Abstract
BACKGROUND AND OBJECTIVES
Generic substitution has become a common practice since the late 1970s in the United States. At that time, many of these generics caused bioavailability problems, which fueled suspicions about their efficacy and safety, and the Food and Drug Administration (FDA) standards for bioequivalence. In Saudi Arabia, the increasing number of local products raised several concerns with regard to switching from brands to generics. Our objective was to review and examine the basis of the controversy surrounding brand and generic interchangeability and to explore a practical approach in pursuing a switch.
DATA SOURCES
Articles indexed initially under terms such as generic medications, generic substitution, bioequivalence and bioinequivalence were identified. These terms were used to search the indexing service, MEDLINE (1966–2006). References from the extracted articles, and additional data sources, including the Code of Federal Regulations and Regulatory Guidance from the FDA Center for Drug Evaluation and Research were also reviewed.
DATA SYNTHESIS
For most drugs, bioequivalence testing generally should enable clinicians to routinely substitute generic for innovator products. However, for narrow therapeutic, critical dose drugs, or for highly variable drugs, safe switching between products cannot be assured. These drugs need special precautions and blood level monitoring upon switching. FDA firmly believes that approved generic and brand drugs can be dispensed with the full expectation that the consumer will receive the same clinical benefit.
CONCLUSION
Performing the switch process is an advisable practice to reduce health care costs in countries with strong post-marketing surveillance program, but caution is to be exercised when narrow therapeutic index drugs or highly variable drugs are prescribed.
To understand the basis of the controversy surrounding brand and generic interchangeability, one must have a thorough understanding of the terms associated with generic drugs. A drug is generic if it is identical or bioequivalent to a brand name drug in dosage form, safety, strength, route of administration, quality, performance characteristics and intended use.1 The generic drug is meant to be used interchangeably with the innovator’s drug product. A generic drug is usually manufactured without a license from the original innovator. A generic drug product hits the market-place after the expiry of all patent and marketing exclusivity rights of an innovators product.1
Bioequivalence is defined as “the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study”.1
A product may also be considered bioequivalent to an innovator product if the difference in rate of absorption of the drug between the two products is intentional and there is no significant difference in the extent of absorption of the two products when they are administered in the same molar dose under similar experimental conditions.2
Bioavailability refers to the “the rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of action. For drug products that are not intended to be absorbed into the bloodstream, bioavailability may be assessed by measurements that reflect the rate and extent to which the active ingredient or active moiety becomes available at the site of action”.1
Pharmaceutical equivalents are drug products that contain the same amount of the same active substance(s), in the same dosage form, for the same route of administration and meeting the same or comparable standards”.3 Pharmaceutical alternatives are drug products that contain the same active moiety but contain different chemical forms such as salts or esters of the active moiety or they may differ from the innovator’s product in the dosage form or strength.4
A historical perspective
Historically, generic products manufacturers of such drugs as codeine sulphate and phenobarbital were allowed to formulate, manufacture, and sell their products without submitting bioequivalence or efficacy data to the Food and Drug Administration (FDA). The 1938 Act founded a “new drug” category, mandating manufacturers to document the safety of a product to the FDA. Until 1962, generic versions of post-1938 drugs were marketed based on a “general recognition” of safety. Classically, this labelling rested on a history of safe use of the innovator product. Such generic products were designated as “not new drugs”.5
The 1950s witnessed an explosion in the growth of new drugs and were the beginning of generic substitution for brand name medications by pharmacists. This resulted in pharmaceutical manufacturer trade groups pressing states to pass laws forbidding replacement of a prescribed brand name product. With the 1960s thalidomide disaster, Congress added a requirement for efficacy standards for new medications. Congress passed the Kefauve-Harris Drug Amendments Act in 1962. As a result of this, ruling scientists had to prove that a drug was safe and effective before it could be sold to the American public. Costs of brand medications began to rise in the 1970s and this provided reason to repeal the previous laws forbidding generic substitution, which have amplified the generic substitution practice again.1
In the 1970s, many generic drugs had problems with bioavailability. Therefore, the FDA established the Abbreviated New Drug Application (ANDA). ANDA waives the need for preclinical testing, safety and efficacy clinical studies of the generic drug as long as it is proved to be bioequivalent to the innovator drug.2
In 1984 the Hatch-Waxman Act has bestowed authority upon the FDA to approve generic versions as safe and effective for drugs approved after 1962. For bioequivalence, the FDA requires that the population mean values for the area under the curve (AUC) and the peak concentration of the drug in the blood (Cmax) of the generic product be not less than 20% or greater than 25% of the population mean values of the innovator product.2
In 1986, the FDA conducted a three-day public hearing to discuss the agency’s method of determining bioequivalence of generic drugs. The hearing concluded that the −20%/+25% rule is satisfactory and shown to be clinically acceptable.6 Although both pro-substitution and anti-substitution clinicians felt that an acceptable degree of bioavailability variation for generic drugs should be 11% for most medications and 5% for a critical dose medication.7
The scandal of generics in 1989 has rejuvenated suspicion about the safety and efficacy of generic medications. At that time, numerous disturbing facts about generic submissions to the FDA were unveiled, among them: some FDA employees were bribed to expedite the regulatory approval of submitted files, there were fraudulent submissions to the FDA for approval of generic drugs by several firms and violations of the good manufacturing practice (GMP) regulations. This has resulted in extensive FDA inspections. Data from hundreds of generic drug applications (including several narrow therapeutic index drugs such as aminophylline tablets) were re-examined. The FDA determined that only 27 samples, or approximately 1% of those tested, did not comply with standards of potency, dissolution, content uniformity, product identification, moisture determination, or purity. Only five of the samples (all aminophylline tablets) failed to meet United States Pharmacopoeia standards. None of the defects in the generic drugs were judged to pose a public health hazard.5
Upon prudent scrutiny of all the recommendations from expert panels in addition to public comments, Legislator bodies such as the FDA and the European Medicines Agency (EMEA) issued a final guidance for industry entitled Bioavailability and Bioequivalence Studies for Orally Administered Drug Products-General Considerations.1,4 These guidelines set the grounds to assure bioequivalence of generic drugs and to safeguard against the recurrence of events learned from history. In addition to data from bioequivalence studies, other data may need to be submitted to meet regulatory requirements for bioequivalence. Such evidence may include analytical methods of validation and the in vitro-in vivo correlation studies.
In a letter to health practitioners in 1998, the FDA stated that:
Additional clinical tests or examinations by the health care provider are not needed when a generic drug product is substituted for the brand-name product.
Special precautions are not needed when a formulation and/or a manufacturing change occurs for a drug product provided that the change is approved according to applicable laws and regulations by the FDA.
As noted in the “Orange Book”, in the judgment of the FDA, products evaluated as therapeutically equivalent are expected to have equivalent clinical effect whether the product is a brand name or a generic drug product.
It is not necessary for the health care provider to approach any one therapeutic class of drug product differently from any other class, when there has been a determination of therapeutic equivalence by FDA for the drug products under consideration.8
Perception of the Generic Switch Concept Among End-users
Physicians, pharmacists and patients were surveyed regarding their views on generic substitution. In this survey, pharmacists in general supported the use of generic drugs. The most important factors that pharmacists cited for selecting a product were quality, price and supplier consistency. Seventy-two percent of the patients accepted generic medication when pharmacists recommend them and 75.8% of patients agreed on generic medications when a physician suggested them. Of particular significance, only 17% of physicians who were surveyed correctly identified the FDA standards for bioequivalence.9,10
FDA Regulations for Generics
The FDA firmly believes that drug products, which have gone through the approval process, brand name or generic, can be dispensed and used with the full expectation that the consumer will receive the same clinical benefit. However, the FDA has specific requirements that generic products must fulfill prior to obtaining approval.11–13
Assuring the acceptability of a given generic launched into the market
Post-marketing surveillance (PMS) is one of the best mechanisms to protect patients from problems associated with drugs and generic brand medication variations. 14 Because all possible side effects of drugs cannot be anticipated based on pre-approval studies involving only several hundred to several thousand patients, FDA maintains a system of PMS and generates assessment programs to identify adverse events that did not appear during the drug approval process. The agency uses this information to update drug labeling, or occasionally, to reevaluate the approval or marketing decisions. There are systems used by the FDA to assure ongoing safety and effectiveness of drug products currently marketed in the United States, that include the Spontaneous Reporting System, the Adverse Events Reporting System, MedWatch, phramcoepidemiology, prescription drug advertising and promotional labeling, pharmaceutical industry surveillance, medication errors, drug shortage, and therapeutic inequivalence reporting systems.
The Spontaneous Reporting System is a computerized database containing reports from the late 1960s through January 1997, of more than 1 million adverse drug reactions primarily reported by health professionals. The primary purpose for maintaining this database was to serve as an early warning system for adverse drug reactions not detected during pre-marketing testing. This system has been replaced by the Adverse Events Reporting System, which offers electronic submission options and international compatibility. The MedWatch program is for health professionals and the public to voluntarily report serious reactions and problems with medical products, drugs, and medical devices. Another safety measure is the FDA Therapeutic Inequivalence Action Coordinating Committee (TIACC). TIACC was created to identify and evaluate reports of therapeutic failures and toxicity that could indicate that one product is not equivalent to another similar product. The committee also provides a mechanism for timely follow up on reports of therapeutic inequivalence and, when appropriate, conducts a full-scale investigation of these issues. Once an inequivalent product is identified, TIAAC can take a number of actions. These include:
Removing inequivalent products from the market.
Evaluating and changing the therapeutic equivalence rating of a product
Recommending that a grandfathered product submit a new drug application
Testing and evaluating the relationship of dissolution and bioequivalence
Recommending appropriate dissolution specifications for narrow therapeutic drugs and evaluating the toxicity profile of injectables and mandating appropriate controls.
What data source should be used to assure the acceptability of a generic medication?
Perhaps the best source of bioequivalence information is the Approved Drug Products with Therapeutic Equivalence Evaluations, “the Orange Book”. In the Orange Book, drug products are rated as either A (substitutable) or B (non-interchangeable). Secondary letters can indicate the type of study by which a product was determined to be bioequivalent, i.e., in vitro or invivo studies or the type of formulation that is not considered bioequivalent.15 For example, “A” drug products that FDA considers to be therapeutically equivalent to other pharmaceutically equivalent products, i.e., drug products for which:
There are no known or suspected bioequivalence problems. These are designated as A, AA, AN, AO, AP, or AT, where the second letter indicate the dosage form, e.g. AT means the topical dosage form of the generic drug is bioequivalent to the topical dosage form of the reference drug and so on.
Actual or potential bioequivalence problems have been resolved with adequate in vivo and/or in vitro evidence supporting bioequivalence. These are designated as AB.
“B” drug products that the FDA at this time considers NOT being therapeutically equivalent to other pharmaceutically equivalent products. This includes drug products for which actual or potential bioequivalence problems have not been resolved by adequate evidence of bioequivalence. Often the problem is with specific dosage forms rather than with the active ingredients. These are designated BC, BD, BE, BN, BP, BR, BS, BT, BX, where the second letter indicate the dosage form.16
The Impact of Generic Pharmaceutical Industry on Healthcare Expenditures
Generic drugs are chemically identical to their branded counterparts, yet they are typically sold at substantial discounts from the branded price. According to the Congressional Budget Office, generic drugs save consumers an estimated $8 to $10 billion a year at retail pharmacies. Even more billions are saved when hospitals use generics.15
The Kingdom’s imports of pharmaceutical products have witnessed a surge between 2001 and 2005, and have recorded an average growth of 16%. The value of pharmaceutical imports has recorded a large jump in 2005 and has reached 6229 million SR which equals to $1.6 billion. Local Saudi economic sources have pointed out that local pharmaceutical plants have contributed to only 20% of the local Saudi pharmaceuticals market which means that foreign pharmaceutical imports have occupied 80% of this market. It is noteworthy to mention that the Saudi pharmaceuticals market is considered the largest pharmaceuticals market in this part of the world hitting the limits of $2 billion annually.17
Health expenditure consumes a significant part of the governmental budget. Using imported pharmaceutical products incurs a costly burden on the shoulders of the governmental budget. Prudent substitution of expensive innovators products with more economic generic options, when appropriate, shall reduce health cost and boost the Saudi economy.
Saudi Arabian Ministry of Health Regulations18
In general, the Saudi Arabian Ministry of Health (SMOH) regulations governing bioequivalence studies are very similar to FDA regulations, which guarantee the need for worldwide harmonization. On the other hand, minor exceptions were adopted to meet the specific needs for Saudi Arabia especially regarding the reference product and number of subjects. These exceptions can be summarized as follows:
-
Study Design
-
Immediate release products:
For immediate release oral solid dosage forms (e.g., tablets and capsules) and for oral suspensions, a single-dose, two-way, two-treatment, two-period, two-sequence crossover study should be conducted under fasting conditions using the highest strength of the dosage form available. An interesting exception to that rule is the oral hypoglycemic agent glimepiride (Amaryl) where the reference listed drug is 1 mg and NOT the highest strength.16 The plausible explanation for this exception is the fact that this product is a potent oral hypoglycemic agent that will be administered to healthy volunteers, hence, and from the ethical standpoint and to consider safety issues, the lowest strength is considered to be the reference against which a generic copy is to be compared.
-
Modified release products:
Delayed release pharmaceutical products such as enteric coated dosage forms and sustained (prolonged and controlled) release dosage forms are categorized into a group called modified release dosage forms. For this group, two types of studies are required:
A single dose study comparing the bioavailability of the highest strength of the generic product to that of the reference product under fasting conditions.
A single dose study comparing the bio-availability of the highest strength of the generic product to that of the reference product under fed conditions.
-
FDA may waive bioequivalence requirements when data demonstrate that formulations are identical and bioavailability is self-evident, i.e. injectables, ophthalmic solutions, and oral solutions. The waivers are based on the fact that these formulations are solutions and dissolution is not a concern.2
-
The reference product:
The FDA guidelines define the reference drug product as a currently marketed, brand-named product with a full new drug application approved by the FDA. For the SMOH, the reference product would normally be the innovator product or the market leader product provided that its safety, efficacy and quality have been established and documented and this product is currently marketed in Saudi Arabia, i.e. all generic drug products either international or local must be compared to the reference product marketed in the Saudi market to assure interchangeability. The SMOH definition of the reference product has been proven to be very problematic for some international pharmaceutical companies who rightfully conduct their bioequivalence studies using the reference product in the country of origin (for example, USA and Europe). However, these bioequivalence studies are not acceptable in Saudi Arabia because the reference product is not the same reference product in Saudi Arabia. An outstanding example on the importance of the reference product for bioequivalence studies is the innovator product for the oral hypoglycemic agent gliclazide manufactured by Servier under the brand name Diamicron. This product is manufactured in France and the UK under the same brand name and using the same dose. However, the release profiles of the two products (Diamicron, France and Diamicron, UK) are totally different and therefore they can be viewed as two different drug products. Diamicron from France is the brand product registered in Saudi Arabia and therefore to assure interchangeability with this drug product, any generic product of gliclazide must be compared to Diamicron from France and not Diamicron from the UK.18
-
Subjects:
The FDA guidelines recommend that the number of subjects enrolled in bioequivalence studies should be sufficient to ensure adequate statistical outcomes. This number is based on the power function of the parametric statistical test procedure applied and should be a minimum of 12 subjects. However, since the patient risk of erroneously accepting bioequivalence is of primary concern for the SMOH, it was decided that a minimum of 24 subjects should be enrolled in bioequivalence studies to be considered for acceptance.
-
Statistical analysis and acceptance criteria:
For a single dose bioequivalence study, it is recommended that sampling should be extended at least 4 to 5 terminal elimination half lives of the drug of interest. The collected samples are processed and stored under conditions that guarantee integrity of the samples until they are analyzed. Biological samples are then analyzed using an accurate, precise, selective and reproducible method of analysis validated according to international guidelines.19 The following pharmacokinetic parameters are then calculated and subjected to statistical analysis:
Cmax: Maximum drug concentration
Tmax: Time to maximum drug concentration
AUCo–t: Area under the curve from time zero to last quantifiable concentration
AUCo–∞: Area under the curve from time zero to infinity
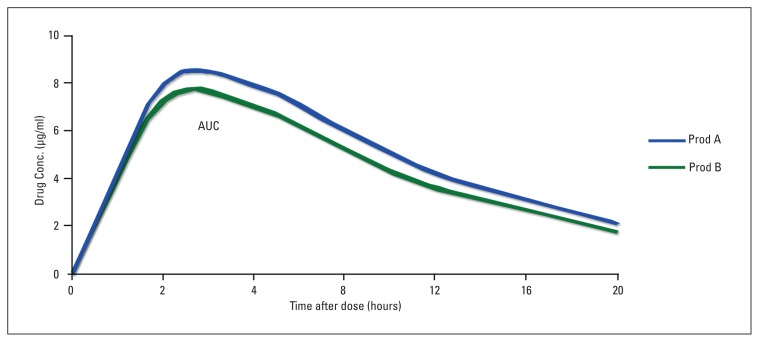
For AUCo–t and AUCo–∞, the parametric 90% confidence interval of the ratio log transformed data should be within acceptance range of 80–125% (Figure 1). For Cmax, the acceptance range is 70–143% when log transformed data is used. The 90% confidence interval (acceptance range of 80–125%) is only conducted for Tmax if there is an established relation between release of the drug to adverse effects or if there is a clinically relevant claim based on the rapid release of the drug from the dosage form.
Figure 1.
Bioequivalence of a generic product (B) and a reference product (A). Product A is the reference product. Product B is the test (generic) product. The relevant parameters are: Drug A: Cmax=8.1 μg/ml; Tmax=2.6 h; AUCo-∞=124.9 μg.h/ml Drug B: Cmax=7.6 μg/ml; Tmax=2.1 h; AUCo-∞=112.4 μg.h/ml The ratio of areas (generic:reference), and therefore the relative bioavailability, is 0.9 To be accepted as bioequivalent, the 90% Confidence Intervals for the area ratio would need to fall within the 0.8–1.25 range* Adopted from Ref 3.
Organizations that develop standards for generic drug substitution
Some major professional pharmacy organizations have not issued written policies for generic substitution, while all other pharmacy organizations that do have written policies, e.g. American Pharmaceutical Association (http://www.aphanet.org) and American Society of Health-System Pharmacists (www.ashp.org), support appropriate generic substitution and emphasize the important role of the pharmacist in this regard. Of note, none of these organisations supported unilateral switch decisions performed by pharmacists to exchange different products within the same therapeutic class.9
Practical hints in handling selected brand-generic switches
Anti-arrhythmics (amiodarone)
Given the uncertain impact of the combination of advanced age, current diseases, and concomitant drug therapy on bioequivalence, it may be advisable to initiate antiarrythmic drug therapy with the innovator preparation. Presumed bioequivalence dosage forms should be avoided and the patient should continue to be treated with the drug product that was used in the titration to an effective antiarrythmic response.20,30
Anticoagulants: (Warfarin)
Controversy continues to exist regarding the substitution of generic warfarin for the innovator’s product or vice versa. Recommendations vary from an affirmative “Yes” from the FDA to a “No” from anticoagulation therapy guidelines issued by organizations such as the Institute for Clinical Systems Improvement (ICSI). Based on bioequivalence studies, generic warfarin can be used safely at the initiation of therapy. It is recommended that patients should take a single warfarin product whenever possible. Furthermore, additional INR monitoring should occur in the days and weeks after substitution of one warfarin product for another to allow timely detection of those patients who experience significant changes in anticoagulation response.21
Immunosuppressants (Cyclosporine)
Neoral cyclosporine capsules or solution for microemulsion and SangCya may be started at the same daily dose as the previously used oral Sandimmune. The Neoral, and SangCya dose is then adjusted to attain the preconversion cyclosporine blood trough concentration. Cyclosporine blood trough concentrations should be monitored every 4 to 7 days after conversion to Neoral or SangCya. Cyclosporine blood trough concentrations should be measured at least twice a week when converting patients to Neoral at doses greater than 10 mg/kg/day until the concentration stabilizes.22
Antiepileptics (Phenytion, Carbamezapine)
Generic substitution can be approved only if safety and efficacy are not compromised. Patient safety and drug efficacy may be unduly compromised by indiscriminate switching to, from, or between generic drugs for patients taking phenytoin or carbamazepine. Physicians should avoid switching between formulations of antiepileptic medications except when medically necessary, particularly with carbamezapine or phenytion. Blood levels are to be monitored closely at the time of any known or suspected switch to a different formulation. Medication dose should be readjusted accordingly. Unilateral switch decisions performed by pharmacists to exchange different products are not encouraged.23
Thyroxine
Levothyroxine has been on the market for nearly 50 years since the introduction of Synthroid in 1955. According to the Orange Book, only Unithroid or Mylan’s generic levothyroxine can be substituted. All other levothyroxine products are BX rated and require the prescriber’s permission for substitution. Package labelling for levothyroxine products recommend obtaining a thyroid stimulating hormone (TSH) level within eight to twelve weeks of changing a levothyroxine dose or brand and every six to twelve months thereafter.24
Highly variable drugs
These are drug products that demonstrate a high variability in pharmacokinetic parameters, and thus pose a challenge in bioequivalence testing. One explanation for this phenomenon is the high intrasubject variability of the drug product. Examples of these drug products include propafenone immediate release, verapamil, and nadolol. It is challenging to extrapolate the current rules of bioequivalence (−20/+25%) on this subset of medications. A different approach to evaluate such products has been proposed by the FDA.2
National concerns and opportunities for improvement
Lack of national post-marketing surveillance program and under-reporting of medication quality
The lack of a PMS program is a challenge to the integrity of our health system. PMS is a necessity mandated by the crucial need to monitor for the safety of marketed products (both generics and innovators). It is our opinion that such a program should be started by the Saudi FDA in the years to come. Health care professionals need to be properly trained for such a program. As for generics, and after they pass bioequivalence testing, consistent quality remains a question of debate. PMS will identify such generic products of low quality (i.e. inferior efficacy) or those with lower safety profile (i.e. more toxicity) if they are launched into the market, and via the PMS, we will thus ascertain the required level of consistent bioequivalence. The FDA TIACC is a model that can be locally simulated to achieve the goal of integration of the health care system.
On the other hand, underreporting of adverse findings in quality is an ever existing issue, let aside the lack of a defined framework of quality reporting system. Numerous factors stand behind the underreporting attitude of healthcare professionals, among them, the intricate and multi-task nature of the jobs performed, the unawareness of the necessity and implications of quality reporting, the elective nature of reporting, and the lack of incentives to reporting.
The underreporting attitude leaves us blinded in many angles of healthcare and counteracts the relentless efforts exerted to improve healthcare in terms of service provided, and in attempts to cut down unnecessary health costs.
The” Yoyo” puzzle of switching-non switching
Brand-generic substitution
Generic substitution is a practice advocated by health authorities, healthcare professionals, and policy makers principally for economic reasons. For instance, in 2002, the Italian Ministry of Health saved an estimated €25 million as a result of introducing generic drugs.25
In 1997, and in support of this concept, the FDA examined all generic drug applications approved, and the observed differences between the innovator’s and generic products for AUCo-∞ was +3.25% (SD, 2.97), for AUCo-t it was +3.47% (SD, 2.84) and for Cmax it was +4.29% (SD, 3.72). These results confirmed an earlier review performed by the FDA in 1984 through 1986 in which the average difference between generic and branded products in AUC was about 3.5%.26
On the other hand, in 1995–1996, the UK Medicines Control Agency (MCA) examined 2427 generic product samples, and a total of 228 deficiencies were discovered. The MCA requested 84 product quality improvements with respect to labelling, packaging, methods of analysis, and products specifications.27
The concept of generic substitution rests on demonstrating bioequivalence via passing the criteria set by international regulators, and this in turn, relies on the concept that demonstrating bioequivalence equates with comparable clinical efficacy and tolerability (i.e. demonstrating bioequivalence means that the generic bioequivalent product is equi-effective and as safe as the branded reference product). Several concerns and questions challenge the current bioequivalence guidelines, such as the use of single regulatory acceptance range for all drug products. Would demonstration of bioequivalence mean that the bioequivalent version possess the same efficacy and safety profile of the branded reference product? Should extrapolation of bioequivalence results demonstrate in normal healthy volunteer subjects apply to all patients populations? Bioequivalence studies are performed on a small number of subjects.24–26 The use of single dose studies in bioequivalence testing, while all drugs are used in multiple doses.27,28
Generic-generic substitution
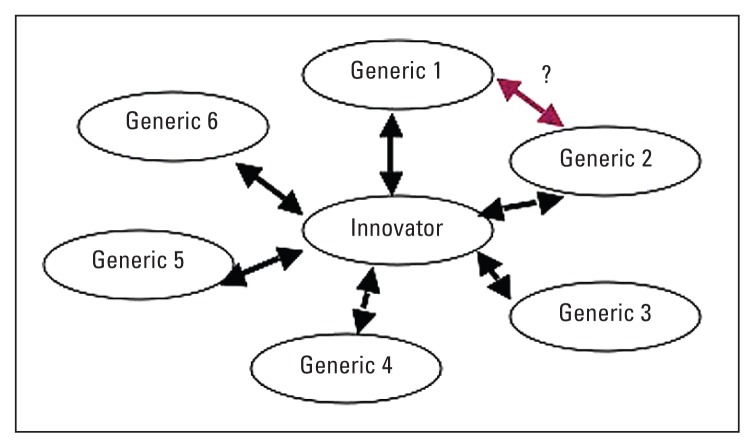
Full impunity is granted to pharmacists to switch among different generic versions of a branded reference product should they prove bioequivalent. Figure 2 depicts the concern, which is based on the concept that should two different generic versions of a branded reference product prove bioequivalent to the reference product, it is assumed that these two generic products are freely interchangeable. However, no data are available to suggest that this theme is tenable.29
Figure 2.
Generic-generic substitution concept.
Interestingly, the FDA does not openly indicate that a generic drug product can be substituted by another generic drug product, even though both of these generic products have demonstrated bioequivalence to the same branded reference product.28 Thus concerns arise when the concept of substitution is adopted.
Most local generic manufacturers in Saudi Arabia limit their production lines to a selected category of pharmaceutical products, which has resulted in a suffocated portfolio of generic products in the local pharmaceuticals market and which is counter to the long-term objective of pharmaceutical security in the region. Hence, all generic manufacturers are strongly encouraged to enrich their portfolio of productions by considering different pharmaceutical categories of medications, and strengthening their alliances with international manufacturers to help transfer the knowledge and technology in order to eventually secure our needs of this indispensable commodity.
Conclusion
Brand-to-generic switching is a plausible option should bioequivalence become evident. Narrow therapeutic index, critical dose, and highly variable medications are not freely interchangeable with their innovator counterparts, and thus demand closer laboratory and clinical monitoring than others.
Strict SMOH regulations and a thorough evaluation of generic application should minimize the bioinequivalence problems should they exist. Health care providers, particularly pharmacists, should contribute significantly in reporting any bioequivalence problems to the SMOH and the Saudi FDA through their post-marketing surveillance systems and to counsel the patient when the switch takes place.
REFERENCES
- 1.Guidance for Industry Bioavailability and Bioequivalence Studies for Orally Administered Drug Products - General Considerations. U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER); Mar, 2003. [As accessed May, 8 2007]. Available at http://www.fda.gov/cder/guidance/5356fnl.pdf. [Google Scholar]
- 2.Welage Lynda S, Kirking Duane M, Ascione Frank J, Gaither Caroline A. Understanding the Scientific Issues Embedded in the Generic Drug Approval Process. J AM Pharm Assoc. 2001;41(6):856–867. doi: 10.1016/s1086-5802(16)31327-4. [DOI] [PubMed] [Google Scholar]
- 3.Donald J. Birkett, Generics - equal or not? Aust Precr. 2003;26(4) [Google Scholar]
- 4.Note for guidance on the investigation of Bioavailability and bioequivalence. The European Agency for Evaluation of Medicinal Products; Jul 26, 2001. [As accessed May, 8 2007]. Evaluation of Medicines for Human use. Available at http://www.emea.eu.int/pdfs/human/ewp/140198en.pdf. [Google Scholar]
- 5.American Medical Association; [As accessed May, 8 2007]. Featured Report: Generic Drugs (A-02) Full Text report, presented as Council on Scientific Affairs Report 6 at the 2002 AMA Annual Meeting, represents information and AMA policy on this subject as of June 2002. available at http://www.ama-assn.org/ama/pub/category/15279.html. [Google Scholar]
- 6.Therapeutic Equivalence of Generic Drugs, Response to National Association of Boards of Pharmacy. U.S. Department of Health and Human Services, Food and Drug Administration; Apr 16, 1997. [As accessed May, 8 2007]. Available at http://www.fda.gov/cder/news/ntiletter.htm. [Google Scholar]
- 7.Banahan BF, 3rd, Kolassa EM. A Physician Survey on Generic Drugs and Substitution of Critical Dose Medications. Arch Intern Med. 1997;157(18):2080–2088. [PubMed] [Google Scholar]
- 8.Therapeutic Equivalence of Generic Drugs, Letter to Health Practitioners. U.S. Department of Health and Human Services, Food and Drug Administration; Jan 28, 1998. [As accessed May 8, 2007]. Available at http://www.fda.gov/cder/news/nightgenlett.htm. [Google Scholar]
- 9.Kirking Duane M, Gaither Caroline A, Ascione Frank J, Welage Lynda S. Physicians’ Individual and Organizational Views on Generic Medications. J Am Pharm Assoc. 2001;41(5):718–722. [Google Scholar]
- 10.Gaither Caroline A, Kirking Duane M, Ascione Frank J, Welage Lynda S. Consumers’ Views on Generic Medications. J Am Pharm Assoc. 2001;41(5):718–722. doi: 10.1016/s1086-5802(16)31281-5. [DOI] [PubMed] [Google Scholar]
- 11.Abbreviated New Drug Application (ANDA) Process for Generic Drugs. U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER); [as accessed May, 8 2007]. Available at http://www.fda.gov/cder/ogd/anda_checklist.pdf. [Google Scholar]
- 12.Wagner JG. Biopharmaceutics: Absorption aspects. J Pharm Sci. 1961;50:359–87. doi: 10.1002/jps.2600500502. [DOI] [PubMed] [Google Scholar]
- 13.Singh P, Guillory JK, Sokoloski TD, Benet LZ, Bhatia VN. Effect of inert tablet ingredients on drug absorption. I. Effect of PEG 4000 on intestinal absorption of four barbiturates. J Pharm Sci. 1966 Jan;55(1):63–8. doi: 10.1002/jps.2600550114. [DOI] [PubMed] [Google Scholar]
- 14.Post drug-Approval Activities. U.S. Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER); [As accessed May 8, 2007]. Available at http://www.fda.gov/cder/regulatory/applications/Postmarketing/activitespost.htm. [Google Scholar]
- 15.Food and Drug Administration Centre for Drug Evaluation and Research approved drug products with Therapeutic Equivalence Evaluations. [As accessed May, 8 2007]. last Updated: April 20, 2001. Available at http://www.fda.gov/cder/ob/docs/preface/ecpreface.htm#Therapeutic%20Equivalence-Related%20Terms.
- 16.The Orange Book. U.S. Department of Health and Human Services, Food and Drug Administration; [As accessed May 8, 2007]. Approved Drug Products with Therapeutic Equivalence Evaluations. Available at http://www.fda.gov/cder/ob/default.htm. [Google Scholar]
- 17.Saudi Ministry of Planning and economy. Central Department of Statistics; 2005. [Google Scholar]
- 18.Saudi Food and Drug Authority. Drug Sector. Kingdome of Saudi Arabia; [as accessed November 2007]. Bioequivalence Requirement Guidelines. Available at http://www.sfda.gov.sa/En/Drug/Topics/Regulations+-+Guidelines.htm. [Google Scholar]
- 19.Shah VP, et al. Bioanalytical method validation- A revisit with a decade of progress. Pharm Res. 2000;17:121551–1557. doi: 10.1023/a:1007669411738. [DOI] [PubMed] [Google Scholar]
- 20.Kowey PR. What to do About Prescribing Generic Antiarrythmic Drugs. Clinical Dialogues on Arrhythmias. 1990;2:1–3. [Google Scholar]
- 21.Boehinger Sherri K. Can Patients be Safley Switched from Brand to Generic Warfarin. Pharmacists Letter. 2003 Sep;19 (190902) [Google Scholar]
- 22.Subatini S, Ferguson RM, Heiderman JH, Hull AR, Kirkpatrick BS, Barr WH. Drug Substitution in Transplantiation: a National kidney Foundation White Paper. Amj Kidney Dis. 1999;33:389–397. doi: 10.1016/s0272-6386(99)70318-5. [DOI] [PubMed] [Google Scholar]
- 23.Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: Generic Substitution for Antiepileptic Medications. Neurology. 1990;40:1641–1643. [PubMed] [Google Scholar]
- 24.Gayle NS. Levothyroxine Substitution. Pharmacists Letters. 2003 Aug;19 (190811) [Google Scholar]
- 25.Borgherini G. The Bioequivalence and Therapeutic Efficacy of Generic Versus Brand-Name Psychoactive Drugs. Clin Ther. 2003;25(6):1578–1592. doi: 10.1016/s0149-2918(03)80157-1. [DOI] [PubMed] [Google Scholar]
- 26.Henney J. Review of generic bioequivalence studies. JAMA. 1999;282(21):1995. [PubMed] [Google Scholar]
- 27.Meredith P. Bioequivalence and other unresolved issues in generic drug substitution. Clin Ther. 2003;25(11):2875–2890. doi: 10.1016/s0149-2918(03)80340-5. [DOI] [PubMed] [Google Scholar]
- 28.Schachter SC, Gordon J. Generic and Brand name AEDS - considerations for clinicians and patients. [As accessed May 8, 2007]. Available at http://professionals.epilepsy.com/page/generic_considerations.html.
- 29.Midha KK, Rawson MJ, Hubbard JW. Bioequivalence: Switchability and scaling. EurJ Pharm Sci. 1998;6:87–91. doi: 10.1016/s0928-0987(97)00080-8. [DOI] [PubMed] [Google Scholar]
- 30.Reiffel JA. Issues in the use of generic antiarrhythmic drugs. Curr Opin Cardiol. 2001 Jan;16(1):23–9. doi: 10.1097/00001573-200101000-00004. [DOI] [PubMed] [Google Scholar]