Anemia in pregnancy remains a major problem in nearly all developing and many industrialized countries. The World Health Organization estimates that 58% of pregnant females in developing countries are anemic.1 In the Arab Gulf countries, maternal anemia, especially iron deficiency anemia has been considered as of the important public health problems with a prevalence ranging from 22.6% to 54.0%.2 High parity with iron deficiency was found to be an important risk factor for maternal mortality at King Fahad University Hospital, Al-Khobar, Saudi Arabia, during the 20 year-period from 1983 to 2002.3 Several studies have reported the risks of pregnancy anemia on the mother and her offspring. In a review of those studies, Scholl and Hediger suggested that anemia during early pregnancy increases the likelihood of poor outcomes such as preterm deliveries, low birth weight and perinatal mortality.4 A higher risk of urinary tract infection, pyelonephritis and pre-eclampsia has been reported in observational studies on iron-deficient women who are not necessarily anemic.5 There is lack of recently published data on maternal anemia from the urban area of Al-Khobar (Eastern province). This study was therefore conducted to determine a) the magnitude of anemia among pregnant women attending primary health care centers (PHCCs) of Al-Khobar and b) the association of pregnancy anemia with certain socio-demographic, biological and dietary factors.
METHODS
Nine PHCCs in three centers serving 22.7%, 14.9% and 12.9% (total 50.5%) of the registered population of Al-Khobar were selected for this cross-sectional, descriptive study. All Saudi and non-Saudi pregnant females who visited the centers during a 1-year period (March 2006 to February 2007) were included.
Data were collected from antenatal records and questionnaires that were interview-administered for all consecutive pregnant women who visited the three PHCCs during a 2-week period in February 2007. The latter group comprised a subsample of 80 mothers. Information collected from antenatal records included socio-demographic and biological data: age, nationality, gravida, inter-pregnancy interval, trimester of pregnancy when last hemoglobin was tested, history of sickle-cell trait/disease and level of hemoglobin last recorded. The non-cyanide hemoglobin analysis method was used and results read by a spectrophotometer (Symex KX-21, Germany). A hemoglobin level of <11g/dL was considered anemia. Criteria for mild, moderate and severe anemia were hemoglobin levels of >10–10.9 g/dL, 7–10 g/dL and <7 g/dL, respectively.6 Data from the questionnaire included additional information from the sub-sample: educational level, history of polymenorrhea or menorrhagia prior to the index pregnancy, intake of non-nutritious substances (pica), tea consumption soon after meals, regularity of iron supplementation and intake of iron-containing foods during the second and third trimester of pregnancy. A semi-quantitative food frequency questionnaire (FFQ) was used for mothers to determine their dietary intake of iron. The FFQ was a checklist of 15 food items, each containing at least 3% of the recommended dietary allowance of iron.7 A score was assigned for each food item. The total score for each mother was calculated according to the number and frequency of food servings she consumed per week. Data processing involved a check for accuracy and completeness of data followed by statistical analysis with the help of the SPSS version 11 program. Univariate analysis of data was done by the χ2 and t tests as appropriate. A P value of ≤.05 was considered as significant.
RESULTS
During the study period, 498 women attended the three PHCCs for antenatal care. Despite a protocol of routine hemoglobin estimation at each trimester during antenatal check-up, data was missing in 34 (6.8%) records and hence information for 464 women was included in the analysis. Three hundred fifty-three (76%) of the women were Saudi. The mean and standard deviation was 26.7±5.4 years for age and 23.2±10.0 weeks for gestational age, and gravida was 3.7±2.7. Forty of 80 women (50%) in the subsample had completed a secondary education or higher.
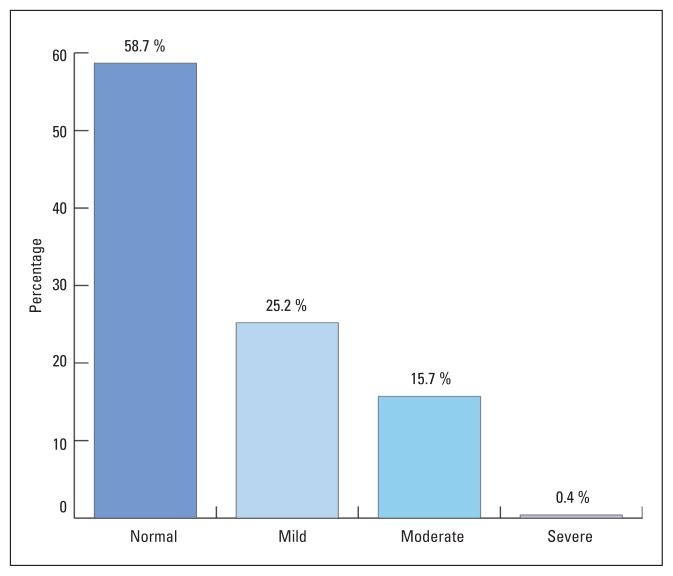
Figure 1 shows that 192 (41.3%) of 464 pregnant women attending PHC facilities for antenatal care were anemic. Mild, moderate and sever anemia was present in 117 (25.2%), 73 (15.7%) and 2 (0.4%) women, respectively. Thirteen of 192 anemic cases (6.7%) had sickle cell trait/disease. Table 1 shows that although more Saudi women were anemic than non-Saudis, this was not statistically significant (P>.05). Anemia was highest among women in their third trimester of pregnancy (P<.01)
Figure 1.
Prevalence of anemia among pregnant women attending primary health care facilities in Al-Khobar in 2006.
Table 1.
Distribution of anemia cases by nationality and trimester pregnancy.
| Anemia | Present (n=192) | Absent (n=272) | Total (n=464) | P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
|
| |||||||
| Nationality | |||||||
|
| |||||||
| Saudi | 153 | 43.3 | 200 | 56.7 | 353 | 100 | >.05 |
|
|
|||||||
| Non-Saudi | 39 | 35.1 | 72 | 64.9 | 111 | 100 | |
|
| |||||||
| Trimester | |||||||
|
| |||||||
| First | 24 | 27.7 | 64 | 72.3 | 88 | 100 | <.01 |
|
| |||||||
| Second | 60 | 37.3 | 101 | 62.7 | 161 | 100 | |
|
| |||||||
| Third | 108 | 50.2 | 107 | 49.8 | 215 | 100 | |
The mean frequency score for food items rich in iron was higher in non-anemic (44.2) than anemic women (38.3), but the difference was statistically insignificant (P>.05). Insignificant differences by mean age, gravida and last inter-pregnancy interval were found between anemic and non-anemic pregnant women. Nineteen out of 54 pregnant women (35%) in their second and third trimesters of the sub-sample were non/irregular takers of iron supplementation and anemia was more common among them (57.9%) than in regular takers (14.3%) (P<.05) (Table 2). Reasons given by mothers for no/irregular intake of iron supplementation included “forgetfulness” (52.9%), “unnecessary” (17.6%) or “harmful” for the fetus (5.9%). No relationship was observed between anemia and tea consumption immediately after main meals. Of the 80 pregnant women in the subsample, 9 (11.3%) mothers were indulging in pica. There was no statistical association of anemia to pica. Only 6 mothers (7.5%) reported a history of polymenorrhea/menorrhagia prior to pregnancy, hence correlation analysis of this variable with anemia was not done.
Table 2.
Distribution of anemia cases by regularity of iron supplementation and tea consumption habits.
| Anemia | Present | Absent | Total | P c | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
|
| |||||||
| Iron supplementationa | |||||||
|
| |||||||
| None | 4 | 44.4 | 5 | 55.6 | 9 | 100 | <.01 |
|
| |||||||
| Irregular | 7 | 70.0 | 3 | 30.0 | 10 | 100 | |
|
| |||||||
| Regular | 5 | 14.3 | 30 | 85.7 | 35 | 100 | |
|
| |||||||
| Tea consumption immediately after main mealsb | |||||||
|
| |||||||
| No | 13 | 28.9 | 32 | 71.1 | 45 | 100 | >.05 |
|
| |||||||
| Yes | 4 | 28.6 | 10 | 71.4 | 14 | 100 | |
Women in first trimester of pregnancy were excluded.
Non-responders=21.
Fisher’s exact test
DISCUSSION
A prevalence rate of 41.3% for anemia in pregnancy in the current study is substantially high and is a reflection of the nutritional health of predominantly Saudi Arabian pregnant women attending PHCCs. This high figure is surprising considering the routine practice at PHCCs to provide pregnant women with prophylactic elemental iron of 60 mg/day and up to 180 mg/day in cases of anemia. Though adequate supplies of iron medication were freely available in all health centers we visited, more than one-third of the second and third trimester pregnant women of our study sub-sample were non/irregular takers of iron supplementation. Major barriers to consuming medication were lack of motivation and misconceptions. Perhaps this was a result of inadequate counseling by the health care providers. Mothers need to be educated that dietary sources do not meet the daily requirement of iron during pregnancy7 and iron supplementation is important especially in the second and third trimesters of the gestational period. Other studies have also reported an increased risk of anemia in mothers who were non/irregular takers of iron pills.8,9
The magnitude of anemia (41.3%) in the study population is slightly higher than that reported in an earlier study (1994) on pregnant women of the Southwestern region of Saudi Arabia (31.9%),10 but is similar to findings of small-scale studies conducted in the neighboring countries of Kuwait (36.8%),11 Oman (43.6%)12 and Bahrain (49.6%)13 as well as those from other Afro-Asian countries such as Mali (47%),14 rural Vietnam (43.2%)9 and Malaysia (34.6%).15 Large-scale studies from India16 and rural Bangladesh17 have reported a higher anemia prevalence of 84.9% and 50% respectively, indicating a poorer state of nutritional health among mothers in these developing countries. On the other hand, studies from economically developed countries have shown a lower frequency of pregnancy anemia such as those from the USA (22%)18 and Belgium (31%).19 It was encouraging to note that most of the maternal anemia cases in the current study were of the mild/moderate and not severe type, which is similar to findings from Malaysia,15 rural Vietnam9 and Indonesia.8 Severe anemia is more prevalent in countries where infections such as malaria or diarrhea are common.14,20
The prevalence of sickle cell trait/disease (6.7%) in the current study is close to that reported by the National Premarital Screening Program (4.46%) in 2007 for Saudi Arabia.21 As the disease is more pronounced in the eastern region of Saudi Arabia,21 all PHCCs routinely monitor the sickle cell status of pregnant women; this protocol should continue in order to provide better care for this high-risk group. Age was not a risk factor for anemia in our study. Mahfouz et al found that Saudi teenage pregnant females were not at a higher risk of anemia than older women if good prenatal care was provided.22 Unlike some studies,10,12 we did not observe any variation in anemia by gravida and last pregnancy interval. However, advancing gestational age significantly increased the risk of anemia, which is similar to the findings of other studies.8–10,19 Compared to the first trimester, a lower hemoglobin level in the second and third trimesters is partly artifactual and is due to a physiological expansion of maternal plasma volume, making it more or less difficult to separate out women who are truly anemic. If iron intake is not adequate during this period to meet the increased demands of the mother and the growing fetus, further reductions in hemoglobin occur due to iron deficiency.
Our study showed that anemic women had a lower mean food frequency score for iron-intake than those who were not anemic. The results were, however, not significant. This finding is consistent with the literature.23 Some of the reasons include imprecise estimation of iron-intake by checklists and variations in iron absorption related to enhancers/inhibitors in food. Further, the levels of iron stores in the body may outweigh any effect of iron-intake on anemia in relatively well-nourished populations.23 Accurate laboratory data versus dietary intake remain the best tools to determine the iron status of individuals. Compulsive intake of nonnutritive substances such as earth, clay, chalk, soap and ice by 11.3% of the sub-sampled women compares with published data on pica prevalence (8%–65%).24 Though pica has frequently been associated with anemia or iron deficiency in pregnancy, we did not see this relationship in our study.
In conclusion, our study showed that a sizable proportion of pregnant women were found to be anemic. Non/irregular intake of iron medication by mothers was significantly associated with anemia. Health education programs at the PHCCs should address the importance of compliance for iron supplementation along with adequate intake of iron-rich dietary sources during pregnancy and for 3 months postpartum as per recommendations of the WHO for countries with a high prevalence (≥40%) of pregnancy anemia.25
REFERENCES
- 1.Galloway R, Dusch E, Elder L, Achadi E, Grajeda R, Hurtado E, et al. Women’s perceptions of iron deficiency and anemia prevention and control in eight developing countries. Soc Sci Med. 2002 Aug;55(4):529–44. doi: 10.1016/s0277-9536(01)00185-x. [DOI] [PubMed] [Google Scholar]
- 2.Musaiger AO. Iron deficiency anemia among children and pregnant women in the Arab Gulf countries: the need for action. Nutr Health. 2002;16:161–71. doi: 10.1177/026010600201600302. [DOI] [PubMed] [Google Scholar]
- 3.Al-Suleiman SA, Al-Sibai MH, Al-Jama FE, El-Yahia AR, Rahman J, Rahman MS. Maternal mortality: a twenty-year survey at the King Faisal University Hospital, Al-Khobar, Eastern Saudi Arabia. J Obstet Gynaecol. 2004 Apr;24(3):259–63. doi: 10.1080/01443610410001660742. [DOI] [PubMed] [Google Scholar]
- 4.Scholl TO, Hediger ML. Anemia and iron deficiency anemia: compilation of data on pregnancy outcome. Am J Clin Nutr. 1994;59( Suppl):492–501. doi: 10.1093/ajcn/59.2.492S. [DOI] [PubMed] [Google Scholar]
- 5.Kitay DZ, Harbort RA. Iron and Folic acid deficiency in pregnancy. Clin Perinatol. 1975;2:255–73. [PubMed] [Google Scholar]
- 6.DeMaeyer EM, Dallman P, Gurney JM, Hallberg L, Sood SK, Srikantia SK. Preventing and Controlling Iron Deficiency Anemia through Primary Health Care: A Guide for health administrators and programme managers. WHO; Geneva, Switerland: 1989. [Google Scholar]
- 7.Guthrie HA. Introductory Nutrition. 4th ed. St. Louis: The CV Mosby Company; 1979. [Google Scholar]
- 8.Suega K, Dharmayuda TG, Sutarga IM, Bakta IM. Iron-deficiency anemia in pregnant women in Bali, Indonesia: a profile of risk factors and epidemiology. Southeast Asian J Trop Med Public Health. 2002;33:604–7. [PubMed] [Google Scholar]
- 9.Aikawa R, Ngyen CK, Sasaki S, Binns CW. Risk factors for iron-deficiency anemia among pregnant women living in rural Vietnam. Public Health Nutr. 2006;9:443–8. doi: 10.1079/phn2005851. [DOI] [PubMed] [Google Scholar]
- 10.Mahfouz AA, El-Said MM, Alakija W, Badawi IA, Al-Erian RA, Moneim MA. Anemia among pregnant women in the Asir region, Saudi Arabia: an epidemiological study. Southeast Asian J Trop Med Public Health. 1994;25:84–7. [PubMed] [Google Scholar]
- 11.Dawood JS, Prakash P, Shubber KM. Iron deficiency among pregnant Arab women. J Kuwait Med Assoc. 1990;24:167–72. [Google Scholar]
- 12.Afifi M. Anemia in pregnancy at South Sharqiya health centers, Oman. J Egypt Public Health Assoc. 2003;78:39–54. [PubMed] [Google Scholar]
- 13.Amine EK. Bahrain Nutrition Status Survey. UNICEF Gulf Area Office; Abu Dhabi, United Arab Emirates: 1980. [Google Scholar]
- 14.Ayoya MA, Spiekermann-Brouwer GM, Traore AK, Stoltzfus RJ, Garza C. Determinants of anemia among pregnant women in Mali. Food Nutr Bull. 2006;27:3–11. doi: 10.1177/156482650602700101. [DOI] [PubMed] [Google Scholar]
- 15.Hassan R, Abdullah WZ, Nik Hussain NH. Anemia and iron status of Malay women attending an antenatal clinic in Kubang Kerian, Kelantan, Malaysia. Southeast Asian Trop Med Public Health. 2005;36:1304–7. [PubMed] [Google Scholar]
- 16.Toteja GS, Singh P, Dhillon BS, et al. Prevalence of anemia among pregnant women and adolescent girls in 16 districts of India. Food Nutr Bull. 2006;27:311–5. doi: 10.1177/156482650602700405. [DOI] [PubMed] [Google Scholar]
- 17.Hyder SM, Persson LA, Chowdhury M, Lonnerdal BO, Ekstrom EC. Anemia and iron deficiency during pregnancy in rural Bangladesh. Public Health Nutr. 2004;7:1065–70. doi: 10.1079/PHN2004645. [DOI] [PubMed] [Google Scholar]
- 18.Alper BS, Kimber R, Reddy AK. Using ferritin levels to determine iron deficiency anemia in pregnancy. J Fam Pract. 2000;49:829–32. [PubMed] [Google Scholar]
- 19.Massot C, Vanderpas J. A survey of iron deficiency anemia during pregnancy in Belgium: analysis of routine hospital laboratory data in Mons. Acta Clin Belg. 2003;58:169–77. doi: 10.1179/acb.2003.58.3.004. [DOI] [PubMed] [Google Scholar]
- 20.Khosla AH, Dahiya P, Dahiya K. Burden of chronic anemia in obstetric patients in rural north India. Indian J Med Sci. 2002;56:222–4. [PubMed] [Google Scholar]
- 21.Al-Hamdan NA, Al-Mazrou YY, Al-Swaidi FM, Choudhry AJ. Premarital screening for thalassemia and sickle cell disease in Saudi Arabia. Genet Med. 2007;9:372–7. doi: 10.1097/gim.0b013e318065a9e8. [DOI] [PubMed] [Google Scholar]
- 22.Mahfouz AA, el-Said MM, al-Erian RA, Hamid AM. Teenage pregnancy: are teenagers a high risk group? Eur J Gynecol Reprod Biol. 1995;59:17–20. doi: 10.1016/0028-2243(94)02012-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhou SJ, Schilling MJ, Makrides M. Evaluation of an iron specific check list for the assessment of dietary iron in pregnant and postpartum women. Nutrition. 2005;21:908–13. doi: 10.1016/j.nut.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Lopez LB, Ortega Soler CR, de Portela ML. Pica during pregnancy: a frequently underestimated problem. Arch Latinoam Nutr. 2004;54:17–24. [PubMed] [Google Scholar]
- 25.WHO. Iron and Folate Supplementation. A document of Standards for Maternal and Neonatal Care. 2006:1–6. [Google Scholar]