Abstract
BACKGROUND
Treatment outcomes from HIV/AIDS programs in resource-limited settings mostly describe short-term follow-up. We report 10-year treatment outcomes in an HIV clinic in Kano, Nigeria.
METHODS
Using paper medical charts, the authors conducted a retrospective cohort study of patients that initiated ART from June 1, 2004 to December 31, 2007, and were followed up until June 30, 2014. The authors abstracted data from patient case files and did a time-to-event analysis on ART failure and loss to follow-up, and determined immunologic trends.
RESULTS
The authors studied 345 patient records (29,860 person months of follow-up); 82 records (23.7%) indicated that patients failed their first-line ART regimen at the rate of 2.75 failures per 1000 person-months. The estimates of durability on first-line ART regimen were 99.1% at 1 year and 59.0% at 10 years. Of the studied patients, 83.0% were still in care at the end of the 10-year period. Only being on abacavir (hazard ratio: 8.0) was a positive predictor of ART failure. CD4 increment at 4 years (hazard ratio: 0.9) and 5 years (hazard ratio: 0.9) were negative predictors.
CONCLUSION
A high rate of long-term ART durability and modest long-term retention in care were achieved among our cohort. Improved availability of low-cost virologic and immunologic monitoring tools and provision of resistance testing technology will go a long way in improving early detection of treatment failure in the developing world.
Human immunodeficiency virus (HIV) infection still constitutes an unresolved public health challenge in sub-Saharan Africa (SSA).1 In 2012, Nigeria had an HIV prevalence of 3.4% making it the second highest in the world and the highest in West Africa due to its large population size.2 Furthermore, there are wide geographic differences in the HIV epidemic in Nigeria. The national HIV & AIDS and Reproductive Health Survey showed that the prevalence ranged from 0.2% in Ekiti state to 15.2% in River State.3
The US President Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) are the largest contributors to the fight against HIV/AIDS.4 Nigeria began its antiretroviral treatment (ART) program in 2002 and by 2004 made antiretroviral medications available for its citizens at no cost.5 According to the Joint United Nations Programme on HIV/AIDS (UNAIDS) report: 2009–2013, the number of ART sites grew from 392 to 820, which was accompanied by a steady expansion in coverage putting 639 397 patients on treatment as of 2013. However, out of 1.4 million persons infected with HIV/AIDs, in 2012, only approximately 400 000 in need of treatment received it.6 Barriers exist as efforts are made to increase and maintain a durable ART coverage and include, for example, poorly coordinated health systems, poverty, stigma, and an unreliable supply chain.7
On their report of ART treatment retention programs in Nigeria, Charurat et al identified factors associated with lower risk of lost to follow-up (LTFU) to include, CD4 counts >200 cells/5L, total ART duration of more than 6 months and being on a tenofovir- or zidovudine-based regimen.8 Reduction in the effectiveness of ART due to development of viral resistance in the setting of sub-optimal adherence levels compromises future treatment options. Moreover, poor adherence reverses the earlier gains made in curbing HIV transmission and increases the likelihood of developing resistance to ART.9 New generation ART medications do not readily find their way into treatment programs in Africa due to cost barriers.10 Thus strategies should focus on increasing and prolonging retention to avoid limiting ART options and improving effectiveness. Retaining people in ART programs often provides an opportunity to provide behavioral change counselling, treatment of other sexually transmitted diseases (STDs), and TB.
As of writing, there are no published data on long-term (10 years) durability of first-line ART in adult cohorts in Nigeria. However, Grimsrud et al, on behalf of Medecins Sans Fronti-res had reported a 10 year-outcome of 25 cohorts of African and Asian HIV Programs, that did not include Nigeria. They reported a nine percent mortality over five years and 21% LTFU.11 Nevertheless they did not report on ART durability. Equally, a study from Netherlands reported on a seven-year trend in CD4 count. They reported on the feasibility of restoring CD4 counts up to 800 cells/5L within 7 years of ART.12 Bashi et al reported that 54% and 64% of patients commencing ART in 1996–2000 and 2005–2006 had CD4 count <200 cells/μl, respectively.13 They also, did not report on the durability of the ART used, and Nigeria was not one of the countries studied.
Given the paucity of studies on long-term outcomes of ART programs in Nigeria, the authors sought to determine the effectiveness of first-line ART, cohort retention in care, immunological trends and potential treatment failure predictors.
METHODS
Study design and setting
The authors conducted a 10-year retrospective cohort study using paper medical records from 2004–2014. The last patient record was recorded on 30 June 30 2014. Data was collected at Aminu Kano Teaching Hospital Kano (AKTH), Nigeria, a 600-bed tertiary care hospital providing primary care as well as specialized services. The HIV treatment center at AKTH is a PEPFAR/CDC-funded program. It is the main tertiary referral center for Kano state (with a population of 15 million).
Inclusion and exclusion criteria
The authors included all patients aged 18 years or older who were ART nave and enrolled in the AKTH ART program between June 2004 and December 2007 with a minimum of one baseline CD4 count. Patient records were also included for analysis if there was one CD4 count result documented for each year of follow-up and a clinical evaluation on at least two visits. Patient charts were excluded if there was evidence of prior ART exposure or chronic medical ailments like diabetes mellitus, hypertension or end-organ damage.
Source population and study participants
We determined that with a power of 80% and 95% confidence interval that a sample of 345 would detect a difference of 10% between comparator groups.
The source population comprised of all HIV-positive persons who were enrolled in the AKTH HIV/AIDS care program since inception. A total of 345 (8.5%) patients were selected using a random sampling technique from among 4072 persons enrolled in care.
HIV care protocol
Patients evaluated for ART initiation also had a routine medical examination, including screening for TB and STDs and hepatitis. The center provided comprehensive HIV care services such as provision of ART, prevention and treatment of opportunistic infections and laboratory services. Liver and renal function tests, lipid profiles, complete blood and CD4 counts, as well as viral load assays were offered to patients. Due to programmatic constraints viral load testing was only done on patients with sub-optimal adherence or those with evidence of immunological or clinical failure. Patients were mostly switched to second-line therapy based on evidence of immunological failure. No patient had genotypic HIV resistance testing prior to ART switch, because the programme could not provide the test.
The choice for first-line ART regimen consisted of triple agents with either a non-nucleoside reverse transcriptase inhibitor [NNRTI, such as efavirenz (EFV) or nevirapine (NVP)] backbone). This is added to any of a nucleoside or nucleotide reverse transcriptase inhibitor (NRTI/NtRTI ), such as zidovudine (AZT), or stavudine (D4T), or abacavir (ABC) or Tenofovir disoproxil fumarate (TDF with either lamivudine (3TC) or emtricitabine (FTC). A typical first-line ART regimen in Nigeria would be: AZT/3TC/NVP or TDF/FTC/EFV. Those on a second-line ART regimen received ritonavir-boosted lopinavir- (LPV/r) or atazanavir- (ATV/r), which was combined with at least two other active NRTIs.
Definition of terms
First-line ART refers to a combination of medications prescribed to treatment nave patients commencing HIV treatment. Second-line ART regimen refers to a combination of medications used in treatment-experienced patients with first-line ART regimen failure. Regimen change denotes substitution of an ART medication with another due to drug toxicity. Regimen switch refers to replacement of first-line ART regimen with a second-line ART regimen due to treatment failure. Any patient with >95% usage of prescribed ART was said to have a good adherence.
Outcomes
The primary outcome measure was immunologic failure. It was defined as a fall in CD4 count to pre-therapy baseline (or below); or a 50% fall from the on-treatment peak value; or failure of CD4 counts to rise above 100 cells/5L.14 Based on programmatic criteria, we defined HIV virologic failure using a cut-off viral load mark of >400 viral copies/5L. However, because of the limited number of patients with viral load testing we did not include it in this report. The secondary outcome measure was lost to follow-up, defined as a patient who did not return within 60 days after their expected visit.15
Data collection
The authors abstracted data from patient case files onto a proforma. This information had been prospectively captured on patient management and monitoring (PMM) forms. Data collected included weight, height, baseline WHO clinical stage, baseline CD4 count, type of regimen at commencement of therapy, duration on therapy, occurrence of TB infection, change or switch in ART, and serial CD4 count testing after initiation of ART.
The authors transferred data from the proforma into an Excel database. The data was subsequently imported into a Stata database after validation. We assessed data for completeness, and any missing information was later captured from the case files. In situations where such data were not available we assumed that the data were missing, and imputed replacements with median values of the variables.
Data analysis
Quantitative variables were summarized using descriptive statistics and categorical variables were tabulated using frequencies and percentages. Differences in means were determined by t and Mann-Whitney tests. Differences in categorical variables were determines by chi-square and Fisher’s exact tests.
The authors used Kaplan-Meier analysis to estimate the time from initiation of ART to immunologic failure. Predictors of treatment failure were investigated by Cox proportional hazard regression with commencement of ART as time zero. Variables associated with the outcome on univariate analysis (defined as a P value <.25) were included in a multivariate analysis, where a P value <.05 was considered statistically significant. Analysis was done using Stata version 11 (Stata Corporation, College Station, USA). The study was approved by the ethics committee of AKTH.
RESULTS
Study population
The study population comprised 345 patients. There were 205 (59%) females and 231 (66.9%) were married. The remaining were either single (10.4%), divorced (6.7%), or widowed (15.7%). Thirty-seven percent of the population were unemployed. There were 217 (63%) employed persons in the cohort. The demographic characteristics were similar between those who failed their first-line ART and those who did not (Table 1). There were a total of 29 860 person-months of follow-up. The median age at time of enrollment was 40 years. The median CD4 count at ART initiation was 300 cells/μL with 31 % of the patients having had a CD4 count <200 cells/μL. Sixty percent of patients lived further than 30 kilometers from the hospital. The median time on ART was 102 months (25th–75th percentile: 94–105 months). The records showed that 91% of the patients were on ART for more than 60 months, 6% between 13 and 59 months, and 3 % on ART for 12 months or less (Table 2).
Table 1.
Sociodemographic characteristics of all HIV-positive study participants and estimated effect on treatment failure.
| Characteristics | Combined frequency (%) | Active on 1st line ART (%) | Failed 1st line ART (%) | P | |
|---|---|---|---|---|---|
|
| |||||
| Age | 18–29 | 20 (5.8) | 14(70.0) | 6(30.0) | .52 |
| 30–39 | 129 (37.4) | 94 ( 72.9) | 35 ( 27.1) | ||
| 40–49 | 143 (37.4) | 114 (79.7) | 29 (20.3) | ||
| 50 and above | 53 (15.4) | 41 (77.4) | 12 (22.6) | ||
|
| |||||
| Sex | Male | 140 (40.6) | 104 (74.3) | 36 (25.7) | .483 |
| Female | 205 ( 59.4) | 159 (77.6) | 46 (23.8) | ||
|
| |||||
| Marital status | Single | 36 (10.4) | 25 (69.4) | 11 (30.6) | .330 |
| Married | 231 (67.0) | 174 (75.3) | 57 (24.7) | ||
| Divorced | 24 (7.0) | 18 (75.0) | 6 (25.0) | ||
| Widowed | 54 (15.7) | 46 (85.2) | 8 (14.8) | ||
|
| |||||
| Employment status | Employed | 217 (62.9) | 165 (76.0) | 52 (24.0) | .912 |
| Not employed | 128 ( 37.1) | 98 (76.6) | 30 (23.4) | ||
|
| |||||
| Distance from treatment center | < 30 km | 137 (39.71) | 106 (77.37) | 31 (22.63) | .686 |
| > 30 km | 208 (60.29) | 157 (75.48) | 51 (24.52) | ||
Table 2.
Clinical and immunological profile of study participants.
| Parameters | Sub-types | All patients (%) | Active on 1st Line ART | Failed 1st Line ART | P |
|---|---|---|---|---|---|
|
| |||||
| 263 (76.23) | 82 (23.77) | - | |||
|
| |||||
| Baseline WHO clinical stage | I | 201 (58.26) | 153 (76.0) | 48 (24) | .985 |
| II | 66 (19.13) | 51 (77.27) | 15 (22.73) | ||
| III | 70 (20.29) | 53 (75.71) | 17 (24.29) | ||
| IV | 8 (2.32) | 7 (75.00 | 2 (25) | ||
|
| |||||
| First line ART regimen | ZDV/3TC/NVP or EFV | 251 (72.75) | 214 (85.26) | 37(14.74) | .001 |
| D4T/3TC/NVP or EFV | 22 (6.38) | 19 (86.36 ) | 3 (13.64) | ||
| TDF/3TC/NVP or EFV | 68 (19.71) | 29 ( 42.65) | 39 ( 57.35) | ||
| ABC/3TC/NVP or EFV | 4 (1.16) | 1 ( 25.00) | 3 (75.00) | ||
|
| |||||
| ART duration | <12 months | 11 (3.19) | 11 (100) | 0 (0) | .022 |
| −59 months | 19 (5.51) | 18 (94.74) | 1 (5.26) | ||
| >60 months | 315 (91.30) | 234 ( 74.29) | 81 (25.71) | ||
|
| |||||
| CD4 count at enrolment | <200 | 106 (30.72) | 70 (66.04) | 36 (33.96) | .001 |
| 200–349 | 101 (29.28) | 73 (72.28) | 28 ( 27.72) | ||
| ≥350 | 138 (40.00) | 120 (86.96) | 18 (13.04) | ||
|
| |||||
| Baseline BMI | <25 | 218 (63.19) | 85 (68.00) | 40 (32.00) | .025 |
| 25–29.9 | 85 (24.64) | 87 (80.56) | 21 (19.44) | ||
| ≥30 | 42 (12.17) | 91 (81.25) | 21 (18.75) | ||
|
| |||||
| Adherence level | <95 | 232 (67.25) | 97(85.84) | 16 (14.16) | .003 |
| >95 | 113 (32.75) | 166 (71.55) | 66 (28.45) | ||
|
| |||||
| Change in 1st line ART regimen | No change ART regimen change |
236 (80.55) 57 (19.45) |
166 (70.34) 48 (84.21) |
70 (29.66) 9 (15.79) |
.034 |
Most patients 218 (63%) had a BMI of <25 kg/m2 at entry of care. Although 232 (67%) of patient records indicated a self-reported adherence of <95%, only 82 (24%) had a regimen switch. This implies that 12 persons experienced immunological failure but without switching regimens. Except for WHO clinical staging, there were significant differences in clinico-immunologic characteristics between those that failed 1st line ART and those that did not (Table 2).
Durability of first-line ART and retention in care During 29,860 person-months of follow-up, 82 (24%) persons failed their first-line ART regimen, at an incidence density of 2.75 failures per 1000 person-months (0.22 per 1000 person-years). Early failure rate in the first five years was lower: 2.04 (1.49–2.79) per 1000 person-months (0.17 per 1000 person years) compared with 3.98 [2.96 – 5.38] per 1000 person-months (0.33 per 1000 person-years in the last five years.
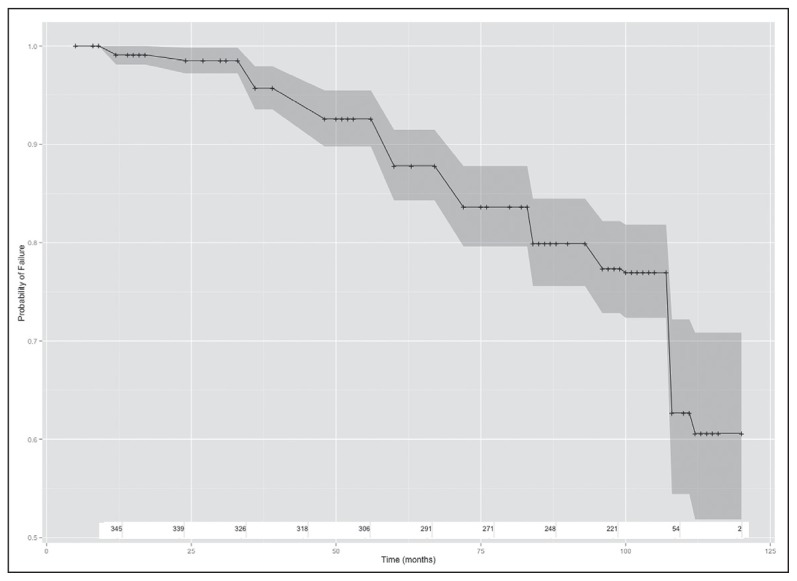
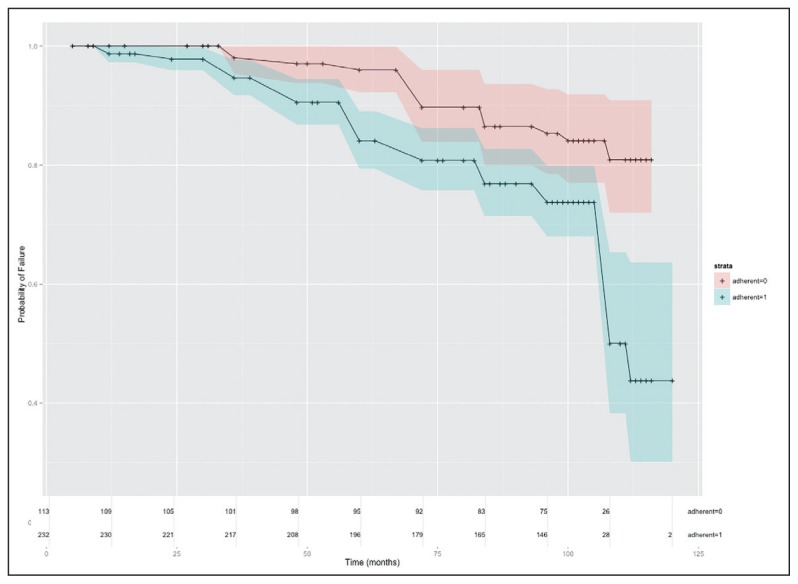
The Kaplan-Meier survival estimates of durability on first-line ART regimen and CI 95% were 99.1% (0.97–0.99) at one year, 87.8% (83.7–90.9) at five years and 59.0% (48.4–68.2) at ten years. This corresponds to monthly failure rates of 0.25 (ART failures) per month in the first 12 months of ART, 0.45 (ART failures) per month in the first five years, and overall 0.68 (ART failures) per month over the 10 years as shown (Figure 1a). There was a significant difference in time to first-line ART failure when stratified by baseline CD4 count categories (I <200, II 200–350, III >350 [P<.01]). Those that reported adherence levels of >95% had higher ART failure rates compared with those with lower adherence rates (P<.01) (Figure 1b). Those with >95% adherence level had significantly higher duration on therapy (P<.02).
Figure 1a.
Kaplan-Meier time-to-failure estimates: (a) Crude time-to-failure estimate and 95% confidence intervals (b) Time-to-failure estimate and 95% confidence intervals stratified by adherence (Adherent =0 is adherence <95%; adherent =1 is adherence > 95%), showing numbers at risk at each time point.
Figure 1b.
Kaplan-Meier time-to-failure estimates: (a) Crude time-to-failure estimate and 95% confidence intervals (b) Time-to-failure estimate and 95% confidence intervals stratified by adherence (Adherent =0 is adherence < 95%; adherent =1 is adherence > 95%), showing numbers at risk at each time point.
Records indicated that 236 patients (81%) had a regimen change, with no gender differences. The most common substitution was from a d4T- to an AZT-based regimen.
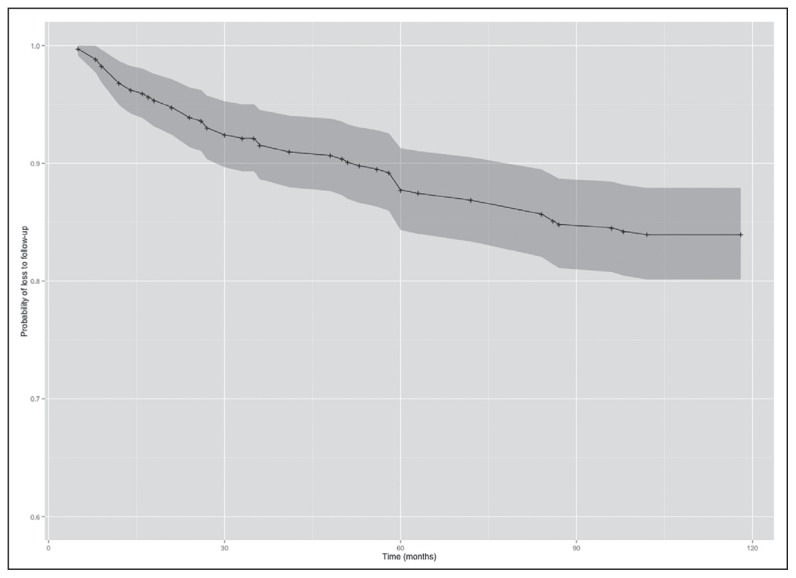
At the end of 10 years of follow-up, 83% of all patients in the study were still in care (Figure 2). There were no gender differences between those lost to follow-up (LTFU) and those in care. However, there was a significant difference in time to LTFU between those with and without ART treatment failure (P<.05). Overall attrition rate was 1.52 per 1000 person-months (PM) with 2.68/1000 PM in the first year, 1.81/1000 PM in the first 5 years, and 0.75 in the last 5 years.
Figure 2.
Estimate of time to loss to follow up of study cohort.
Immunologic trend
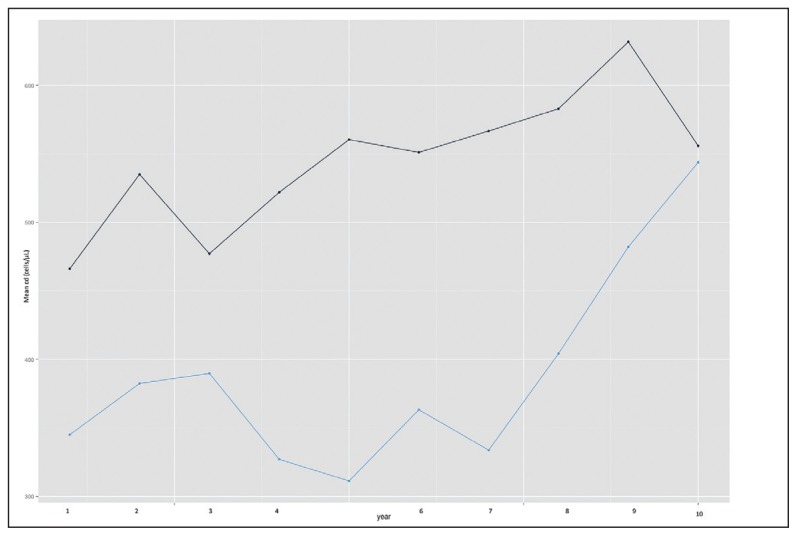
Patients with treatment failure had a lower baseline mean CD4 count and a lower incremental rate. By the seventh year all those that had failed first-line ART regimens had been switched to a second-line ART regimen, and started experiencing a rise in CD4 count with values approaching those who had remained active on first-line ART (Figure 3).
Figure 3.
Mean CD4 count over time stratified by treatment failure status. Active on first-line ART (black line); Not active on first-line ART ( blue line).
Predictors of treatment failure
Of all variables that were significantly associated with treatment failure on univariate analysis, only being on an ABC-based regimen (HR. 7.8, 95% CI 2.6–23.8) was a significant positive predictor of treatment failure on multivariate analysis; while negative (protective) predictors were CD4 count increment at 4 years (HR. 0.99, 95% CI. 0.996–0.999) and CD4 count increments at 5 years (HR 0.99, 95% CI 0.996–0.999). CD4 count increments at 10 years (HR.1.00 95% CI 1.00–1.01) was a neutral predictor (Table 3).
Table 3a.
Predictors of antiretroviral treatment failure (univariate analysis).
| Characteristics | Univariate hazard ratios (reference value) | P |
|---|---|---|
|
| ||
| Age | 0.95 | .91 |
| 0.68 | .39 | |
| 0.79 | .63 | |
|
| ||
| Sex | 0.83 (0.54–1.29) | .420 |
|
| ||
| Marital status | 0.80 (0.42–1.5 ) | .508 |
| 0.74 (0.27–2.01) | .564 | |
| 0.46 (0.18–1.15)a | .097 | |
|
| ||
| Employment status | 0.97 (0.61–1.52 ) | .895 |
|
| ||
| Distance from treatment center | 1.11 (0.71–1.75) | .633 |
|
| ||
| CD4 count at enrolment in care | 0.79 (0.48–1.29) | .353 |
| 0.34 (0.19–0.60)a | .000 | |
Significant on univariate analysis.
Table 3b.
Predictors of antiretroviral treatment failure (multivariate analysis).
| Parameters | Sub-types | Univariate hazard ratio | P | Adjusted hazard ratio | P |
|---|---|---|---|---|---|
|
| |||||
| WHO clinical stage | I | 1.72 (0.45–4.89)a | .001 | 5.67 (0.23–8.79)b | .000 |
| II | 1.80 (0.23–3.4)a | .001 | 6.00 (0.123–2.34)b | .000 | |
| III | 1.98 (0.45–4.56)a | .001 | 5.28 (0.45–3.67)b | .000 | |
| IV | 2.30 (1.23–4.56)a | .000 | 1.15 (0.67–4/67)b | .000 | |
|
| |||||
| First-line ART regimen | ZDV/3TC/NVP or EFV | Ref | |||
| D4T/3TC/NVP or EFV | 2.7 (0.082–8.82)a | .100 | |||
| TDF/3TC/NVP or EFV | 4.9 (3.16–7.82) | .001 | |||
| ABC/3TC/NVP or EFV | 6.67 (2.04–21.76)a | .002 | 5.75 (1.5–22.03)b | .05 | |
|
| |||||
| ART duration | <12 months | ||||
| −59 months | 8.29 | ||||
| >60 months | 6.64 (0.92–10.2)a | .001 | 6.55 (0.77–8.96)b | .000 | |
|
| |||||
| CD4 count | <200 | Ref | |||
| 200–349 | 0.79 (.48–1.29) | .353 | |||
| ≥350 | 0.34 (0.19–.60)a | .001 | |||
|
| |||||
| Body mass index | <25 | Ref | |||
| 25–29.9 | 0.67(0.23–2.34)a | .140 | |||
| ≥30 | 0.53 (0.14–1.89)a | .021 | |||
|
| |||||
| Adherence | < 95% | Ref | |||
| > 95% | 2.28 (1.31–3.97)a | .003 | |||
|
| |||||
| Change in first-line ART regimen | No change | ||||
| Changed | 0.56 (0.27–1.12)a | .103 | |||
|
| |||||
| Duration on therapy | 0.99 (0.97–1.01) | .511 | |||
|
| |||||
| BMI | 0.98 (0.94–1.04) | .623 | |||
|
| |||||
| CD4 change at 1 year | 0.99 (0.99–1.00) | .843 | |||
| CD4 change at 2 year | 0.99 (0.99–1.01) | .605 | |||
| CD4 change at 3 year | 1.00 (0.99–1.01) | .391 | |||
| CD4 change at 4 year | 0.99 (0.99–1.00)a | .022 | .99 (0.996–0.999)b | .036 | |
| CD4 change at 5 year | 0.99 (0.98–1.01)a | .001 | .99 (.996–.999)b | .004 | |
| CD4 change at 6 year | 0.99 (0.98–1.01)a | .065 | |||
| CD4 change at 7 year | 0.99 (0.97–1.01)a | .002 | |||
| CD4 change at 8 year | 0.99 (0.96–1.00)a | .103 | |||
| CD4 change at 9 year | 0.99 (0.97–1.01) | .819 | |||
| CD4 change at 10 year | 1.00 (0.99–1.00)a | .005 | 1.003 (1.00–1.01)b | .000 | |
Significant on univariate analysis
Significant on multivariate analysis, CD4 change: increment or decrease in CD4 count from baseline value.
DISCUSSION
Our study showed the practicability of effectively implementing and rapidly scaling up HIV ART services within the resource constraints of a Nigerian hospital-based ART program. The evaluation of 345 patients out of the first cohorts enrolled in care demonstrated good long-term clinical and immunologic outcomes, with good retention in care, but a high rate of first-line ART regimen change, mainly due to failure. To our knowledge, this is the first report of a 10-year follow-up study of ART clinical outcomes from SSA.
Overall, the study showed a high (83%) retention rate into care over the study period, with no remarkable differences between those that failed treatment and those that did not. Attrition was highest in the first year and subsequently declined. A meta-analysis in 2007 reported a 60% retention rate in ART programs in SSA.16 Our figure is higher than earlier reports from ART programs in parts of Africa with 74% and 76% from South Africa17 and Nigeria,9 respectively. However, while these studies evaluated all cohorts, this study focused on patients with lengthy treatment experience. Furthermore, time to loss to follow up was shorter in the sub-group who remained on a potent first-line ART perhaps out of the feeling of being cured since their medications have remained potent for so long a time.
The authors found those self-reporting good adherence had a higher risk of treatment failure. Within the context of HIV care, patient reported good adherence that results in an unfavorable outcome reflects a lack of strict compliance to timing of medication usage. While delaying an hour or two with other antibiotics might not have remarkable deleterious consequences, in HIV care such delays might result in treatment failure. In the early years of HIV care, this message was not properly appreciated by patients on ART in Nigeria. Thus they might not have considered such timing glitches to be a cause for concern, and would have considered themselves as having good adherence. However, during the latter part of HIV care programs in Nigeria, adherence issues received boosted attention with the formation of dedicated adherence and LTFU tracking units. This was augmented by better patient preparation before commencement of ART. These interventions may explain the lower rate of treatment failure and attrition seen in the latter part of the follow-up period.18 There would be a need for improved and sustainable programmatic methods of tracking adherence by using pill counts or therapeutic drug level monitoring within the context of Nigeria economic challenges.
After a decade of ART, more than half of patients in the first cohort who commenced ART in our PEPFAR-supported HIV treatment program were still in care, with an active first-line ART regimen. This represents a fairly good durability, considering the myriad of developmental challenges faced in SSA. Although confronted with limited resources and a dearth of trained healthcare personnel, the programs have struggled to offer some level of quality care.
This estimates reported here may differ from prior studies because of differences in sample size and universal usage of viral load testing in the referred adult studies. The introduction of intensified adherence counseling very early in the course of therapy, to enhance treatment efficacy, may also explain the differences.
As in several other studies, patients with treatment failure had a much lower CD4 count response from baseline.19,20 Likewise, although the mean CD4 count continued to rise for both groups, it rose at a slower rate for those that failed ART during the course of treatment. Perhaps, those that failed ART had other mitigating factors, in addition to starting with a lower CD4 count. These may have included the occurrence of opportunistic infections and sub-optimal adherence, which hindered their ability to reach their full immunologic recovery potentials. Indeed, time to first-line ART failure was shorter for those starting with a lower CD4 count, perhaps due to diminished ability to mount an appropriate immune reconstitution. It is also important to consider the impact of secular trend on immune response to ART. Since the study has assessed a span of ten years, viral evolution may have influenced variation in CD4 count.21 We found that by the seventh year of ART, the decline in CD4 count was obvious and switching to second-line therapy most likely occurred at that time. As expected, after the switch, the CD4 count stopped declining and even increased, almost attaining values close to those that never failed. Other studies have suggested that mean CD4 counts could continue to increase even when the viral load is detectable.22 Thus, it is pertinent to have low-cost viral load monitoring tools for low-income countries that would allow universal timed viral load monitoring.
Poor adherence to ART, use of some NRTIs, previous virologic failure, high baseline HIV viral copies (viral load), lower baseline CD4 cell count, missed clinic appointments and commencing ART at a young age are some of the identified predictors of ART failure.23–25 As in earlier studies, we found the use of NRTIs to be predictors of early treatment failure. This may be related to their side effect profile and loss of potency resulting from genetic mutations. Stavudine (D4T) has been associated with lactic acidosis, peripheral neuropathy and lipoatrophy.26 These toxicities are not only cumulative but also irreversible. Thus, they could impair adherence and may even lead to withdrawal from treatment. Accordingly, the Nigerian HIV program had withdrawn its use except for a select few patients, under special circumstances.
The level of adherence to therapy may decline with time. This has been depicted in several studies where number of patients failing a particular regimen steadily increase with time.27 In line with this, our study also found that being on ART for more than 60 months as well as being on therapy for 10 years to be the anticlimax points in ART. Possible reasons for this observation in our study may include, for example, a cumulative development of resistance and age-related decline in immune function.
We have found the CD4 increment at the fourth and fifth years to be protective against treatment failure. By intuition, we would assume that this implies that patients who had maintained good adherence with sustained CD4 count rise at this time frame are less likely to experience treatment failure. Studies on adherence suggest that patients with good long-term adherence to ART are more likely to have sustained HIV suppression, a lower risk of drug resistance and better chances of survival.28,29
Our study has a number of limitations. These include missing data, limited availability of viral load monitoring due to programmatic constraints and use of routine data collected from a high turnover clinic. Generalizability may be limited because of the use of data from a single tertiary care hospital with higher access to technical expertise, as compared with many other SSA settings.
CONCLUSION
We have established evidence for good long-term clinical and immunologic outcomes, as well as good retention in care, from an HIV treatment program. These results add to the body of evidence supporting the need for early commencement of patients on ART before CD4 cell counts decline, as well as improved access to low cost immunologic and viral load monitoring technology, to enhance early detection of treatment failure. Whereas the effectiveness of ART on virologic suppression is best determined from randomized trials, durability in real world settings may be better evaluated in cohort studies. Hence, this study suggest that larger multicenter cohort studies would clearly establish long-term outcomes of ART programs in resource-limited settings.
REFERENCES
- 1.World Health Organization. HIV fact sheet. Geneva, Switzerland: 2013. [Accessed 30th October 2014]. [Online] Available at: http://www.who.int/mediacentre/factsheets/fs360/en/ [Google Scholar]
- 2.Federal Republic of Nigeria Federal Ministry of Health Abuja, Nigeria. National HIV&AIDS and Reproductive Health Survey(NARHS Plus II, 2012) [Accessed 30th October 2014]. [online] http://nascp.gov.ng/demo/wp-content/uploads/2014/02/NARHS-Plus-2012-Final-18112013.pdf.
- 3.Federal Ministry of Health Abuja Nigeria. National HIV&AIDS and Reproductive Health Survey (NARHS Plus II, 2012) [Accessed 21st May 2015]. Available at: http://nascp.gov.ng/demo/wp-content/uploads/2014/02/NARHS-Plus-2012-Final-18112013.pdf.
- 4.The United States Presidents Emergency Plan for AIDS Relief. Nigeria Nigeria 2014 Country Operational Plan Executive Summary. 2014. [Accessed 21st May 2015]. Available at : http://www.pepfar.gov/countries/cop/240154.htm.
- 5.National Agency for AIDS Control. Federal Government of Nigeria. Nigeria: 2014. [Accessed 2 June 2015]. Global AIDS response; pp. 37–38. [online] http://www.unaids.org/sites/default/files/country/documents/NGA_narrative_report_2014.pdf. [Google Scholar]
- 6.Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, Ele P, et al. Patient Retention and Adherence to Antiretrovirals in a Large Antiretroviral Therapy Program in Nigeria: A Longitudinal Analysis for Risk Factors. PLoS One. 2010 May 11;5(5):e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner LA, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000 Mar 10;14(4):357–66. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 8.Badri M, Maartens G, Mandalia S, Bekker L-G, Penrod JR, Platt RW, et al. Cost-Effectiveness of Highly Active Antiretroviral Therapy in South Africa. PLoS Med. 2006;3(1):e4. doi: 10.1371/journal.pmed.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimsrud A, Balkan S, Casas EC, Lujan J, Cutsem GV, Poulet E, et al. Outcomes of Antiretroviral Therapy Over a 10-Year Period of Expansion: A Multicohort Analysis of African and Asian HIV Programs. J Acquir Immune DeficSyndr. 2014;67(2):e55–66. doi: 10.1097/QAI.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 10.Gras L, Kesselring AM, Griffin JT, van Sighem AI, Fraser C, Ghani AC, et al. CD4 Cell Counts of 800 Cells/5/L or Greater After 7 Years of Highly Active Antiretroviral Therapy Are Feasible in Most Patients Starting With 350 Cells/5/L or Greater. J Acquir Immune DeficSyndr. 2007;45:183–92. doi: 10.1097/QAI.0b013e31804d685b. [DOI] [PubMed] [Google Scholar]
- 11.volution des conditions denitiation du traitement antiretroviral des patients infects par le VIH en Afrique de luest [Time trends in demographic and clinical characteristics of adult] patients on HAART initiation in West Africa] Bashia J, Balestreb E, Messouc E, Maigad M, Coffieb PA, Zannoua DM. Med Mal Infect. 2010 Aug;40(8):449–55. doi: 10.1016/j.medmal.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Intiretroviral therapy for HIV infection in adults and adolescents, Recommendations for a public health approach. 2006. [Accessed 18th November 2014]. [online] http://www.who.Int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 13.Benjamin HC, Ronald AC, Albert M, Andrew OW, Wilbroad M, Mohammed L, et al. An Empirical Approach to Defining Loss to Follow-up Among Patients Enrolled in Antiretroviral Treatment Programs. Am J Epidemiol. 2010;171:924–31. doi: 10.1093/aje/kwq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: a prospective cohort study. J Int AIDS Soc. 2009;12:38. doi: 10.1186/1758-2652-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haley DF, Lucas J, Golin CE, Wang J, Hughes JP, Emel L, et al. Retention strategies and factors associated with missed visits among low income women at increased risk of HIV acquisition in the US (HPTN 064) AIDS Patient Care STDS. 2014 Apr;28(4):206–17. doi: 10.1089/apc.2013.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajasekaran S, Jeyaseelan L, Vijila S, Gomathi C, Raja K. Predictors of failure of first-line antiretroviral therapy in HIV-infected adults: Indian experience. AIDS. 2007 Jul;21(Suppl 4):S47–53. doi: 10.1097/01.aids.0000279706.24428.78. [DOI] [PubMed] [Google Scholar]
- 18.Dragsted UB, Mocroft A, Vella S, Viard JP, Hansen AB, Panos G, et al. Predictors of immunological failure after initial response to highly active antiretroviral therapy in HIV-1-infected adults: a EuroSIDA study. J Infect Dis. 2004 Jul 1;190(1):148–55. doi: 10.1086/420786. [DOI] [PubMed] [Google Scholar]
- 19.Rajasekaran S, Jeyaseelan L, Vijila S, Gomathi C, Raja K. Predictors of failure of first-line antiretroviral therapy in HIV-infected adults: Indian experience. AIDS. 2007 Jul;21(Suppl 4):S47–53. doi: 10.1097/01.aids.0000279706.24428.78. [DOI] [PubMed] [Google Scholar]
- 20.Dragsted UB, Mocroft A, Vella S, Viard JP, Hansen AB, Panos G, et al. Predictors of immunological failure after initial response to highly active antiretroviral therapy in HIV-1-infected adults: a EuroSIDA study. J Infect Dis. 2004 Jul 1;190(1):148–55. doi: 10.1086/420786. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien TR, Hoover DR, Rosenberg PS, Chen B, Detels R, Kingsley LA, et al. Evaluation of secular trends in CD4+ lymphocyte loss among human immunodeficiency virus type 1 (HIV-1)-infected men with known dates of seroconversion. Am J Epidemiol. 1995 Sep 15;142(6):636–42. doi: 10.1093/oxfordjournals.aje.a117687. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J, Sirisanthana T, Kiertiburanakul S, Chen YM, Han N, Lim PL, et al. Trends in CD4 counts in HIV-infected patients with HIV viral load monitoring while on combination antiretroviral treatment: results from The TREAT Asia HIV Observational Database. BMC Infectious Diseases. 2010;10:361. doi: 10.1186/1471-2334-10-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 24.Lundgren JD, Mocroft A, Gatell JM, et al. A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis. 2002;185:178–187. doi: 10.1086/338267. [DOI] [PubMed] [Google Scholar]
- 25.Deeks SG, Hecht FM, Swanson M, et al. HIV RNA and CD4 cell count response to protease inhibitor therapy in an urban AIDS clinic: response to both initial and salvage therapy. AIDS. 1999;13:35–43. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- 26.Shibuyama S, Gevorkyan A, Yoo U, et al. Understanding and avoiding antiretroviral adverse events. Curr Pharm Des. 2006;12(9):1075–90. doi: 10.2174/138161206776055796. [DOI] [PubMed] [Google Scholar]
- 27.The Antiretroviral Therapy Cohort Collaboration (ART-CC) Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and North America 2002–2009. AIDS. 2013;27(5):803–13. doi: 10.1097/QAD.0b013e32835cb997. [DOI] [PubMed] [Google Scholar]
- 28.Chesney MA. The elusive gold standard. Future perspectives for HIV adherence assessment and intervention. [Accessed 30th November 2014];J Acquir Immune DeficSyndr. 2006 43(Suppl 1):S149–155. doi: 10.1097/01.qai.0000243112.91293.26. [Online] Available at http://www.ncbi.nlm.nih.gov/pubmed/17133199. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO) Adherence to long term therapies-evidence for action. 2003. [Accessed 2th December 2014]. [Online] Available at http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf.