Abstract
BACKGROUND AND OBJECTIVES
Allergic fungal sinusitis (AFS) is a relatively newly recognized entity consisting of a pansinusitis with allergic mucinous infiltrates in all involved sinuses. Historically mistaken for a paranasal sinus tumor, AFS is believed to be an allergic reaction to aerolized environmental fungi, usually of dematiaceous and Aspergillus species, in an immunocompetent host. We determined the occurrence of AFS in patients with chronic rhinosinusitis (CRS) to identify accurate preoperative parameters for AFS, as well as to identify the common fungi causing AFS in Saudi Arabia.
METHODS
We conducted a retrospective chart review of 406 cases of CRS undergoing functional endoscopic sinus surgery from 2001 to 2005. Data regarding patient demographics, presenting symptoms, ENT examination, laboratory and radiological features, histopathological features and fungal culture was collected and analyzed.
RESULTS
Fungal cultures were positive in 69 (16.9%) cases of CRS. Based on radiological features, histopathologic findings and culture results, AFS was diagnosed in 59 (14.5%) cases. Nasal polyposis was present in 56 (94.9%) cases; multiple sinuses were affected in all cases. Aspergillus species was the commonest causative fungal pathogen, being isolated in 40 (67.8%) cases, whereas dematiaceous fungi were isolated in 19 (32.2%) cases.
CONCLUSIONS
AFS has been an underdiagnosed clinical entity. Only increased awareness among physicians of fungal involvement will increase accuracy of diagnosis.
Traditionally, fungal infections of the paranasal sinuses have been considered uncommon and were thought to occur only in immunocompromised individuals. However, the occurrence of fungal sinusitis has increased recently in the immunocompetent population.1 It is now believed that fungi are important etiologic agents of sinusitis.2 Over the last two decades the incidence of fungal sinusitis has increased dramatically.3 The American Academy of Otolaryngology-Head and Neck Surgery lists four types of fungal sinusitis: 1) mycetoma fungal sinusitis, 2) allergic fungal sinusitis (AFS), 3) chronic indolent sinusitis, and 4) fulminant sinusitis.4 AFS is the most common form of fungal sinusitis.4
AFS is a unique, probably under-diagnosed condition similar to the lower airway disorder allergic bronchopulmonary aspergillosis.5–7 Nonetheless, AFS is a distinct clinical entity.8 Historically mistaken for a paranasal sinus tumor, AFS is now believed to be an allergic reaction to aerolized environmental fungi, in an immunocompetent host.3 AFS is characterized by a typical ‘allergic mucin’ which is usually greenish brown with a peanut butter-like consistency, prominent eosinophilia, Charcot-Leyden crystals, sloughed respiratory epithelium and fungal hyphae without tissue invasion.9 The most common causes of AFS are the dematiaceous molds, including Curvularia, Drechslera, Bipolaris, Exserohilum, Alternaria, Helminthosporium, Fusarium; and Aspergillus species.7,10
First described in 1981, AFS has become the most commonly diagnosed and least understood form of fungal sinusitis.11 With subsequent increasing awareness and understanding of the disorder, various diagnostic criteria for AFS have been proposed.12 No consensus exists amongst rhinologists concerning diagnostic criteria for AFS.3 However, the five diagnostic criteria proposed by Bent and Kuhn have been the most accepted. These include: 1) the presence of nasal polyps, 2) hypersensitivity as indicated by atopic history, skin tests, or serological testing, 3) characteristic CT scan features, 4) histological features of allergic mucin, and 5) noninvasive fungal hyphae as evidenced by histological examination or culture.12 De Shazo and Swaim have also proposed more or less similar diagnostic criteria, with the exception of atopy.13,14
Early diagnosis and treatment are keys to good outcome and this requires a high index of suspicion. An accurate preoperative diagnosis, should it be available, would guide the surgeon on the surgical approach, extent of resection, and also any preoperative adjuvant medical therapy.12 With these objectives in mind we undertook a retrospective study of 406 cases of chronic rhinosinusitis who underwent functional endoscopic sinus surgery (FESS) at King Abdul Aziz University Hospital, Riyadh. The aim of the study was to determine the frequency of AFS in patients with chronic rhinosinusitis (CRS) and to identify accurate preoperative diagnostic parameters for AFS by evaluating their clinical and radiological features, as well as to identify the common fungi causing AFS in this part of the world.
METHODS
We conducted a retrospective chart review of 406 cases of chronic rhinosinusitis (CRS) undergoing FESS at King Abdul Aziz University Hospital, Riyadh from the period of June 2001 to June 2005. King Abdul Aziz University Hospital (KAUH) is a tertiary care academic hospital attached to the College of Medicine, King Saud University, Riyadh, Saudi Arabia. Information on presenting symptoms, an ENT examination, CT findings, histopathological features, and fungal staining and culture was collected. Inclusion criteria included: 1) presence of allergic mucin within sinuses, 2) detection of fungi by means of histological examination and/or culture, 3) absence of fungal invasion of submucosa, blood vessels or bone, and 4) an absence of diabetes or immunodeficiency state. All patients were cancer free, HIV negative with a normal WBC count and none had received immunosuppressive therapy and were not diabetic. A CT had been done in all cases and an MRI in 21 cases. Positive fungal cultures were obtained in 69 (16.9%) cases. However, only 59 cases were included in the study as the other 10 cases did not meet the inclusion criteria.
RESULTS
Fifty-nine cases of AFS were treated between June 2001 and June 2005. There were 22 males and 37 females. Fifty-four were Saudi, 5 were non-Saudi and most were from the Riyadh area (Table 1). Ages ranged from 12 to 56 years, with a mean age of 24.2 years. All patients had history of allergic nasal symptoms. Sixteen (27.1%) patients had a strong history of atopy (Table 2). AFS was confirmed by pathology and positive fungal culture in 59/406 (14.5%). All patients were immunocompetent. Twenty-five of 59 (42.3%) had previous surgery. Nasal polyposis was present in 56/59 (94.9%) cases. Multiple sinuses were affected in all cases. Thirty-nine patients had bilateral disease while 20 had unilateral disease. Hyperattenuating areas on CT (Figures 1 and 2) were seen in all cases. Sinus expansion was observed in 37 (62.7%) cases while bone erosion was present in 21 cases (35.6%). Intraorbital spread of disease was seen in 16 cases and intracranial spread in 5 cases. MRI revealed extradural intracranial spread in 4 cases.
Table 1.
Geographical distribution of 59 allergic fungal sinusitis cases in Saudi Arabia.
| City or area | No. of patients |
|---|---|
| Riyadh | 39 |
| Qasim | 5 |
| Jizan | 5 |
| Najran | 4 |
| Haai’l | 2 |
| Waadi Dawasir | 2 |
| Dahran | 1 |
Table 2.
Demographic, clinical and radiological features in 59 cases of allergic fungal sinusitis.
| Number of cases (%)* | |
|---|---|
| Age, range (years) | 24.2 (12–56) |
| Sex (F:M) | 37:22 |
| Previous sinonasal surgery | 25 (42.3) |
| Associated atopic illness | 16 (27.1) |
| Asthma | 13 (22.0) |
| Aspirin allergy | 1 (1.7) |
| Peripheral blood eosinophilia | 2 (3.4) |
| Nasal polyposis | 56 (94.9) |
| CT findings | |
| Bilateral:unilateral | 39:20 |
| Hyperattenuation | 59 (100%) |
| Sinus expansion | 37 (62.7) |
| Bony erosion | 21 (35.6) |
| Orbital spread | 16 (27.1) |
| Intracranial spread | 5 (8.4) |
Unless noted otherwise.
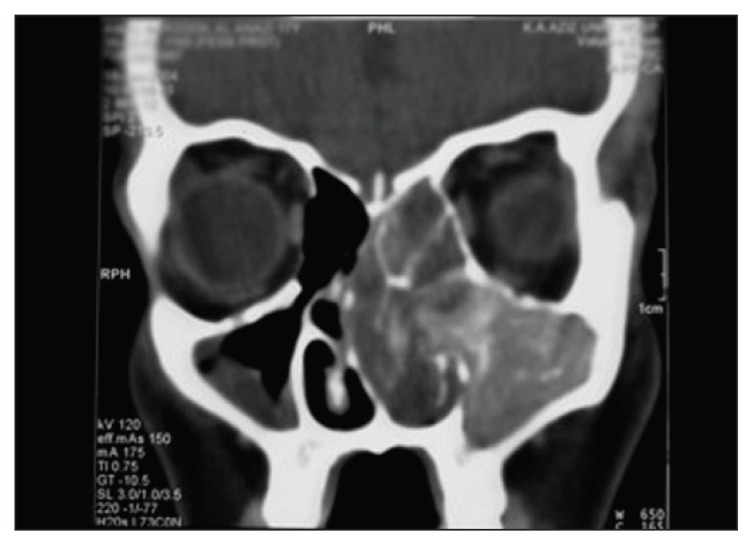
Figure 1.
Coronal noncontrast CT film of a patient with left-sided allergic fungal sinusitis. Note the typical ‘hyperattenuation’ areas within the sinuses, and bowing of the septum to the right.
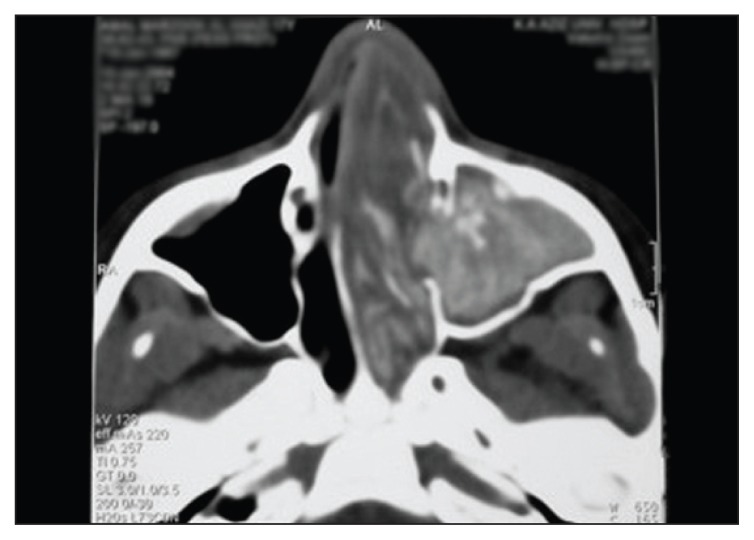
Figure 2.
Axial noncontrast CT film of the same patient.
Histologically, all patients had copious mucin, abundant eosinophils, Charcot-Leyden crystals and fungal hyphae without tissue invasion. Fungal cultures revealed Aspergillus as the commonest organism, being isolated in 40 (67.8%) cases whereas dematiaceous fungi were isolated in 19 cases (32.2%) (Table 3). Amongst the Aspergillus species, A. flavus was the commonest subclass, isolated in 33 cases. Amongst the dematiaceous fungi, Bipolaris/Drechelera was the commonest subclass, isolated in 12 cases (20.3%).
Table 3.
Histological and microbiological features of allergic fungal sinusitis.
| Histopathology | |
|---|---|
| Allergic mucin | 59 (100%) |
| Inflammatory cells | 59 (100%) |
| Fungal hyphae | 38 (64.4%) |
| Angioinvasion by fungi | 0 |
| Fungal culture | 59 (100%) |
| Aspergillus flavus | 33 (55.9%) |
| Aspergillus niger | 4 (6.8%) |
| Aspergillus terreus | 2 (3.4%) |
| Aspergillus species | 1 (1.7%) |
| Bipolaris/Drechelera | 12 (20.3%) |
| Alternaria | 3 (5.1%) |
| Penicillium | 2 (3.4%) |
| Saccharomyces cerevisine | 1 (1.7%) |
| Epicoccum | 1 (1.7%) |
DISCUSSION
AFS is a relatively newly recognized entity consisting of pansinusitis with eosinophilic mucinous infiltrates in all involved sinuses.15 AFS has been considered rare, but in essence it is an underdiagnosed condition. Underdiagnosis is likely since many clinicians and pathologists are unfamiliar with this newly described disorder. 11 With heightened awareness of the disease, an increased number of reports have been published more recently. Most rhinologists believe AFS is a combination of both a Gell and Coombs type I (IgE-mediated) and type III (immune-complex mediated) hypersensitivity reaction13 in response to fungi causing subsequent tissue edema, resulting in obstruction of the sinus ostia3 of immunocompetent individuals. Despite documentation of specific immunologic hypersensitivity in a few case reports, controversy exists16 over whether Ig-E mediated type I hypersensitivity is actually involved in the pathophysiology of AFS. Thus the most widely accepted diagnostic criteria for AFS are 1) CRS, 2) presence of allergic mucin (clusters of eosinophils and their by products, e.g. Charcot-Leyden crystals and major basic proteins); and the 3) presence of fungal organisms within that mucin, confirmed by histology, culture, or both.13
Deutsch and Hevron observed that 4% to 7% of all patients with CRS requiring surgery have AFS.17 Ponikau et al found the incidence to be 6% to 7%13 whereas Schubert estimated the incidence to be 5% to 10%.18 Retrospective studies have estimated that 5% to 10% of cases requiring surgical intervention are due to AFS.19,20 At 14.5% the incidence of AFS in the present series, although slightly higher, is still comparable to that mentioned in the literature.
AFS is more common in adolescents and young adults, with a mean age of 21.9 years in one study.3 In other studies the mean age was 25 years.8,10 In our series the mean age was 24.2 years. The male-female ratio has been found to be age dependant. Males predominate in children, while in adults females predominate.3 This is consistent with our study, where females predominated.
CT findings of patients with AFS include intrasinus high attenuation areas, sinus expansion (complete opacification), bone erosion of the involved sinus, remodeling and thinning of bony sinus walls, bone erosion of sinus walls and extension of disease into the adjacent sinuses.8 In most series an incidence of 20% bone erosion with extension into the brain and orbit has been reported.3 In the present series sinus expansion was observed in 37 (62.7%) cases and bone erosion in 21 (35.6%). Heterogeneous ‘high-attenuation’ areas within sinuses, reflecting allergic mucin, on CT scans are relatively characteristic for AFS.12 This observation is consistent with our series where all the cases had hyperattenuating areas in the involved sinuses. In fact, AFS may present with nonspecific symptoms and the diagnosis may be initially suggested by the characteristic CT findings. These findings should alert the clinician to the possibility of AFS and prompt other diagnostic studies to clinch the diagnosis. The incidence of nasal polyposis has been reported as between 73% and 100% in patients of AFS in various studies. In our study nasal polyposis was present in 56/59 (94.9%) cases. Allergic mucin, containing clusters of degenerating eosinophils and their by-products, was found in 96% of surgical cases by Ponikau et al. In our study it was present in all cases (100%). Allergic mucin is the characteristic histological feature of AFS.
Etiologically, most reported cases have been attributed to the pigmented dematiaceous fungi.7,10 However, there is a geographic vulnerability with regard to the predominately incriminated fungi in AFS. In Sudan, North India and along the Atlantic coast of the USA, Aspergillus species are the commonest etiological agents,21 whereas in the southwest of the USA, the dematiaceous fungi are the commonest.14,22 In the present study, Aspergillus was isolated in 40 (67.8%) cases and dematiaceous fungi in 19 (32.2%) cases. This differs from the experience from North America, where dematiaceous fungi are isolated more often.12 However, our results are consistent with studies from North India and Northern Sudan. The predominance of Aspergillus has been attributed to the dry and hot climatic conditions in these areas,21 which is similar to the climate in Saudi Arabia. In contrast, AFS in the United States is generally associated with demetiaceous fungi, which have been found to be of greater incidence in humid climates.
We identified areas from which AFS was diagnosed in Saudi Arabia, including Riyadh, Qasim, Najran, Gizan and Haai’l. However, a prospective study is needed to estimate the exact incidence and prevalence of AFS in these areas.
In conclusion, AFS is probably an underdiagnosed clinical entity. Only increased awareness among physicians to look for fungal involvement will increase accuracy of diagnosis. The diagnostic clinical triad of nasal polyposis, hyperattenuation foci on CT scan, and the presence of “allergic mucin” is a reliable preoperative diagnostic indicator for AFS. There exists a geographic diversity regarding the most commonly implicated fungi in AFS.
REFERENCES
- 1.Usamah H, Ray H, Raja S, Rola H, Issam R. Fungal sinusitis in the immunocompetent patient: Risk factors and Surgical Management. Surg Infect. 2003;4(2):199–204. doi: 10.1089/109629603766956997. [DOI] [PubMed] [Google Scholar]
- 2.Granville L, Chirala M, Cernoch P, Ostrowsk M, Truong LD. Fungal sinusitis: histologic spectrum and correlation with culture. Hum Pathol. 2004;35(4):474–81. doi: 10.1016/j.humpath.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Clay Mc, et al. Clinical Presentation of Allergic Fungal Sinusitis in Children. Laryngoscope. 2002;112:565–569. doi: 10.1097/00005537-200203000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Understanding Fungal sinusitis, Fungal Sinusitis Information. Sinus news. 2006. www.sinusnews.com.
- 5.Manning SC, Schaefer SD, Close LG, Vuitch F. Culture positive Allergic Fungal Sinusitis. Arch Otolaryngol Head Neck Surg. 1991;117(2):174–178. doi: 10.1001/archotol.1991.01870140062007. [DOI] [PubMed] [Google Scholar]
- 6.Dolen WK. Risk factors for Allergic Aspergillus Sinusitis. Med Mycol. 2006;44:273–275. doi: 10.1080/13693780600776316. [DOI] [PubMed] [Google Scholar]
- 7.Manning SC, Merkel M, Kriesel K, Vuitch F, Marple B. Computed Tomography and magnetic resonance diagnosis of Allergic Fungal sinusitis. Laryngoscope. 1997;107:170–176. doi: 10.1097/00005537-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Mukherji SK, et al. Allergic fungal sinusitis: CT findings. Radiology. 1998;207(2):417–422. doi: 10.1148/radiology.207.2.9577490. [DOI] [PubMed] [Google Scholar]
- 9.Chrzanowski RR, Rupp NT, Kuhn FA, Philips A, Dolen WK. Allergenic fungi in allergic fungal sinusitis. Ann Allergy Asthma Immunol. 1997;79(5):431–5. doi: 10.1016/S1081-1206(10)63039-6. [DOI] [PubMed] [Google Scholar]
- 10.Torres C, Jy Ro, el Naggar AK, Sim SJ, Weber RS, Ayala AG. Allergic fungal sinusitis: a clinicopathologic study of 16 cases. Hum Pathol. 1996;27(8):793–9. doi: 10.1016/s0046-8177(96)90451-7. [DOI] [PubMed] [Google Scholar]
- 11.James FM, Ned IR, Will KD, et al. Fungal Sinusitis: an update. Annals of Allergy, Astma and Immunol. 1996;70:128–36. doi: 10.1016/S1081-1206(10)63411-4. [DOI] [PubMed] [Google Scholar]
- 12.Dhiwakar M, Thakar A, Bahadur S, et al. Preoperative diagnosis of allergic fungal sinusitis. Laryngoscope. 2003;113:688–94. doi: 10.1097/00005537-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Ponikau JU, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo clinic Proc. 1999;74(9):877–84. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 14.de Shazo RD, Swaim RE. Diagnostic criteria for Allergic Fungal Sinusitis. J Allergy Clin Immunol. 1995;96:24–35. doi: 10.1016/s0091-6749(95)70029-3. [DOI] [PubMed] [Google Scholar]
- 15.Bartynski Jm, Mc Caffrey TV, Frigas F. Allergic fungal sinusitis secondary to Dematiaceous fungi - Curvularia lunata and Alternaria. Otoloryngol Head and Neck Surg. 1990;103(1):32–9. doi: 10.1177/019459989010300105. [DOI] [PubMed] [Google Scholar]
- 16.Manning SC, Mabry RL, Schaefer SD, Close LG. Evidence of IgE mediated hypersensitivity in allergic fungal sinusitis. Laryngoscope. 1993;103(7):717–21. doi: 10.1288/00005537-199307000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Deutsch F, Heveron I. Allergic fungal sinusitis: diagnostic, therapeutic and prognostic evaluation. Harefuah. 2004;143(6):394–7. 464. [PubMed] [Google Scholar]
- 18.Schubert Ms, Goetz DW. Evaluation and treatment of allergic fungal sinusitis: demographics and diagnosis. J Allergy Clin Immunol. 1996;102(3):387–94. doi: 10.1016/s0091-6749(98)70125-3. [DOI] [PubMed] [Google Scholar]
- 19.Ence BK, Gourley DS, Jorgensen Nl, et al. Allergic fungal sinusitis. Am J Rhinology. 1990;4:169–78. [Google Scholar]
- 20.Schweitz LA, Gourley DS. Allergic fungal sinusitis. Allergy Proc. 1992;13:3–6. doi: 10.2500/108854192778878980. [DOI] [PubMed] [Google Scholar]
- 21.Chakrabarti A, Sharma SC. Paranasal sinus mycoses. Indian J Chest Dis Allied Sci. 2000;42(4):293–304. [PubMed] [Google Scholar]
- 22.de Shazo RD. Fungal Sinusitis. Am J Med Sci. 1998;316(1):39–45. doi: 10.1097/00000441-199807000-00006. [DOI] [PubMed] [Google Scholar]