Abstract
Hemoglobin A1c (HbA1c) has been used for decades to monitor the control of glycemia in diabetes. Although HbA1c is currently undergoing a reassessment, and major developments have been underway in recent years, HbA1c is not recommended at present for diabetes screening or diagnosis. The objective of this review is to summarize the recent developments and to review a potential diagnostic role for HbA1c. Implementation of changes in HbA1c results and units of measurements have been suggested for the purpose of test standardization. These include lower reference ranges (by about 1.5–2 points) and measurement units expressed in percentage (%), as mg/dL (mmol/L) or mmol/mol (or a combination of these units). In diabetes screening and diagnosis, the current diagnostic guidelines use measurement of plasma glucose either fasting or after glucose load. These diagnostic methods have shortcomings warranting a potential diagnostic role for HbA1c. While recent developments in HbA1c methodologies are acknowledged, it is not yet known which changes will be implemented, and how soon. Given the recent literature supporting HbA1c diagnostic abilities, and given the shortcomings of the current guidelines, it is possible that a diagnostic role for HbA1c may be considered in future practice guidelines, globally. Very recently, the first of such recommendations has been proposed by an expert panel, as announced by the US Endocrine Society.
The “worldwide explosion in the prevalence of type 2 diabetes mellitus1” has turned diabetes into a global epidemic.2 Epidemiological data have shown that diabetes prevalence skyrocketed in recent years; for example, in the US, a 61% increase in the prevalence of diabetes between 1990 and 2001 has been reported.2 The prevalence of pre-diabetes, defined as impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT), is also increasing globally.1,3 Recent studies2 have shown pre-diabetes which eventually progresses to diabetes in up to 70% of cases, to be a risk factor for cardiovascular disease.2
It follows that there is a critical need to develop effective methods to improve early detection and treatment of diabetes and pre-diabetes, in an effort to avert the well-known complications of diabetes. These micro-and macrovascular complications continue to claim more lives and to further exhaust national budgets. Control of hyperglycemia, as monitored by glycated hemoglobin (HbA1c), has been recommended by various international diabetes organizations which recommend HbA1c management targets.4 These targets vary from one organization to another, ranging roughly between 6.0% and 7.0%.4
It is notable, however, that HbA1c has been associated with controversies and confusion among patients and physicians alike. Among the scientific community, HbA1c has traditionally served as an indicator of glycemic control over the preceding 2- to 3- month period. In the real world, patients with diabetes measure their glucose levels at home obtaining results such as “120 or 200” (mg/dL), while physicians measure HbA1c with different results that many patients cannot fully comprehend, e.g., 7.6% or 8.8%. Among the diabetes scientific community, the main controversies about HbA1c concern standardization of the test and artifactual interferences with some assay methods.5
Another controversy about HbA1c is whether or not the test can be used for screening or diagnosis of diabetes. The current screening and diagnostic methods for diabetes have known shortcomings, which warrant the consideration of HbA1c as a possible alternative.5–7 These shortcomings are: 1) the fasting plasma glucose (FPG), the main screening method, suffers from inadequate sensitivity; 2) the oral glucose tolerance test (OGTT), a suggested alternative diagnostic test, is considered cumbersome and is not commonly used in clinical settings; 3) both tests require fasting, which may not be feasible, especially in busy clinical settings and in population screening.
In response to the aforementioned concerns, there have been major developments in HbA1c methodologies and terminologies over the last few years. Similarly, there have been developments regarding a potential diagnostic role for HbA1c, revisiting this controversial issue. In the forthcoming sections, we will provide an overview of these developments and discuss the validity of HbA1c as a potential diagnostic test for diabetes.
HbA1c Terminology and Methodology, Past and Present
The chemistry and clinical interpretation of HbA1c
During unrelated work in the late 1960s, Rahbar discovered a glycated species of hemoglobin that he called the “diabetic hemoglobin” as reported by Miedema.8 Different subtypes of these compounds have since been described. The collective overall entity, previously termed glycosylated hemoglobin, is referred to in modern laboratory terms as glycated hemoglobin (GHb), but that term or glycosylated hemoglobin is not used commonly at present in cinical settings and day-to-day communication among health care providers. Hemoglobin A1 (HbA1) is a derivative of adult hemoglobin (HbA), with monosaccharide (fructose or glucose) attachments. HbA1c is the major and the most extensively studied subtype of the three known HbA1 species (HbA1a, b and c). In strict chemical terms, the molecular structure of HbA1c is β-N-(1-deoxy)-fructosyl-hemoglobin8 or N-(1-deoxyfructose-1-yl) hemoglobin beta chain.9 HbA1c is formed via a posttranslational nonenzymatic attachment of glucose to hemoglobin10 in an irreversible fashion and at a rate dependent on the ambient blood glucose during the lifespan (120 days) of the red blood cell.8 Hence, HbA1c is traditionally looked at as an indicator of the mean blood glucose (MBG) in the preceding 2 to 3 months. However, it has been recently recognized that the MBG in the preceding 1 to 2 months is the major contributor to HbA1c.11–13 In this regard, it has been found that MBG in the preceding 30 days has the largest contribution to HbA1c,11 and that up to 70% is determined by the preceding 2-month MBG.12 According to Tahara et al, monthly contributions of MBG to HbA1c are as follows: 50% from the most recent 30 days and 25% from each of the preceding 30 and 60 days.13 This concept has been referred to as the “weighted” average of blood glucose as related to HbA1c.11,13,14 Furthermore, until recently it had not been established how variations in glucose profiles on a daily basis would influence HbA1c; for example, fasting versus postprandial glucose levels. In a recent analysis of the Diabetes Control and Complications Trial (DCCT) cohort, Service and O’Brien concluded that the MBG contributed more to overall HbA1c than did variations in 7-point daily glucose measurements.15
At present, HbA1c is measured by three basic types of assay methods.8,10 These include immunoassay and the two types of high performance light chromotagraphy (HPLC), the cation-exchange and the boronate affinity methods. The unit of HbA1c measurement is the percentage unit (%), i.e., the percentage of HbA1c to HbA. The reference range used by many laboratories is roughly 4.0% to 6.0%.
HbA1c as the Time-Tested Cornerstone in Glucose Monitoring in Diabetes
Once it became known that HbA1c closely reflected the preceding glycemic average, it became the cornerstone of monitoring of glycemic control, in addition to the method for glucose self-monitoring by patients. Furthermore, almost all outcome studies on diabetes complications are now based on HbA1c. The most famous of such studies, which displayed the relationship of HbA1c to diabetic complications, are the Diabetes Control and Complications Trial (DCCT),16 and the United Kingdom17 Prospective Diabetes Study. Both studies have established a direct link between HbA1c levels and retinopathy, nephropathy and neuropathy.10 Various diabetes and other health organizations have since issued management guidelines defining HbA1c targets.4,10 Thus, and soon after the publication of the DCCT, the American Diabetes Association (ADA) began in 1994 to recommend a HbA1c target of <7 % for patients with diabetes.18 It is notable that subsequent ADA guidelines amended the earlier HbA1c target by calling for individualization of this target (by relaxing or tightening this target), considering age, comorbidities, life expectancy and hypoglycemia risks.19 Other health organizations, worldwide, recommended similar HbA1c guidelines, overall.20–22
Problems and Concerns About HbA1c Measurement and How These Were Addressed
The clinical use of HbA1c has encountered several road blocks since it became available. These obstacles have included:
Standardization
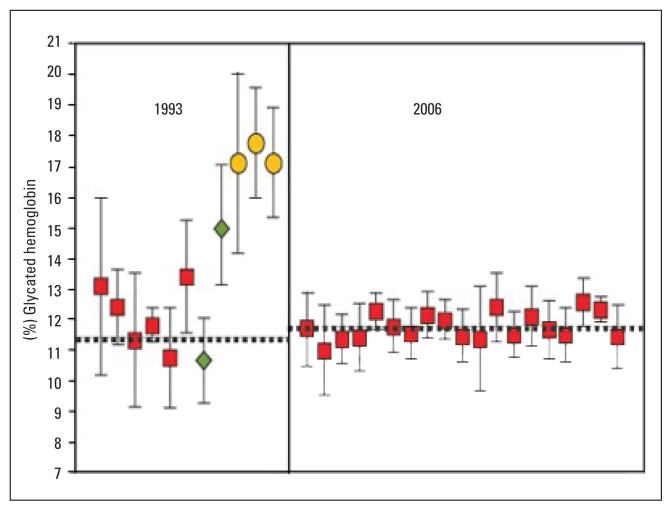
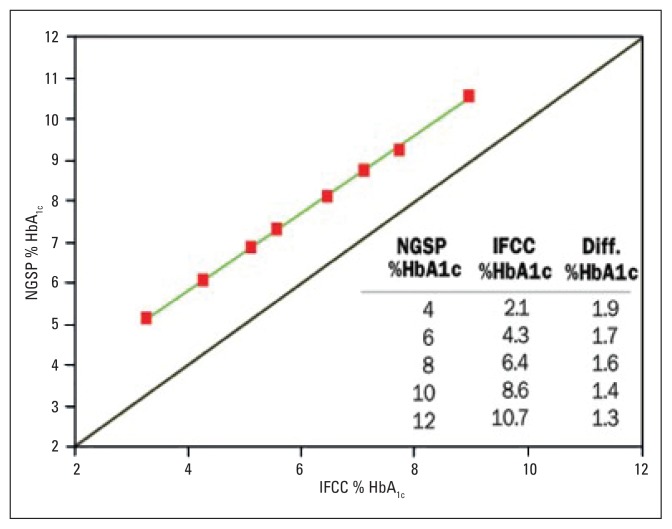
Measurement of glycated hemoglobin from different assay methods used by laboratories varied considerably,10 with over 20 methods in clinical use as recently as 2004.23 Early assay methods measured and reported different glycated fractions (total GHb, HbA1, and HbA1c).10 This heterogeneity in reported results caused concerns about reliability and reproducibility of HbA1c. The call for test standardization was critical. In response, the DCCT method (Rex 70 ion-exchange HPLC) was recommended by the National Glycohemoglobin Standardization Program (NGSP) as the preferred method for US laboratories in the mid 1990s.10,24 Compliance with this program was reported to be very satisfactory, as noted in Figure 1.10 Outside the US, the NGSP standardization method has been adopted rather universally, but local (somewhat similar) standardization programs have recently been adopted in Sweden25 and Japan.26 At about the same time that the NGSP initiated its program, the International Federation of Clinical Chemistry (IFCC) developed the so called “definitive reference method”, which was aimed toward global HbA1c standardization.8,10,23 This method involves a sophisticated technical, 2-step procedure that further purifies the HbA1c assay by removing impurities from the tested blood samples.8 It should be noticed that the NGSP and IFCC results are very tightly correlated (r=0.99), as noted in Figure 2.10 The results are interchangeable by a master equation as follows (23–27): To convert HbA1c from IFCC units to NGSP units=(0.915×IFCC)+2.15. The IFCC results are about 1.9, and 1.3 points lower than the NGSP results at normal and elevated HbA1c values, respectively.27
Figure 1.
Glycated hemoglobin results from the College of American Pathologists in 1993 and 2006.10 Each point and bar represent the mean ±2 standard deviation of results for a single method. Diamonds represent results reported as HbA1, squares represent results reported as HbA1c, and circles represent results reported as total GHb. The dotted horizontal line represents the NGSP/DCCT target value. (Reproduced with permission: From the IVD (In Vitro Devices) Technology in Los Angeles, CA, and the University Of Missouri School Of Medicine in Columbia, MO).
Figure 2.
The relationship between HbA1c measured by the NGSP and IFCC networks.10 The upper green line is the regression line. The solid line is y=x. (Reproduced with permission: From IVD (In Vitro Devices) Technology in Los Angeles, CA, and the University of Missouri School of Medicine in Columbia, MO).
Interference with assays
Concerns have been raised about the possible effects of hemoglobinopathies,28,29 uremia30 and ethnic variations5,31 on HbA1c assays. Concerns about other possible interferences have been raised, particularly in regards to recent circulatory and hematological changes, such as in patients with recent blood regeneration.32 These concerns were real and they certainly needed to be addressed. While interfering substances raise concerns about test reliability, recent technologies have introduced more accurate commercial analyzers into clinical use reportedly not affected by common hemoglobinopathies or uremeia.33,34 It is prudent that physicians be aware of the functionality of analyzers used in their local laboratories. They should also be aware of recent circulatory changes that may influence HbA1c, such as a recent blood transfusion or hemolytic anemia.30
Finally, in regards to the potential effects of race or ethnicity on HbA1c measurements, a recent study by Herman et al (n=3819) has addressed this issue.31 In this study of individuals with IGT, mean HbA1c was found to be slightly higher in minority US individuals, with the highest differences observed between Caucasians and African Americans (5.8% vs 6.2%, respectively). Therefore, this should be taken into consideration when analyzing HbA1c results.
Confusing terminologies and measurement units
As anecdotally observed, the fact that the HbA1c test includes hemoglobin (literally and by name) has often created confusion among patients. While they understand that “diabetes is a disease of blood sugar”, they wonder what hemoglobin (referred to as blood count by patients) has to do with diabetes? One of our patients once referred to HbA1c as “that sugar thing on the blood count”! Similarly, patients wonder what the percentage (%) unit appearing in HbA1c result means. Finally, patients often wonder “how come” a blood sugar of 130 on their glucose meters, for example, translates into 6.5% or 7% as reported by their physicians as HbA1c laboratory results! It seems, therefore, as if patients and their physicians are testing two different entities. For better education, in the quest for better patient participation in their disease management, there is no doubt that a common, easily understandable language of communication between patients and physicians, is highly desirable. To reconcile the concepts of what HbA1c actually measures and what patients measure at home, major developments in HbA1c have occurred in the recent few years. These developments will be covered in the next section.
Summary of the Recent Developments in HbA1c Terminology, Methodology and Units of Measurements
Technical and laboratory developments
To capture the aforementioned developments in HbA1c testing, various national and international laboratory organizations participated in collaborating and then reporting these developments. After a series of expert consensus meetings10 it was agreed that the proper scientific name for the measurement (test) should be “hemoglobin beta chain (blood) - N-(1-deoxyfructos-1-yl) hemoglobin beta chain; substance fraction millmole per mole”. This change in HbA1c units, i.e., mmol/mol, will give totally different HbA1c numbers that are unfamiliar to clinicians, and therefore we opted not include these new units in this review. Readers can learn more about this in a brief and concise recent editorial by Panteghini and John.35 Other changes agreed upon are summarized in the final consensus statement by the aforementioned organizations, and will be reviewed in a subsequent section.
Clinical Developments (The International HbA1c-MBG Study)
To better understand the relationship of HbA1c and glucose levels, an international, multicenter, prospective study was designed. This landmark study was sponsored by major national and international diabetes organizations.35 The main purpose of the study was to firmly define the relationship between HbA1c and MBG. Although this correlation had long been observed in the literature, the concern was that this correlation was not “exceptionally robust”, and that prior studies had not utilized frequent glucose measurements.36
The most important message from the study sponsors during the conduct of the study is that the ultimate purpose of the study was to alleviate the patient confusion about HbA1c terminology and measurement units.36 Thus, if a tight correlation between HbA1c and MBG is confirmed, then HbA1c can be expressed in glucose units, the so called estimated average glucose (eAG), or A1c-derived average glucose (ADAG).
The study was launched in 2004, and utilized monthly HbA1c, continuous glucose monitoring systems and 7-point capillary (fingerstick) glucose profiles, and has just been published.37 The study enrolled 507 subjects: 268 with type 1 diabetes mellitus, 159 with type 2 diabetes mellitus and 80 non-diabetic controls. As expected, the study confirmed the tight correlation between HbA1c and average glucose, per linear regression analysis (P<.0001).
The firmly established tight correlation between HbA1c and a average glucose across the spectrum of glycemia (from normal to extreme hyperglycemia) allowed calculation of an estimated average glucose (eAG) from HbA1c results.37 Thus this calculation can be used to express the results of HbA1c measurements into glucose units that patients are more familiar with, i.e., the same units they get from their glucose meters. An easy formula37 was derived from the study results as follows: eAG (mg/dl)=28.7×HbA1c%–46.7.
It is notable that of the anticipated changes in HbA1c measurement, the idea of co-reporting eAG with HbA1c is not totally new in clinical practice. In fact, many laboratories have been doing that for some time,38 and many physicians have been giving their patients HbA1c-derived glucose equivalents.36
The International Consensus Statement Summarizing the Recent Developments
Following the aforementioned developments, a consensus statement was published jointly by major national and international diabetes and laboratory organizations (ADA, EASD [The European Association for the Study of Diabetes], IFCC [The International Federation of Clinical Chemistry and Laboratory Medicine], IDF [The International Diabetes Federation]). The statement was intended to educate the public on recent developments and anticipated changes in HbA1c. The statement included five recommendations:36
HbA1c test results should be standardized worldwide, including the reference system and results reporting.
The new IFCC reference system for HbA1c represents the only valid anchor to implement standardization of the measurement.
HbA1c results are to be reported worldwide in IFCC units (mmol/mol) and derived NGSP units (%), using the IFCC-NGSP master equation.
If the ongoing “average plasma glucose study” fulfills its a priori-specified criteria, an ADAG value calculated from the A1c result will also be reported as an interpretation of the A1c results (As noted above, the study has already been completed and published).
Glycemic goals appearing in clinical guidelines should be expressed in IFCC units (i.e., mmol/mol), derived NGSP (i.e., %) units, and as ADAG (i.e., mg/dl or mmol/L).
The Role of HbA1c in Screening and Diagnosis of Diabetes and Pre-diabetes
HbA1c and MBG correlation from a diagnostic perspective: Review of the literature on the diagnostic validity of HbA1c
Evidence had shown a satisfactory correlation between MBG and HbA1c39–42 in the hyperglycemic range. It is expected that such evidence will be firmly substantiated by the International HbA1c-MBG Study which was recently published.37 The next question, in regards to the diagnostic issue, was: Does HbA1c follow glycemia as it transits from normal to pre-diabetes and then to diabetes? Research has shown this to be the case indeed. Correlation between HbA1c and MBG did hold true in cohort large studies, including two separate analyses from the population-based NHANES (National Health and Examination Survey), the NHANES III and the IV.43,44 Certainly, the latest international HbA1c-MBG37 substantiated this (tight) correlation across the glycemic spectrum.
It is notable that the diagnostic role of HbA1c is not a new research endeavor. As a matter of fact, this issue was addressed soon after GHb discovery, but has remained controversial.43 In a brief search of the literature since the late 1970s, we came across numerous studies that addressed the validity of HbA1c for screening and diagnosis, in both type 2 diabetes 45–61 and gestational diabetes mellitus (GDM).62–71 These studies were heterogeneous in design and yielded no consensus on the diagnostic validity of HbA1c, overall. An extensive literature search for all relevant studies or appraisal of these studies is beyond the scope of this review. However, we identified published reports that reviewed the available studies in an in-depth scrutiny.
In the case of type 2 diabetes, Perry and associates discussed the ongoing debate about HbA1c diagnostic usefulness, and analyzed large population studies, as compared to their study, the Early Diabetes Intervention Program (EDIP).54 They reported that two large epidemiological studies showed poor sensitivities of HbA1c, as compared to FPG alone. This was in contrast to the findings of the EDIP study,54 as well as the findings of two other large, population-based studies which showed improved sensitivity of combined HbA1c and FPG; their own study yielded a sensitivity of 61% for the combined tests versus 45% for FPG alone.54 Ko et al reported similar advantages of combined HbA1c and FPG in diabetes screening.51
Acknowledging that several studies on HbA1c diagnostic validity were done prior to test standardization, Bennett et al recently published a systematic review of studies done between 1998 and 2004.5 They evaluated primary cross-sectional studies on the accuracy of HbA1c (at a cut-off point of 6.1%) for the detection of type 2 diabetes using the OGTT as the reference standard and FPG (at a cut-off point of 6.1 mmol/L) as a comparison. They cited a total of 63 studies, and included 9 that fulfilled their strict inclusion criteria; 6 were Asian studies and the rest were from Europe and the US. The findings of the systematic review showed that both HbA1c and FPG are equally effective screening tools for the detection of type 2 diabetes, but both were not effective for detection of IGT (sensitivities ~ 50%). At certain cut-off points of HbA1c (6.1%) and FPG (6.1 mmol/L), sensitivities and specificities were 78% to 81% and 79% to 84% for HbA1c, and 48% to 64% and 94% to 98%, respectively.5 The investigators concluded that both HbA1c and FPG were equally effective screening tools.
Overall, Bennett et al concluded that while HbA1c may be more expensive than FPG at present, HbA1c provides less intra-individual variability and better predicts diabetic complications, and thus provides a more favorable argument for cost-effectiveness. Additional benefits of HbA1c included the convenience of non-fasting, availability of point-of-care capillary assays, and the potential for mass population screening given the availability of transporting capillary samples from remote areas to central laboratories.5 While the investigators emphasized the several advantages of HbA1c over FPG and OGTT, they also recapitulated the possible influences of hemoglobinopathies, uremia, and medications on HbA1c measurements.5
Not included in the systematic review were several studies that addressed the use of HbA1c in the retrospective opportunistic detection of undiagnosed diabetes in inpatient and outpatient settings.50–57,60,72 The most impressive report is the recent analysis of the NHANES 1999–2004 cohort reported by Buell et al in late 2007.44 In this study (n=4935; 3280 normal, 1485 with IFG, and 170 with diabetes), a cut-point HbA1c of 5.8% was shown to have a sensitivity and specificity of 86% and 92%, respectively, for diagnosing diabetes.44
The final question in regards to the diagnostic validity of HbA1c is: What is a reliable HbA1c cut-point for screening and diagnosis? We found out that various cut-points have been utilized, and these ranged from 5.8% to 6.2%.5,43,44,54 Bennett et al concluded in their systematic review that this value was noted to be 6.1% in most reviewed studies. However, they emphasized that there was an argument for a population-specific, demographic-adjusted optimum HbA1c diagnostic cutoff point.5 Obviously, this cut-off is arbitrary and will encounter pitfalls in terms of false positives and false negatives; an ideal diagnostic test for diabetes, with high sensitivity and specificity, is desirable but is yet to be found. However, HbA1c has a good biological variability as compared to FPG (2% vs 14%) and is free of laboratory variability.6
Unlike the case with type 2 diabetes, where reasonable evidence exists to suggest a diagnostic role for HbA1c, it seems that the current literature is not conclusive for GDM. Several studies were published about HbA1c in GDM,62–71 but except for few recent studies69–71 the majority of these studies were done 2 to 3 decades ago. With drawbacks in designs and HbA1c methodologies and conflicting conclusions, no consensus could be reached from these studies. Well-designed prespective studies are therefore warranted to settle this issue.
The Current Guidelines for Diabetes Screening and Diagnosis and the Prospective of HbA1c Diagnostic Use
At the moment, none of the health organizations in the USA, or elsewhere, recommend HbA1c for screening or diagnosis of either type 2 diabetes or GDM.19,20,74 According to current ADA guidelines,19 adopted almost globally at present, screening and diagnosis of type 2 diabetes and GDM are based on glucose measurement; these include: a) casual plasma glucose (with symptoms), fasting plasma glucose (FPG) or oral glucose tolerance test (OGTT) for type 2 diabetes on at least two occasions; b) 1-hour glucose challenge test (GCT) for screening, and 3-hour OGTT for diagnosis of GDM. Random blood sugar (RBS) is not recommended by ADA for screening or diagnosis,19 and is not standardized. However, these recommended guidelines for diabetes screening are not usually followed in routine clinical practice.6,72 Ealovega and associates evaluated retrospective opportunistic screening for diabetes in a large managed care system (n=5752), to evaluate how physicians acted on abnormal glycemic tests done either for targeted screening purposes or as part of routine tests. While 69% of the patients in the system were screened, the most commonly used test was RBS (95%), followed by FPG (3%), HbA1c (2%), whole blood glucose measurement (1%), and GTT (< 1%). Unfortunately, follow up on these tests was uncommon, and therefore the yield from these opportunistic screening efforts was low.72 Finally, a survey was conducted by an independent survey company at the 2005 American College of Physicians Annual Meeting.6 Of 258 physicians attending the meeting who were surveyed, 93% reported that they routinely screened for diabetes. HbA1c was the screening or diagnostic method in 49% and 59% of the time, respectively. Interestingly, 49% of these physicians thought that HbA1c was an approved test for screening.6
For GDM, GCT is generally adopted in the US as a screening test. It has been noticed that in other places physicians use other screening methods. For example, in the Netherlands, both RBS and GCT are almost equally used in screening for GDM.75 We have also observed use of RBS for screening in pregnancy elsewhere.76
Problems with the current diagnostic guidelines
For type 2 diabetes and GDM, the current diagnostic methods are suboptimal. The FPG has been shown to have poor sensitivity, missing a significant proportion of subjects with OGTT-confirmed diabetes, ranging from 33% to 50%5,54,77 in the case of type 2 diabetes. The OGTT on the other hand has been regarded as inconvenient and cumbersome, and not well-reproducible.5,54 From anecdotal observation, the OGTT is particularly inconvenient for pregnant women, mainly due to the unpleasant taste of the glucose load used in the test. Furthermore, both of these glucose tests require fasting; this requirement is less easily achievable in busy practices and, in particular, in settings of population screening. Therefore it is not surprising that these “established diagnostic criteria for diabetes are not followed in the community”.6 Given the aforementioned arguments, we believe that HbA1c may provide a reasonable alternative or adjunct in the screening and diagnosis of diabetes. In the case of GDM in particular, we believe that HbA1c provides a more tolerable alternative than the unpleasant glucose load tests, if research confirms its diagnostic validity.
Point-of-Care HbA1c Assays: Another Technological Improvement in HbA1c Methodology
HbA1c can now be reliably measured by portable capillary devices at physicians’ offices,78–81 and immediate feedback can thus be provided to patients. This point-of-care technology has been shown to improve management outcomes in patients with diabetes. The advantages of sharing the results of HbA1c with patients at the time of their visit include better motivation by patients, and better chances that patient take more active roles in their diabetes management. In addition to its role in diabetes management, we believe that this new point-of-care technology, especially the most recent improved devices (small, cheap, simple and fast) will be helpful should HbA1c attain a diagnostic role in diabetes.5,81 This will be of particular importance in population-based diabetes screening globally, especially the advantage of transporting capillary samples from remote areas.5
The Future of HbA1c Diagnostic Potential
During the 2007 ADA annual meeting, the NGSP’s Clinical Advisory Committee posted on the NGSP web site a summary of discussions on the status of the use of HbA1c in diabetes screening.82 It was reported that: “only in Japan was HbA1c used for screening or diagnosis of diabetes at present”. The consensus was that: “many physicians are already using HbA1c for the screening and/or diagnosis of diabetes, but different cutoff levels are being used.” The consensus ascribed multiple advantages, and fewer disadvantages, to HbA1c as a diagnostic test.82 Whether the ADA, or other diabetes organizations, will be revisiting their diagnostic guidelines in regards to HbA1c remains to be seen. The US Endocrine Society announced in a press release that an expert panel recommended new diagnostic guidelines for diabetes. These recommendations, recently published,6 noted that the current ADA diagnostic guidelines were made over a decade ago, dismissing HbA1c as a diagnostic tool based on inadequate test standardization. Given recent evidence, the expert panel believed that it was time to revisit using HbA1c and include it in screening and diagnosis of diabetes.6 These new guidelines recommended incorporating HbA1c into the current criteria for screening and diagnosing diabetes, besides FPG and OGTT. The guidelines recommended a screening HbA1c cut-point of 6.0% as a threshold for close follow up, and diagnostic cut-point of 6.5%, if supported by any glucose test. The guidelines recommended, and for the first time, adding RBS for screening purposes, at a cut-point of 130 mg/dl.
Conclusions
In conclusion, HbA1c has been and continues to be used to monitor the control of glycemia in diabetes management. While HbA1c testing will probably not be abandoned, it is expected to undergo some changes in terms of terminologies and measurement units. It is anticipated that laboratories around the world will either use NGSP % or IFCC (mmol/mol or %) plus MBG (to be called eAG or ADAG) in communicating HbA1c results. In the US, it is anticipated that laboratories will probably continue to report NGSP HbA1c % units, and probably not IFCC (in % units) new units, which are about 2 points lower and may thus cause confusion. Similarly it is not anticipated that the IFCC molar units (mmol/mol), which are quite unfamiliar to clinicians, will be adopted in the US, to avoid further confusion, but this remains to be seen. Changes in other countries that may want to report the IFCC units are expected to be very slow in view of anticipated technical difficulties in achieving such a major transition.
Furthermore, it is anticipated that diabetes organizations may consider adding HbA1c at an appropriate cut-point value as a screening tool for diabetes. This has been rationalized by improved test standardization, and by the observation that a lot of physicians already use HbA1c for screening of type 2 diabetes, and probably for diagnosis confirmation in some cases. The ES has announced in a press release that an expert panel recommended using HbA1c in diabetes screening and diagnosis. Whether other diabetes organizations will follow suit remains to be seen.
Acknowledgments
We would like to thank Jinie Shirey (Department of Medicine, Michigan State University College of Human Medicine, East Lansing, MI), Laura Smith, Steve Kalik, and Michael Simon (Sparrow Hospital Medical Library, Lansing, MI), and Faith Crutscheon (Saint Francis Medical Center Medical Library, Cape Girardeau, MO) for assistance in references retrieval. Special acknowledgment: We would like to express our utmost gratitude to Dr. Randie Little for her time and effort in reviewing this manuscript at 2 stages during the manuscript preparation. Dr. Little is a Professor of Medicine at the Department of Pathology and Anatomical Sciences and the Department of Child Health at the University of Missouri School of Medicine, Columbia, Missouri. Dr. Little is a nationally renowned investigator in the clinical and laboratory aspects of glycohemoglobin.
REFERENCES
- 1.Chiasson JL. Prevention of Type 2 diabetes: Fact or fiction? Expert Opin Pharmacother. 2007;8(18):3147–58. doi: 10.1517/14656566.8.18.3147. [DOI] [PubMed] [Google Scholar]
- 2.Nathan D, Davidson M, Defrenzo R, Heine R, Henry R, Pratley R, Zinman B. Impaired fasting glucose and Impaired glucose tolerance: Implications for care [Consensus Statement: Reviews/ commentaries/ADA Statements] Diabetes Care. 2007;30(3):753–9. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 3.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycemia: The current status on definition and intervention. Diabet Med. 2002;19(9):708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 4.Schneider HG, Goodall I, Colman PG, McLean M, Barker G. New haemoglobin A1c: the way it is reported is about to change. Int Med J. 2007;37:213–215. doi: 10.1111/j.1445-5994.2006.01302.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabetic Med. 2007;24:333–43. doi: 10.1111/j.1464-5491.2007.02106.x. [DOI] [PubMed] [Google Scholar]
- 6.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. J Clin Endocrinol Metab. 2008;93:2447–53. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]
- 7.Bryson CL, Boyko EJ. Review: glycated haemoglobin A1c and fasting plasma glucose screening tests have similar sensitivities and specifities for early detection of type 2 diabetes. Evid Based Med. 2007;12:152. doi: 10.1136/ebm.12.5.152. [DOI] [PubMed] [Google Scholar]
- 8.Miedema K. Standardization of HbA1c and Optimal Range of Monitoring. Scand J Clin Lab Invest Suppl. 2005;240:61–72. doi: 10.1080/00365510500236143. [DOI] [PubMed] [Google Scholar]
- 9.The 2006 NGSP Clinical Advisory Committee Meeting. ADA 66th Annual Meeting; [accessed 2007 December 16]. Available from: http://www.ngsp.org/prog/News/CAC2006.htm. [Google Scholar]
- 10.Little RR, Rohlfing CL. Proposed changes for reporting HbA1c: Various groups are deciding how HbaA1c results are to be reported in the future. In Vitro Diagnost Technol. 2007. May, [accessed 15 December 2007]. p. 18. Available from: http://www.devicelink.com/ivdt/archive/07/05/007.html.
- 11.National Glycohemoglobin Standardization Program. The relationship between GHB and blood glucose. [accessed 16 December 2007]. Available from: http://www.ngsp.org/prog/index.html.
- 12.Davidson MB. Glycosylated hemoglobin as a diagnostic test for Type 2 diabetes mellitus (Letters) JAMA. 2000;283:606. [PubMed] [Google Scholar]
- 13.Tahara Y. On the weighted-average relationship between plasma glucose and HbA1c. Diabetes Care. 2006;29:466–7. doi: 10.2337/diacare.29.02.06.dc05-1941. [DOI] [PubMed] [Google Scholar]
- 14.Trevino G. On the weighted-average relationship between plasma glucose and HbA1c. Diabetes Care. 2006;29:466. doi: 10.2337/diacare.29.02.06.dc05-1941. [DOI] [PubMed] [Google Scholar]
- 15.Service FJ, O’Brien PC. Influence of glycemic variables on hemoglobin A1c. Endcorin Pract. 2007;13:350–4. doi: 10.4158/EP.13.4.350. [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33) The Lancet. 1998;352:837–851. [PubMed] [Google Scholar]
- 18.The American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 1994;17(Suppl):616–23. doi: 10.2337/diacare.17.6.616. [DOI] [PubMed] [Google Scholar]
- 19.The American Diabetes Association. Executive Summary: Standards of medical care in diabetes. Diabetes Care. 2008;31(Suppl):S5–S11. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 20.ACE/AACE Diabetes Road Map Task Force. Road maps to achieve glycemic control in type 2 diabetes mellitus. Endocr Pract. 2007;13:260–268. doi: 10.4158/EP.13.3.260. [DOI] [PubMed] [Google Scholar]
- 21.Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med. 2007 Sep 18;147(6):417–22. doi: 10.7326/0003-4819-147-6-200709180-00012. [DOI] [PubMed] [Google Scholar]
- 22.Harris SB, Lank CN. Recommendations from the Canadian Diabetes Association. 2003 guidelines for prevention and management of diabetes and related cardiovascular risk factors. Can Fam Physician. 2004;50:425–33. [PMC free article] [PubMed] [Google Scholar]
- 23.Miedema K. Towards worldwide standardization of HbA1c determination. Diabetologia. 2004;47:1143–48. doi: 10.1007/s00125-004-1453-0. [DOI] [PubMed] [Google Scholar]
- 24.Little R, Rohlfing C, Wiedmeyer H, Myers G, Sacks D, Goldstein D for the NGSP Steering Committee. The National Glycohemoglobin Standardization Program: A five-year progress report. Clinical Chemistry. 2001;47:1985–92. [PubMed] [Google Scholar]
- 25.Jeppsson J-O, Jerntorp P, Sundkvist G, Englund H, Nylund V. Measurement of hemoglobin A1c by a new liquid chromatographic assay: methodology, clinical utility and relation to glucose tolerance evaluated. Clinic Chem. 1986;32:1867–72. [PubMed] [Google Scholar]
- 26.Hoshino T, Okahashi M, Arai H. Survery and assessment of the actual state of routine measurement of glycohemoglobin/GHb by commercial methods: warning to users and the providers. J Pharm Biomed Anal. 1997;15:1555–62. doi: 10.1016/s0731-7085(96)02050-x. [DOI] [PubMed] [Google Scholar]
- 27.Hoelzel W, Weykamp C, Jeppsson Jo, Miedema K, Barr JR, Goodall I, Hoshino T, John WG, Kobold U, Little R, et al. IFCC Working Group on HbA1c standardization. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: A method-comparison study. Clinic Chem. 2004;50:166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 28.Weykamp CW, Penders TJ, Muskiet FA, van der Slik W. Influence of haemoglobin variants and derivatives on glycohemoglobin determinations, as investigated by 102 laboratories using 16 methods. Clini Chem. 1993;39:1717–23. [PubMed] [Google Scholar]
- 29.Gunton J, McElduff A. Hemoglobinopathies and HbA1c measurement. Diabetes Care. 2000;23:1197–8. doi: 10.2337/diacare.23.8.1197. [DOI] [PubMed] [Google Scholar]
- 30.Koskinen L, Ala-Houhala I, Lahtela J, Laippala P, Koivula T. Does uremia interfere with HbA1c results in the FPLC method with mono S cation exchanger? Clinica Chemica Acta. 1998;273:69–79. doi: 10.1016/s0009-8981(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 31.Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E Diabetes Prevention Program Research Group. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007 Oct;30(10):2453–7. doi: 10.2337/dc06-2003. Epub 2007 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders AM. Glycosylated hemoglobin as a diagnostic test for Type 2 diabetes mellitus (Letters) JAMA. 2000;283:606. [PubMed] [Google Scholar]
- 33.HbA1c and hemoglobin variants (HbS and HbC traits) The NGSP Website. [accessed 16 December 2007]. Available from: http://www.ngsp.org/prog/hbsandc.htm.
- 34.Manufacturer’s pamphlet on the Roche/Hitachi (r) HBA1C II (Tina-quant (r) [a] Hemoglobin A1c II) turbidimetric inhibition immunoassay analyzer. 2001 [Google Scholar]
- 35.Panteghini M, John WG. Implementation of haemoglobin A1c results traceable to the IFCC reference system: the way forward. Clin Chem Lab Med. 2007;45:942–944. doi: 10.1515/CCLM.2007.198. [DOI] [PubMed] [Google Scholar]
- 36.Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007 Sep;30(9):2399–400. doi: 10.2337/dc07-9925. [DOI] [PubMed] [Google Scholar]
- 37.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group. Translating the A1c assay into estimated average glucose values. Diabetes Care. 2008;31:1704–7. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tucker M. Internal Medicine News. 2007. Nov 1, Equation ties HbA1c to average blood glucose. Endocrinology Clinical Rounds; p. 25. [Google Scholar]
- 39.Svendson PA, Lauritzen T, Soegaard U, Nerup J. Glycosylated haemoglobin and steady-state mean blood glocsoe concentration in type 1 (insulin-depenedent) diabetes. Diabetlogia. 1982;23:403–405. doi: 10.1007/BF00260951. [DOI] [PubMed] [Google Scholar]
- 40.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341–346. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 41.Diabetes Control and Complications Trial (DCCT): results of feasibility study. The DCCT Research Group. Diabetes Care. 1987 Jan-Feb;10(1):1–19. doi: 10.2337/diacare.10.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Rohlfing C, Wiedmeyer H, Little R, England J, Tennill A, Goldsetin D. Defining the relationship between plasma glucose and HbA1c. Diabetes Care. 2002;25:275–278. doi: 10.2337/diacare.25.2.275. [DOI] [PubMed] [Google Scholar]
- 43.Rohlfing C, Little R, Wiedmeyer H, England J, Madsen R, Harris M, Flegal K, Eberhardt M, Goldsetin D. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the US population. Diabetes Care. 2000;23:187–191. doi: 10.2337/diacare.23.2.187. [DOI] [PubMed] [Google Scholar]
- 44.Buell C, Kermah D, Davidson MB. Utility of A1c for diabetes screening in the 1999–204 NHANES population. Diabetes Care. 2007;30(9):2233–5. doi: 10.2337/dc07-0585. [DOI] [PubMed] [Google Scholar]
- 45.Santiago JV, Davis JE, Fisher F. Hemoglobin A1c levels in a diabetes detection program. J Clin Endocrinol Metab. 1978;47:578–80. doi: 10.1210/jcem-47-3-578. [DOI] [PubMed] [Google Scholar]
- 46.Dods RF, Bolmey C. Glycosylated hemoglobin assay and oral glucose tolerance test compared for detection of diabetes mellitus. Clin Chem. 1979;25:764–68. [PubMed] [Google Scholar]
- 47.Simon D, Coignet MC, Thibult N, Senan C, Eschwege E. Comparison of glycosylated hemoglobin and fasting plasma glucose with two-hour postload plasma glucose in the detection of diabetes mellitus. Am J Epidemiol. 1985;122:589–593. doi: 10.1093/oxfordjournals.aje.a114138. [DOI] [PubMed] [Google Scholar]
- 48.Little RR, England JD, Wiedmeyer HM, McKenzie EM, Pttitt DJ, Knowler WC, Goldstein DE. Relationship of glycosylated hemoglobin to oral glucose tolerance. Implications for diabetes screening. Diabetes. 1988;37:60–64. doi: 10.2337/diab.37.1.60. [DOI] [PubMed] [Google Scholar]
- 49.McCance DR, Hanson RL, Charles MA, Jackobson LTH, Pettit DJ, Bennett PH, Knowler WC. Comparison of tests for glycated hemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods. BMJ. 1994;308:1323–28. doi: 10.1136/bmj.308.6940.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters A, Davidson MB, Schriger D, Hasselblad V for the Meta-analysis Research Group on the Diabgonsis of Diabetes Using Glycated Hemoglobin Levels. A clincial Approach for the diagonosis of diabetes mellitus. JAMA. 1996;276:1246–52. [PubMed] [Google Scholar]
- 51.Ko GT, Chan JC, Cockram CS. Supplement to the use of a paired value of fasting plasma glucose and glycated hemoglobin in predicting the likelihood of having diabetes. Diabetes Care. 1998;21:2032–3. doi: 10.2337/diacare.21.11.2032. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi Y, Noda M, Tsugane S, Kuzuya T, Ito C, Kadowaki T. Prevalence of diabetes estimated by plasma glucose criteria combined with standardized measurement of HbA1c among health checkup participants on Myako Island, Japan. Diabetes Care. 2000;23:1092–6. doi: 10.2337/diacare.23.8.1092. [DOI] [PubMed] [Google Scholar]
- 53.Ito C, Maeda R, Ishida S, Sasaki H, Harada H. Correlation among fasting plasma glucose, two-hour plasma glucose and HbA1c. Diabetes Res Clin Practice. 2000;50:225–230. doi: 10.1016/s0168-8227(00)00187-x. [DOI] [PubMed] [Google Scholar]
- 54.Perry RC, Shankar RR, Finerberg N, McGill J, Baron AD. HbA1c measurement improves the detection of type 2 diabetes in high-risk individuals with nondiagnostic levelsl of fasting plasma glucose. The early diabetes intervention program (EDIP) Diabetes Care. 2001;24:465–71. doi: 10.2337/diacare.24.3.465. [DOI] [PubMed] [Google Scholar]
- 55.Edelman D. Outpatient diagnostic errors: unrecognized hyperglycemia. Eff Clin Pract. 2002;5:11–6. [PubMed] [Google Scholar]
- 56.Edelman D, Edwards LJ, Olsen MK, Dudley TK, Harris AC, Blackwell DK, Oddone EZ. Screening for diabetes in an outpatient clinic population. J Gen Intern Med. 2002;17:23–8. doi: 10.1046/j.1525-1497.2002.10420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greci LS, Kailasam M, Malkani S, Katz DL, Hulinsky I, Ahmadi R, Nawaz H. Utilitiy of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care. 2003;26:1064–8. doi: 10.2337/diacare.26.4.1064. [DOI] [PubMed] [Google Scholar]
- 58.Davidson MB, Shriger DL, Peters AL, Lorber B. Relationship between fasting plasma glucose and glycosylated hemoglobin: potential for false-positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA. 1999;281:1203–10. doi: 10.1001/jama.281.13.1203. [DOI] [PubMed] [Google Scholar]
- 59.Anand S, Razak F, Vuksam V, Gerstein H, Malmberg K, Quilong Y, Teo K, Yusuf S. Diagnostic strategies to detect glucose intolerance in a multiethnic population. Diabetes Care. 2003;26:290–6. doi: 10.2337/diacare.26.2.290. [DOI] [PubMed] [Google Scholar]
- 60.Silverman RA, Pahk R, Carbone M, Wells E, Mitzner R, Burris K, Kelson JR, Grella R, Katzeff H. The relationship of glucose and HbA1c levels among emergency department patients with no prior history of diabetes mellitus. Acad Emerg Med. 2006;13:722–6. doi: 10.1197/j.aem.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Suwazono Y, Sakata K, Okubo Y, Harada H, Oishi M, Kobayashi E, Uetani M, Kido T, Nogawa K. Long-term longitudinal study on the relationship between alternating shift work and the onset of diabetes mellitus in male Japanese workers. Journal of Occumpational and Environmental Medicine. 2006;48:455–461. doi: 10.1097/01.jom.0000214355.69182.fa. [DOI] [PubMed] [Google Scholar]
- 62.Pollak A, Brehm R, Havelec L, Lubec G, Malamitsi-Puchner A, Simbrunner G, Widness JA. Total glycosylated hemoglobin in mothers of large-for-gestational-age infants: a postpartum test for undetected maternal diabetes. Biol Neonate. 1981;40:129–35. doi: 10.1159/000241481. [DOI] [PubMed] [Google Scholar]
- 63.Shah BD, Cohen AW, May C, Gabbe SG. Comparison of glycohemoglobin determination and one-hour oral glucose screen in the identification of gestational diabetes. Am J Obstet Gynecol. 1982;144:774–7. doi: 10.1016/0002-9378(82)90350-7. [DOI] [PubMed] [Google Scholar]
- 64.Artal R, Artal R, Mosley GM, Dorey FJ. Glycohemoglobin as a screening test for gestational diabetes. Am J Obstet Gynecol. 1984;148:412–4. doi: 10.1016/0002-9378(84)90717-8. [DOI] [PubMed] [Google Scholar]
- 65.Cousins L, Dattel BJ, Hollingsworth Zetner A. Glycosylated hemoglobin as a screening test for carbohydrate intolerance in pregnancy. Am J Obstet Gyne. 1984;150:455–60. doi: 10.1016/s0002-9378(84)90420-4. [DOI] [PubMed] [Google Scholar]
- 66.Frisoli G, Naranjo L, Shehab N. Glycohemoglobin in normal and diabetic pregnancy. Am J Perinatol. 1985;2:183–8. doi: 10.1055/s-2007-999945. [DOI] [PubMed] [Google Scholar]
- 67.Cocilovo G, Guerra S, Colla F, Tomasi F. Glycosylated hemoglobin (HbA1) assays a test for detection and surveillance of gestational diabetes. Diabetes Metab. 1987;13:426–30. [PubMed] [Google Scholar]
- 68.Griffith RJ, Vinall PS, Stickland MH, Wales JK. Hemoglobin A1c in normal and diabetic pregnancies. Eur J Obstet Gynecol Reprod Biol. 1987;24:195–200. doi: 10.1016/0028-2243(87)90018-9. [DOI] [PubMed] [Google Scholar]
- 69.Agarwal MM, Hughes PF, Punnose J, Ezimokhai M, Thomas L. Gestational diabetes screening of a multi-ethnic, high-risk population using glycated proteins. Diabetes Res Clin Pract. 2001;51:67–73. doi: 10.1016/s0168-8227(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 70.Agarwal M, Dhatt G, Punnose J, Koster G. Gestational diabetes: A reappraisal of HBA1c as a screening test. Acta Obstet Gynecol Scand. 2005;84:1159–63. doi: 10.1111/j.0001-6349.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 71.Aldasouqi S, Solomon D, Bokhari S, Khan M, Shareef M. Glycohemoglobin A1c is a promising diagnostic tool in gestational diabetes mellitus. A pilot retrospective study. Endocr Pract. 2006;12(Suppl):8. A-113. [Google Scholar]
- 72.Ealovega M, Tabaei B, Brandle M, Burke R, Herman W. Opportunistic screening for diabetes in routine clinical practice. Diabetes Care. 2004;27:9–12. doi: 10.2337/diacare.27.1.9. [DOI] [PubMed] [Google Scholar]
- 73.The USPSTF. Screening for type 2 diabetes mellitus in adults: Recommendations and rationale. Annals of Internal Medicine. 2003;138:212–4. doi: 10.7326/0003-4819-138-3-200302040-00014. [DOI] [PubMed] [Google Scholar]
- 74.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes provisional report a WHO consultation. Diabetes Medicine. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 75.Leeuwen M, Zweers E, Opmeer B, Ballegooie E, Brugge H, Valk H, Mol B, Visser G. Comparison of accuracy of two screening tests for gestational diabetes mellitus. Diabetes Care. 2007;30:2779–2784. doi: 10.2337/dc07-0571. [DOI] [PubMed] [Google Scholar]
- 76.Aldasouqi S, Solomon D, Bokhari S, Khan M, Shareef M, Gossain V. Glycohemoglobin A1c is a promising screening tool in gestational diabetes mellitus. Forthcoming. doi: 10.4103/0973-3930.45271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barr RG, Nathan DM, Meigs JB, Singer DE. Tests of glycemian for the diagnosis of type 2 diabetes mellitus. Annal Intern Med. 2002;137:263–272. doi: 10.7326/0003-4819-137-4-200208200-00011. [DOI] [PubMed] [Google Scholar]
- 78.Thaler LM, Dunbar VG, Ziemer DC, Phillips LS, Gallina DL, El-Kebbi IM, Cook CB. Diabetes in urban African-Americans. XVII. Availability of rapid HbA1c measurements enhances clinical decision making. Diabetes Care. 1999;22:1415–1421. doi: 10.2337/diacare.22.9.1415. [DOI] [PubMed] [Google Scholar]
- 79.Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin treated type 2 diabetic patients. Diabetes Care. 1999;22:1785–1789. doi: 10.2337/diacare.22.11.1785. [DOI] [PubMed] [Google Scholar]
- 80.Ferenczi A, Reddy K, Lorber DL. Effect of immediate hemoglobin A1c results on treatment decisions in office practice. Endocr Pract. 2001;7:85–88. doi: 10.4158/EP.7.2.85. [DOI] [PubMed] [Google Scholar]
- 81.Mattewal A, Aldasouqi S, Solomon D, Gossain V, Koller A simple method for office-based glycohemoglobin measurement. Diab J Scien Technolo. 2007;1(6):879–884. doi: 10.1177/193229680700100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.The 2007 NGSP Clinical Advisory Committee Meeting. The American Diabetes Association 67th Annual Scientific Sessions; 2007 June; 2007. [accessed 23 December 2007]. Available from: http://ngsp.org/prog/News/CAC2007.htm. [Google Scholar]