different among our different nicotine dependency subjects.
LIMITATIONS
Purposive sampling for subject recruitment and inaccurate information in the self-administered questionnaire.
It is estimated that there are more than 1.3 billion smokers globally and by 2025; it could reach to 1.6 billion.1,2 The Global Adult Tobacco Survey (GATS) 2011 showed the prevalence of Malaysian’s men who currently smoke tobacco which was 43.9%.3
Previous studies have demonstrated the association between cigarette smoking and weight loss while weight gain always occurs due to its cessation.4–6 Nicotine is believed to underlie the effect of smoking on body weight.6,7 It is suggested that nicotine exposure from smoking activities could enhance the release of leptin, an appetite suppressor, and resulted in decreased feeding. 8 Hence suggested the inverse relationship between nicotine and body weight.8 Therefore, the role of leptin might be responsible for weight changes among smokers. 9,10 On the other hand, other researchers speculate that there was a clustering factor such as consumption of cigarette smoke, physical activity level and diet involved in smoking and body weight relationship.11
Nicotine dependency might be a factor which affects the final result of leptin concentration and calorie intake. Low dependence could be the answer why leptin concentration was not affected by smoking as reported in some studies. The effect of smoking on leptin concentration must be studied on the different group of smokers regarding their dependency to ensure the exact results are obtained. Therefore, this study aims to determine the status of leptin and calorie intake among generally healthy Malay male smokers based on their nicotine dependency.
PATIENTS AND METHODS
This was a cross-sectional study conducted at School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kelantan from September 2011 to December 2012. Ethical approval was obtained from the Research Ethics Committee (Human) of Universiti Sains Malaysia and this study wasn conducted in compliance with the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to the study. Volunteers among Malay male smokers were invited by advertisement and selected by purposive sampling. The inclusion criteria were currently active smoker, age between 30 to 50 years old, non-obese (BMI<30), no history of chronic diseases such as hypertension, diabetes or kidney problem, not on any medication, not participating in a weight loss program and no alcohol consumption.
Anthropometry, body composition and blood pressure
Height was measured barefoot using a portable body meter (SECA 206, Germany) to the nearest 0.1 cm.12 Body weight, body fat percentage (BF), visceral fat (VF) and basal metabolic rate (BMR) were measured using a body composition analyzer (Tanita SC330, Tokyo, Japan), which uses bioelectrical impedance analysis. Waist circumference (WC) and hip circumference (HC) were measured in the standing position using the same measurement tape. WC was measured at a level midway between the lower rib margin and iliac crest, while HC was measured as maximal circumference over the buttocks.13 Waist-hip ratio (WHR) was defined as the ratio of WC to HC. After 10 minutes of rest, the measurement of right arm systolic and diastolic blood pressure (SBP, DBP) was done using digital blood pressure monitors (HEM-780, Omron, Japan) while seated. The second reading was taken after five minutes, and the average value was recorded.13
Lipid profile and serum leptin
Fasting peripheral blood was drawn in the morning and allowed to clot at room temperature. The sample was then centrifuged to obtain a serum aliquot that was stored at −80°C. Total cholesterol (TC), triglyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were analyzed with an automated biochemistry analyzer (Vitalab Selectra E, Netherlands).13 TC and TG were measured by colorimetric methods: oxidase-peroxidase (CHOD-PAD)(Randox, UK) for TC and glycerol oxidase-peroxidase (GPO-PAP) for TG (Randox, UK). HDL-C was estimated with a direct clearance method (Randox, UK), while low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation.14 Serum leptin was measured in duplicate with commercial 96 wells enzyme-linked immunosorbent assays (ELISA) kits (Cusabio, China), based on direct sandwich ELISA technique. The optical density was determined by using a microplate reader (Varioskan Flash, Thermo Scientific, USA) at 450 nm, and a standard curve was generated using the manufacturer software (SkanIt Software 2.4.3, Thermo Scientific, USA).
Nicotine dependence and physical activity level
The Malay version of the Fagerstrom Test for Nicotine Dependence (FTND) questionnaire is valid and reliable. 15 FTND is a short self-report questionnaire consisting of six questions on the smoking habit with specific points for each answer. The points are counted to provide a total score. A score of 7 to 10 points represents high nicotine dependence. A score of 4 to 6 and less than 4 represent moderate and low dependence, respectively. 16 The short version of International Physical Activity Questionnaire (IPAQ) was used to assess the physical activity of the subjects. The total score was calculated by the summation of the duration (in minutes) and frequency (days) of walking, and other moderate-and vigorous-intensity activities.17,18 Based on the total score, subjects were categorized as having low, moderate or high physical activity level.
Total calorie intake
Calorie intake was measured using the 24-hour diet recall. All subjects underwent a guided interview to recount all foods and drinks they had consumed in the past 24 hours or during the previous day. Two recall interviews were conducted on non-consecutive days to obtain a better representation of the usual diet. The total calories from the first interview was combined with the total calories from the second interview to get the average, which was taken to represent the usual total calorie intake per day of the subjects. All data were analyzed with Nutritionist Pro-Diet Analysis software (Axxya Systems, USA).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics software (Version 20, IBM, USA). Data are reported as mean with standard deviation (SD) and considered significant by a P value less than 0.05. One-way analysis of variance (ANOVA) was used to determine the mean difference of studied parameters between smoking dependence groups. Correlation between nicotine dependence (FTND score) and leptin concentration was measured using Spearman correlation test.
RESULTS
One hundred and seven adult Malay male smokers participated in this cross-sectional study. The mean (standard deviation) age was 37.0 (9.4) and BMI was 24.8 (3.7) kg/m2. Based on FTND questionnaire score, 55 subjects were categorized as having low nicotine dependence, followed by moderate nicotine dependence (n=34) and high nicotine dependence group (n=16). For physical activity level, the majority of the subjects had high physical activity level (n=61) followed by moderate (n=29) and low (n=17) physical activity level.
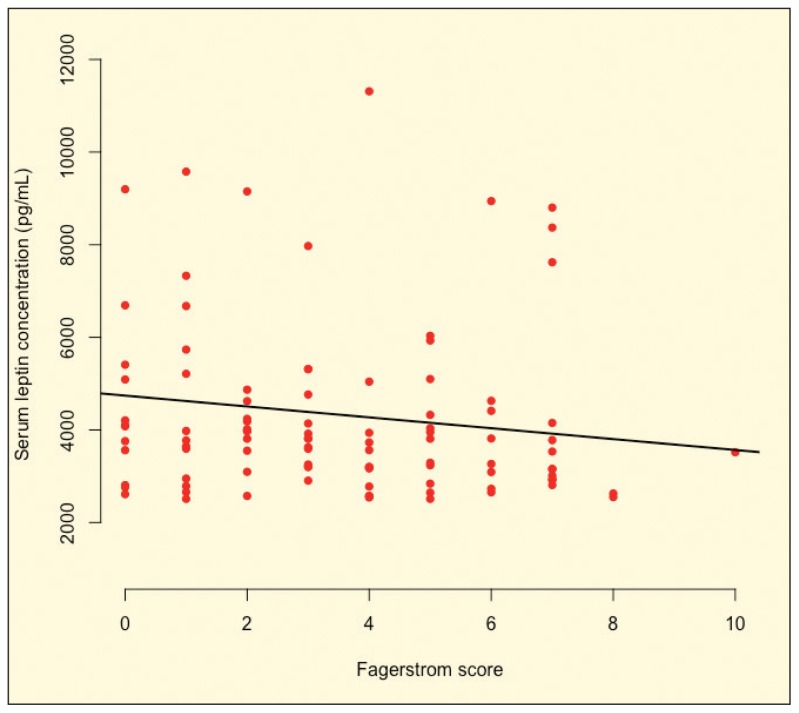
There were no significant differences in anthropometry (body weight, WC, HC, WHR), blood pressure (SBP, DBP), body composition (BF, VF, BMR), lipid profile (TC, TG, LDL-C, HDL-C), and physical activity level (IPAQ score) between low, moderately and highly smoking dependence groups. Furthermore, leptin concentration and total calorie intake were also not significantly different between these groups (Table 1). However, leptin concentration was inversely but weakly correlated with nicotine dependence (FTND score) (r=−0.198, P=.048) (Figure 1).
Table 1.
Mean difference among nicotine dependent groups.
| Parameters | Low Dependence (n=55) | Moderate Dependence (n=34) | High Dependence (n=18) | P |
|---|---|---|---|---|
|
| ||||
| Age (years) | 37.4 (10.1) | 36.7 (9.2) | 36.5 (8.1) | .910 |
| Smoking period (years) | 16.5 (8.1) | 16.6 (7.5) | 18.7 (7.3) | .555 |
| SBP (mm Hg) | 127.8 (15.2) | 122.2 (13.1) | 124.5 (13.8) | .248 |
| DBP (mm Hg) | 79.1 (11.2) | 74.1 (12.6) | 77.3 (10.2) | .202 |
| Weight (kg) | 69.1 (12.9) | 71.7 (12.9) | 69.7 (10.5) | .642 |
| BMI (kg/m2) | 25.0 (3.9) | 24.7 (3.8) | 24.5 (3.2) | .900 |
| WC (cm) | 83.6 (13.3) | 84.9 (10.9) | 83.0 (9.3) | .834 |
| HC (cm) | 97.0 (7.7) | 97.0 (7.5) | 95.9 (7.6) | .851 |
| WHR | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | .867 |
| BF | 22.4 (7.3) | 21.3 (5.7) | 21.0 (4.9) | .658 |
| VF | 9.8 (4.4) | 9.9 (4.8) | 9.7 (3.8) | .990 |
| BMR (kcal) | 1494.8 (206.0) | 1567.9 (216.2) | 1529.5 (179.7) | .266 |
| IPAQ score | 3950.6 (2773.0) | 3300.4 (2799.7) | 4166.0 (3793.0) | .506 |
| TC (mmol/L) | 5.05 (1.1) | 5.4 (0.9) | 5.2 (1.1) | .416 |
| TG (mmol/L) | 1.7 (1.0) | 1.5 (0.7) | 1.7 (0.9) | .468 |
| LDL-C (mmol/L) | 3.2 (0.8) | 3.5 (0.6) | 3.3 (0.9) | .127 |
| HDL-C (mmol/L) | 1.1 (0.2) | 1.2 (0.2) | 1.1 (0.2) | .493 |
| Leptin (ng/mL) | 4561.5 (2028.6) | 4026.6 (1883.7) | 4062.5 (2055.6) | .418 |
| Calorie intake (kcal) | 2019.4 (677.5) | 2034.7 (530.5) | 2311.9 (764.1) | .269 |
Values presented as mean (SD). Analysis using one-way analysis of variance (ANOVA).
SBP=systolic blood pressure, DBP=diastolic blood pressure, BMI=body mass index, WC=waist circumference, HC=hip circumference, WHR=waist hip ratio, BF=body fat, VF=visceral fat, BMR=basal metabolic rate, IPAQ=international physical activity questionnaire, TC=total cholesterol, TG=triglyceride, LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol.
Figure 1.
Spearman correlation of serum leptin concentration by score on Fagerstrom Test for Nicotine Dependence (r=−0.198, P=.048, n=107).
DISCUSSION
The main intention of this study was to verify the leptin status and calorie intake, in addition to the anthropometry measurements, blood pressure, body composition, lipid profile and physical activity status among generally healthy Malay male smokers based on their nicotine dependency. In this study, no significant difference was found in anthropometric measurements, blood pressure, body composition, lipid profile and physical activity level (IPAQ score) between low, moderate and high smoking dependent groups. Nevertheless, all groups recorded acceptable blood pressure, normal BMI, low-risk WC (<102 cm), average BF (20–25%) and healthy VF (<12).19–21 Meanwhile, lipid profile parameters were categorized as optimal or borderline.22
In the present study, no significant difference was found in leptin concentration among low, moderate and high smoking dependent groups. However, there was a significant, although weak inverse correlation between Fagerstrom score with leptin concentration. This finding is consistent with previous studies where the plasma leptin concentration of smokers was lower than non-smokers.23–25 Besides, a cross-sectional study of a multiethnic population revealed a significant inverse relationship between leptin level with the number of cigarettes smoked per day. In that study, the leptin level was predicted to decline at 17.9% with 20 cigarettes smoking per day.26
The lower leptin concentration observed among the highly nicotine dependent group compared with other groups and the inverse correlation between Fagerstrom score with serum leptin concentration contradict the initial theory that exposure to nicotine via cigarette smoking would increase leptin hormone, which is one of the main appetite suppressant hormones in the human body, and reduce food intake. The present finding could suggest that smoking does not directly affect smokers appetite and food intake by changes in leptin.
Elucidation of the mechanism for how cigarette smoking possibly decreases leptin levels is beyond the scope of this study. There are several possible mechanisms. Smoking might increase the basal metabolic rate of smokers due to an effect of nicotine.27 A higher basal metabolic rate would cause the body to burn more energy and reduce body weight. Any loss of body weight or body fat would lead to a decrease in leptin hormone concentration as it is derived from the adipose tissue of body fat.
Another reason for the reduction of serum leptin concentrations in smokers is probably the indirect effect of nicotine of increasing catecholamine,25 which is believed to inhibit leptin expression and secretion.28 Another reason is that factors such as elevation of leptin turnover, distribution alteration or removal from the circulation may cause a decline in serum leptin level among smokers.25,29 Increased transportation of leptin over the blood-brain-barrier is stimulated by adrenaline, which also causes a rise in the uptake and brain/serum ratio of leptin.25,29 Smoking also increases free fatty acid concentration and lipolysis, which may decrease leptin concentration in the blood circulation.30,31 Moreover, oxidative stress induced by smoking has been reported to affect the functions of adipocytes,32,33 resulting in lower leptin production particularly by reduction of leptin gene expression in 3T3-L1 adipocytes.33,34 Additionally, a previous study also reported that the function of adipocytes is reflected by levels of adipokines.33,35
However, there was no significant difference between total calorie intake among the three nicotine dependence groups. This finding is consistent with a previous study where smoking had no major effect on suppressing smokers’ appetite or total calorie intake.36 Our results showed that the highest total calorie was among the highly dependent group, followed by the moderate dependence and low dependence groups. Although there was no significant mean difference among nicotine dependence groups, the value of total calorie intake across the groups is similar to a previous study, which heavy smokers had the highest total calorie intake followed by the moderate and light smokers.37 The high total calorie intake among heavy smokers could be due to their greater consumption of fat and sugar compared with moderate and light smokers. Smokers do not eat less than non-smokers or ex-smokers and in some cases tend to eat slightly more.38
There are a few limitations in the present study. Causation and generalization cannot be inferred because of the purposive sampling. Responses to the questionnaire may have been inaccurate, which could affect classification. Lastly, analysis of macro- and micronutrients should be performed to elucidate the effects of high consumption of fat and sugar among different smoking dependent groups. Apart from the limitations, the present study also had several methodologic strengths. We recruited only generally healthy active Malay male smokers. The apparent dose-dependent association between Fagerstrom score and leptin might indicate a causal relationship.
In conclusion, leptin has an inverse correlation with nicotine dependence, but there were no other differences between the nicotine dependent groups. These findings may indicate that nicotine exposure via smoking may influence leptin concentration. However, any effect is not reflected in total calorie intake.
Acknowledgments
We thank Universiti Sains Malaysia (USM) for funding this study through Short Term Research Grant (304/PPSP/61311093) and for awarding Graduate Assistant (GA) scholarship to Muhammad Zulhusni Suhaimi.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Authors’ contribution
MZS and ZS was involved in the collection, analysis and interpretation of data, and manuscript preparation. HJJM was involved in study design, interpretation of data, and manuscript preparation. HMY conceived the idea and study design, coordinated the study and participated in manuscript preparation. All authors read and approved the content of the manuscript.
REFERENCES
- 1.Jha P. Avoidable death from smoking: a global perspective. Public Health Rev. 2012;33(2):569–600. [Google Scholar]
- 2.Lim HK, Ghazali SM, Kee CC, Lim KK, Chan YY, Teh HC, Yusoff AF, Kaur G, Zain ZM, Mohamad MH, Salleh S. Epidemiology of smoking among Malaysian adult males: prevalence and associated factors. BMC Public Health. 2013;13:8. doi: 10.1186/1471-2458-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Adult Tobacco Survey (GATS) - Malaysia. 2011. [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins KA. Weight gain following smoking cessation. J Consult Clin Psychol. 1993;61(5):768–77. doi: 10.1037//0022-006x.61.5.768. [DOI] [PubMed] [Google Scholar]
- 5.Pisinger C, Jorgensen T. Weight concerns and smoking in a general population: The Inter99 study. Prev Med. 2007;44(4):283–9. doi: 10.1016/j.ypmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Cobanoglu N, Dalkan C, Galip N, Tekguc H, Uncu M, Bahceciler NN. Is calprotectin a marker of tobacco smoke related inflammation?: a pilot study in children. Inhal Toxicol. 2012;24(8):486–91. doi: 10.3109/08958378.2012.693137. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Vlahos R, Bozinovski S, Jones J, Anderson GP, Morris MJ. Effect of short-term cigarette smoke exposure on body weight, appetite and brain neuropeptide Y in mice. Neuropsychopharmacology. 2005;30(4):713–9. doi: 10.1038/sj.npp.1300597. [DOI] [PubMed] [Google Scholar]
- 8.Klein LC, Corwin EJ, Ceballos RM. Leptin, hunger, and body weight: Influence of gender, tobacco smoking, and smoking abstinence. Addict Behav. 2004;29(5):921–7. doi: 10.1016/j.addbeh.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Oeser A, Goffaux J, Snead W, Carlson MG. Plasma leptin concentrations and lipid profiles during nicotine abstinence. Am J Med Sci. 1999;318(3):152–7. doi: 10.1097/00000441-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Miyata G, Meguid MM. Is leptin involved in the acute anorectic effect of nicotine? Nutrition. 2000;16(2):141–2. doi: 10.1016/s0899-9007(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 11.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–9. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 12.Suzana S, Kee CC, Jamaludin AR, Noor Safiza MN, Khor GL, Jamaiyah H, Geeta A, Ahmad Ali Z, Rahmah R, Ruzita AT, Ahmad Fauzi Y. The Third National Health and Morbidity Survey: prevalence of obesity, and abdominal obesity among the Malaysian elderly population. Asia Pac J Public Health. 2012;24(2):318–29. doi: 10.1177/1010539510380736. [DOI] [PubMed] [Google Scholar]
- 13.Sanip Z, Ariffin FD, Al-Tahami BA, Sulaiman WA, Rasool AH. Obesity indices and metabolic markers are related to hs-CRP and adiponectin levels in overweight and obese females. Obes Res Clin Pract. 2013;7(4):e315–20. doi: 10.1016/j.orcp.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 15.Yee AHA, Ng CG, Rusdi AR. Validation of the Malay version of Fagerstrom Test For Nicotine Dependence (FTND-M) among a group of male staffs in a university hospital. Malaysian J Psychiatr. 2011;20(1):1–7. [Google Scholar]
- 16.Ministry of Health Malaysia. Clinical practice guidelines on treatment of tobacco use and dependence. 2003 [Google Scholar]
- 17.International Physical Activity Questionnaire Research Committee. Guideline for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. 2005 [Google Scholar]
- 18.https://ses.library.usyd.edu.au/bitstream/2123/14065/2/2015_Jennifer_McArthur_Thesis.pdf.
- 19. [Assessed on 13 April 2016]. http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/AboutHighBloodPressure/Understanding-Blood-Pressure-Readings_UCM_301764_Article.jsp#.Vw3rNNR95kp.
- 20.Obesity Education NHLBI Initiative. The Practical Guide Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health National Heart, Lung, and Blood Institute. North American Association for The Study of Obesity; 2000. [Google Scholar]
- 21. [Assessed on 28 January 2016]. http://tanita.eu/help-guides/understanding-measurements.
- 22.National Cholesterol Education Program. ATP III Guidelines At-A-Glance Quick Desk Reference. U.S. Department of Health and Human Services. Public Health Service. National Institutes of Health. National Heart, Lung, and Blood Institute; 2001. [Google Scholar]
- 23.Koc B, Bulucu F, Karadurmus N, Sahin M. Lower leptin levels in young non-obese male smokers than non-smokers. Ups J Med Sci. 2009;114(3):165–9. doi: 10.1080/03009730902761631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaleel A, Jaleel F, Majeed R, Alam E. Leptin and blood lipid levels in smokers and ex-smokers. World Appl Sci. 2007;2(4):348–52. [Google Scholar]
- 25.Reseland JE, Mundal HH, Hollung K, Haugen F, Zahid N, Anderssen SA, Drevon CA. Cigarette smoking may reduce plasma leptin concentration via catecholamines. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):43–9. doi: 10.1016/j.plefa.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Donahue RP, Zimmet P, Bean JA, Decourten M, Donahue RAD, Collier G, Goldberg RB, Prineas RJ, Skyler J, Schneiderman N. Cigarette smoking, alcohol use, and physical activity in relation to serum leptin levels in a multiethnic population: the Miami community health study. Ann Epidemiol. 1999;9(2):108–13. doi: 10.1016/s1047-2797(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 27.Gonseth S, Dugas L, Viswanathan B, Forrester T, Lambert V, Plange-Rhule J, Bovet P. Association between smoking and total energy expenditure in a multi-country study. Nutr Metab (Lond) 2014;11:48. doi: 10.1186/1743-7075-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Böttner A, Eisenhofer G, Torpy DJ, Ehrhart-Bornstein M, Keiser HR, Chrousos GP, Bornstein SR. Lack of leptin suppression in response to hypersecretion of catecholamines in pheochromocytoma patients. Metabolism. 1999;48(5):543–5. doi: 10.1016/s0026-0495(99)90047-1. [DOI] [PubMed] [Google Scholar]
- 29.Banks WA. Enhanced leptin transport across the blood-brain barrier by α1-adrenergic agents. Brain Res. 2001;89991–2:209–17. doi: 10.1016/s0006-8993(01)02242-9. [DOI] [PubMed] [Google Scholar]
- 30.Andersson K, Arner P. Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. Int J Obes Relat Metab Disord. 2001;25(8):1225–32. doi: 10.1038/sj.ijo.0801654. [DOI] [PubMed] [Google Scholar]
- 31.Al Mutairi SS, Mojiminiyi OA, Shihab-Eldeen AA, Al Sharafi A, Abdella N. Effect of smoking habit on circulating adipokines in diabetic and non-diabetic subjects. Ann Nutr Metab. 2008;52(4):329–34. doi: 10.1159/000151487. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WZ, Venardos K, Chin-Dusting J, Kaye DM. Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertension. 2006;48(2):278–85. doi: 10.1161/01.HYP.0000231509.27406.42. [DOI] [PubMed] [Google Scholar]
- 33.Hotta Y, Yatsuya H, Toyoshima H, Matsushita K, Mitsuhashi H, Takefuji S, Oiso Y, Tamakoshi K. Low leptin but high insulin resistance of smokers in Japanese men. Diabetes Res Clin Pract. 2008;81(3):358–64. doi: 10.1016/j.diabres.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Kamigaki M, Sakaue S, Tsujino I, Ohira H, Ikeda D, Itoh N, Ishimaru S, Ohtsuka Y, Nishimura M. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;339(2):624–32. doi: 10.1016/j.bbrc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 35.Stern N, Osher E, Greenman Y. Hypoadiponectinemia as a marker of adipocyte dysfunction. Part I: the biology of adiponectin. J Cardiometab Syndr. 2007;2(3):174–82. doi: 10.1111/j.1559-4564.2007.06597.x. [DOI] [PubMed] [Google Scholar]
- 36.Jitnarin N, Kosulwat V, Boonpraderm A, Haddock CK, Poston WS. The relationship between smoking, BMI, physical activity, and dietary intake among Thai adults in central Thailand. J Med Assoc Thai. 2008;91(7):1109–16. [PubMed] [Google Scholar]
- 37.Birkett NJ. Intake of fruits and vegetables in smokers. Public Health Nutr. 1999;2(2):217–22. doi: 10.1017/s1368980099000270. [DOI] [PubMed] [Google Scholar]
- 38.Perkins KA. Effects of tobacco smoking on caloric intake. Br J Addict. 1992;87(2):193–205. doi: 10.1111/j.1360-0443.1992.tb02693.x. [DOI] [PubMed] [Google Scholar]