Abstract
BACKGROUND
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of mortality and morbidity worldwide. However, there are still no easily obtained biomarkers for prognosis. As a high-affinity Fc receptor, CD64 is an early marker of immune response to bacterial infection, but its role in acute exacerbation of COPD (AECOPD) remains incompletely understood.
OBEJECTIVE
We investigated the prognostic role of the neutrophial CD64 (nCD64) index in AECOPD patients.
DESIGN
Retrospective cross-sectional study of all patient admitted between January 2013 to May 2014.
SETTING
Provincial hospitals affiliated with a university.
PATIENTS AND METHODS
Clinical and laboratory data were collected in patients admitted for AECOPD and stable COPD patients, in whom nCD64 index was obtained. A receiver operating characteristics curve was used to determine the optimal cut-off levels for the nCD64 index that discriminated survivors versus nonsurvivors during index hospitalization, and during a post-discharge period of 12 months.
MAIN OUTCOME MEASURES
nCD64 index level.
RESULTS
The white blood cell count, CRP (C-reactive protein (CRP) and PCT (procalcitonin) in AECOPD subjects (n=31) were all significantly higher than in controls (n=18) (P=≤.01). The mean nCD64 index in AECOPD subjects was significantly higher than in control subjects (2.84% [1.0%] vs. 1.50% [0.5%], P=<.001). Moreover, the mean nCD64 index in nonsurvivors was significantly higher than in survivors (2.6%) (2.59±0.85 vs. 3.87±0.65, P<.001). nCD64 index >3.3 predicted in-hospital mortality with a sensitivity and specificity of 80% and 83%, respectively (area under the ROC=0.887; 95% confidence interval [CI]=0.721–0.972, P<.001). An nCD64 index of 3.3 upon admission as the optimal cut-off level to predict post-discharge mortality had a sensitivity and specificity of 83% and 75%, respectively (area under the ROC=0.842; 95% confidence interval [CI]=0.667–0.948, P<.001).
CONCLUSIONS
An elevated nCD64 index was a reliable prognostic biomarker for both short-term and long-term mortality in patients admitted for AECOPD.
LIMITATIONS
Retrospective design prevented collection of enough evidence to demonstrate infectious origin for COPD in every patient. Unsure whether nCD64 differed between bacterial and viral exacerbation.
Chronic obstructive pulmonary disease (COPD) is the fourth main cause of death and global burden of disease worldwide.1 COPD is characterized by an increasing decline in lung function and recurrent exacerbations that require hospitalization.2 The characteristic symptoms of COPD are cough, productive sputum, and dyspnea, which impairs activities of daily life and quality of life. Furthermore, COPD patients often coexist with other systemic comorbid diseases, such as lung cancer, cardiovascular disease, osteoporosis, and diabetes.3 The comorbidities of COPD can also require emergency hospitalization, and increase mortality.4,5
The severity and frequency of exacerbations in COPD patients are the most important factors determining overall prognosis. Several biomarkers have been investigated to predict clinically relevant outcomes such as hospitalization, exacerbations, and mortality.6–9 For example, leukocyte count was significantly associated with hospitalization in COPD patients. C-reactive protein (CRP) was significantly associated with mortality in COPD patients.6–8 D-dimer also was a prognostic biomarker for mortality in AECOPD.9 Nevertheless, there are still no easily obtained biomarkers for short-term and long-term prognosis.
As a high-affinity Fc receptor, CD64 is expressed by monocytes and only weakly on resting neutrophils.10–12 The high-expression of neutrophil CD64 (nCD64) is an early step in the host immune response to bacterial infection.11 Expression of nCD64 is markedly increased about 1 hour after invasion and is stable for more than 24 hours. Previous studies have shown that the nCD64 index might be used as a biomarker for early-onset sepsis or bacterial infection.13–17 Moreover, some reports have demonstrated the role of nCD64 in noninfectious inflammatory diseases, such as acute pancreatitis, adenoid hypertrophy and total joint arthroplasty.18–20 A recent study showed that the diagnostic accuracy of nCD64 index to predict sepsis in critically ill patients was better than that of procalcitonin and CRP.21 These results indicate that the nCD64 index might be an important prediction biomarker in both infectious diseases and noninfectious inflammatory diseases. However, the value of the nCD64 index in COPD prognosis is unknown.
The purpose of the present study was to assess the prognostic value of the nCD64 index in patients with COPD. We also assessed circulating levels of CRP, leukocyte count and procalcitonin as potential prognostic parameters for in-hospital and long-term outcomes in patients with acute exacerbation of COPD (AECOPD) requiring hospitalization.
PATIENTS AND METHODS
AECOPD and stable COPD subjects were recruited from the Department of Respiratory Medicine, the Shandong Provincial Hospital affiliated to Shandong University. All patients admitted between January 2013 and May 2014 were included in the study. A diagnosis of COPD was made by a clinical spirometer (MS-10S, Jaeger, Germany) based on a history of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio of <0.7. The severity of COPD was graded according to the Global Initiative for Chronic Obstructive Lung Disease guidelines (Stage I, mild COPD: FEV1≥80.0% predicted; Stage II, moderate COPD: 50.0%≤FEV1 <80.0% predicted; Stage III, severe COPD: 30.0%≤FEV1 <50.0%; Stage IV, very severe COPD: FEV1 < 30.0% or FEV1 <50.0% predicted with respiratory failure). The exacerbation of COPD was defined as the patient being diagnosed with COPD with two or more of the following three symptoms of exacerbations: new or worsening cough, worsened dyspnea, and worsened sputum volume and/or change in its color.
Blood samples
The following clinical data were collected: age, gender, BMI (body mass index), arterial blood gas analysis, and laboratory data. The blood samples were collected within 24 hours of hospitalization. Blood samples were centrifuged at 1000g/min for 15 minutes, and then the serum was separated and stored under −80°C. Procalcitonin (PCT) was also detected. Ethical approval was provided by the medical ethics committee of the Shandong Provincial Hospital affiliated to Shandong University. Informed consent was obtained either from the patients or the patients’ families.
nCD64 index measurement
Neutrophil CD64 (nCD64) index was measured using flow cytometry using a commercial kit (Quanti BRITE PE, Becton Dickinson). Briefly, phosphate-buffered saline-diluted whole blood (100 μL) was incubated for 20 minutes at room temperature with a combination of anti-CD14-FITC and anti-CD64-PE. After lysis, blood samples were washed and fixed with BD Lyse/Wash Assistant. Neutrophils were identified by electronic gating based forward and side scatter. Interassay standardization and CD64 quantization were performed using Quanti BRITEPE calibration beads with known numbers of PE molecules. Data analysis was performed by using light scatter gating to define the neutrophil population, and the nCD64 index was quantified as mean equivalent soluble fluorescence units using BD Diva software. Corrections for nonspecific antibody binding were performed by subtracting values for the isotope control. Expression of nCD11b was also measured by flow cytometry in the same way.
Statistical analysis
Descriptive data are presented as mean and standard deviation) or median and range. Continuous data were tested for normality. Comparisons between groups were made using the t test (for continuous variables, such as pH, PaCO2, PaO2, hs-CRP, PCT and nCD64) or Mann-Whitney U test (for categorical variables, such as MRC and median length of hospitalization). Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off level for the serum nCD64 index. A Cox proportional multivariate hazards model using all potential predictors of mortality was performed. A two-sided P value of .05 was considered statistically significant. All of the statistical analyses were performed using the statistical software package (SPSS 13.0).
RESULTS
Thirty-one subjects had AECOPD and 18 had stable COPD. There was no significant difference in sex, ages, BMI between the two groups (Table 1). The white blood cell count, hsCRP and PCT in AECOPD subjects were all significantly higher than in stable COPD subjects (P<.01). The nCD64 index in AECOPD patients was significantly higher than in stable COPD subjects (2.54% versus 1.05%, P<.001). In AECOPD patients, there were 12 subjects in GOLD stage III or IV. Most AECOPD patients had one or more comorbidities, such as ischemic heart disease, diabetes, congestive heart failure or osteoporosis. Twenty-seven (87.1%) AECOPD patients received antibiotic therapy during hospitalization. The median length of hospitalization was 11.8 days.
Table 1.
Demographic and clinical characteristics of subjects.
| Stable COPD group | AECOPD group | P | |
|---|---|---|---|
|
| |||
| N | 18 | 31 | NS |
| Age (y) | 63.6 (11.3) | 68.6 (10.4) | .85 |
| Gender (male/female) | 15/3 | 26/5 | .63 |
| BMI (kg/m2) | 21.4 (5.2) | 18.7 (5.7) | .91 |
| GOLD stage (I+II) | 10 | 19 | .69 |
| GOLD stage (III+IV) | 8 | 12 | .69 |
| Ischemic heart disease (n) | 4 | 10 | .45 |
| Diabetes | 5 | 15 | .16 |
| Osteoporosis | 3 | 6 | .81 |
| Length of hospitalization (median, days) | --- | 11.8 (4.6) | NS |
| Antibiotic treatment (n, %) | --- | 27 (87.1%) | NS |
| White blood cell count (109/L) | 7.72 (1.9) | 9.3 (2.5) | <.01 |
| pH | --- | 7.3 (0.1) | NS |
| PaCO2 (mm Hg) | --- | 53.7 (20.0) | NS |
| PaO2 (mm Hg) | --- | 70.0 (10.5) | NS |
| hs CRP (mg/L) | 5.3 (1.9) | 8.0 (3.1) | <.01 |
| PCT (μg/L) | 0.3 (0.1) | 0.49 (0.2) | <.01 |
| nCD64 (%) | 1.50 (0.5) | 2.84 (1.0) | <.01 |
Values are mean (standard deviation). Statistically significant values are bolded.
During hospitalization, 6 patients (19.35%) died. The recorded causes for death were respiratory failure (n=1), congestive heart failure (n=1), sepsis (n=2) or multi-organ failure (n=2). The differences in main parameters between survivors and nonsurvivors are listed in Table 2. There were no significant differences in age, sex, BMI, white blood cell count, CRP, procalcitonin, pH, PaO2 or comorbidities between survivors and nonsurvivors. A significantly higher proportion of nonsurvivors were in GOLD stages III–IV compared with survivors (83.3% versus 36.0%, P<.001). Patients who died during hospitalization had higher PaCO2 and a lower pH upon admission, although the difference was not statistically significant. The nCD64 index upon admission in nonsurvivors was significantly higher than survivors (2.59±0.85 vs. 3.87±0.65, P<.001).
Table 2.
Comparison of clinical and laboratory data between survivors and nonsurvivors during index hospitalization.
| Survivor group | Nonsurvivor group | P | |
|---|---|---|---|
|
| |||
| N | 25 | 6 | NS |
| Age (yrs) | 67.25 (12.63) | 72.84 (15.23) | .45 |
| Gender (male/female) | 19/6 | 5/1 | .69 |
| BMI (kg/m2) | 21.05 (7.66) | 18.37 (5.79) | .87 |
| GOLD stage (I + II) | 18 | 1 | .012 |
| GOLD stage (III + IV) | 7 | 5 | .012 |
| Ischemic heart disease (n) | 8 | 3 | .41 |
| Diabetes (n) | 9 | 4 | .17 |
| Osteoporosis (n) | 6 | 3 | .21 |
| Length of hospitalization (median, days) | 9.68 (3.59) | 19.72 (6.58) | <.001 |
| Antibiotic treatment(n) | 21 | 6 | .29 |
| White blood cell count (109/L) | 8.83 (3.25) | 11.33 (5.57) | .15 |
| pH | 7.35 (0.07) | 7.30 (0.16) | .25 |
| PaCO2 (mmHg) | 50.96 (18.08) | 65.17 (25.11) | .14 |
| PaO2 (mmHg) | 71.48 (9.85) | 64.34 (11.85) | .11 |
| hs CRP (mg/L) | 7.48 (2.93) | 9.97 (3.05) | .091 |
| PCT (μg/L) | 0.46 (0.18) | 0.62 (0.34) | .15 |
| nCD64 (%) | 2.59 (0.85) | 3.87 (0.65) | .002 |
Values are mean (standard deviation). Statistically significant values are bolded.
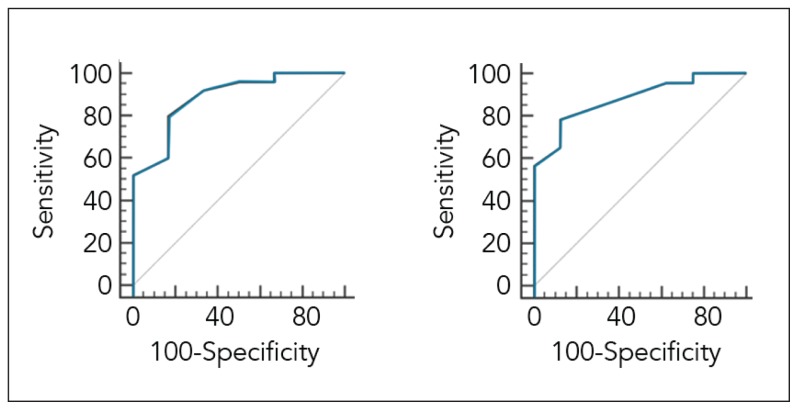
ROC analysis (Figure 1a) identified an nCD64 index of 3.3 upon admission as the optimal cut-off level to discriminate survivors from nonsurvivors during hospitalization (area under the ROC= 0.887; 95% confidence interval [CI]=0.721–0.972, P<.001). The nCD64 index above 3.3 had a sensitivity and specificity of 80% and 83.3%, respectively, for predicting hospital mortality (Table 3). After discharge, overall median survival for the entire cohort was 10.8 months. ROC analysis based on post-discharge survival data (Figure 1b) identified a nCD64 index of 3.3 upon admission as the optimal cut-off level to predict post-discharge mortality with a sensitivity and specificity of 82.6% and 75.0%, respectively (Table 3) (area under the ROC= 0.842; 95% confidence interval [CI] = 0.667–0.948, P<.001).
Figure 1.
Receiver operating characteristic (ROC) curve for nCD64 as marker of in-hospital mortality (1a) and post-discharge mortality (1b) in patients with COPD.
Table 3.
Test characteristics of nCD64 upon admission as a biomarker for predicting in-hospital and long-term mortality.
| Criteria | In-hospital mortality | Long-term mortality |
|---|---|---|
|
| ||
| Cut-off point (%) | 3.3 | 3.3 |
| AUC | 0.887 | 0.842 |
| 95% CI | 0.721–0.972 | 0.667–0.948 |
| Sensitivity | 80.0% | 82.6% |
| Specificity | 83.3% | 75.0% |
DISCUSSION
nCD64 expression is an early marker of bacterial infection. In this study, our results demonstrated a increased nCD64 index in AECOPD patients. Moreover, the elevated nCD64 index was a reliable prognostic biomarker for both short-term and long-term survival in patients admitted for AECOPD. These results show the critical prognostic role of nCD64 in the COPD process.
Although previous studies have found many biomarkers for evaluation of COPD, few were effective in the short-term and long-term prognosis of COPD. Our results indicate a role for the nCD64 index in both short-term and long-term prognosis of COPD patients. As a marker of host immune response to bacterial infection, the nCD6 expression increases about 1 hour after invasion and is stable for more than 24 hours.10–13,22 Previous studies have shown that the nCD6 index acts as a biomarker for the early-onset sepsis and acute pancreatitis.10–13,18 Infections, including bacterial infection and viral infection, are the major causes of AECOPD, so it is not surprising that the nCD64 index increased significantly in AECOPD patients compared with controls. The increased nCD64 index, suggesting airway infection, was correlated with a worsened short-term prognosis in AECOPD patients. As this is a retrospective study, we could not collect enough evidence to demonstrate an infectius origin for every patient, so we cannot show whether nCD64 differed between infectious and non-infectious patients. Furthermore, we are unsure whether nCD64 differed between bacterial exacerbation and viral exacerbation. As increased nCD64 is mainly an early immune response to bacterial infection, infectious patients might show a higher nCD64 level than non-infectious patients, but this should be proven with further research.
Our results also showed the role of the nCD64 index in the estimation of long-term prognosis in COPD patients, indicating that a higher nCD64 index might predict not only the present exacerbation but also a risk of future exacerbation, which could result in a serious prognosis in COPD patients. This result also indicated that nCD64 might play a critical role in COPD development. The CD64 index was an early marker of bacterial infection. However, it has also been shown that increased CD64 expression was associated with noninfectious inflammatory processes, such as acute pancreatitis, adenoid hypertrophy and total joint arthroplasty. Thus, CD64 might be considered not only a general marker of bacterial infection, but also a characteristic of noninfectious inflammation. Thus, we suggest that the elevation of nCD64 in our study might be due to infection and non-specific inflammation. An increased nCD64 index might be a marker of systemic inflammation in COPD patients. However, the prognostic value of nCD64 in chronic COPD remains unclear. There is much work to do before we learn the molecular mechanism of nCD64 in COPD.
In conclusion, the elevated nCD64 index was a potential prognostic biomarker for both short-term and long-term survival in patients admitted for AECOPD. Our findings should further be confirmed by randomized prospective studies.
REFERENCES
- 1.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 2.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–63. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corsonello A, Antonelli Incalzi R, Pistelli R, Pedone C, Bustacchini S, Lattanzio F. Comorbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2011;17(Suppl 1):S21–8. doi: 10.1097/01.mcp.0000410744.75216.d0. [DOI] [PubMed] [Google Scholar]
- 4.Lee HM, Lee J, Lee K, Luo Y, Sin DD, Wong ND. Relation between COPD severity and global cardiovascular risk in US adults. Chest. 2012;142(5):1118–25. doi: 10.1378/chest.11-2421. [DOI] [PubMed] [Google Scholar]
- 5.Mullerova H, Maselli DJ, Locantore N, Vestbo J, Hurst JR, Wedzicha J, et al. Hospitalized Exacerbations of Chronic Obstructive Pulmonary Disease: Risk Factors and Outcomes in the ECLIPSE Cohort. Chest. 2015 Apr;147(4):999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen M, Ingebrigtsen TS, Marott JL, Dahl M, Lange P, Vestbo J, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA. 2013;309(22):2353–61. doi: 10.1001/jama.2013.5732. [DOI] [PubMed] [Google Scholar]
- 7.Warwick G, Thomas PS, Yates DH. Noninvasive biomarkers in exacerbations of obstructive lung disease. Respirology. 2013;18(5):874–84. doi: 10.1111/resp.12089. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen M, Dahl M, Lange P, Vestbo J, Nordestgaard BG. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(10):982–8. doi: 10.1164/rccm.201206-1113OC. [DOI] [PubMed] [Google Scholar]
- 9.Fruchter O, Yigla M, Kramer MR. D dimer as a Prognostic Biomarker for Mortality in Chronic Obstructive Pulmonary. Am J Med Sci. 2015;349(1):29–35. doi: 10.1097/MAJ.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 10.Dimoula A, Pradier O, Kassengera Z, Dalcomune D, Turkan H, Vincent JL. Serial determinations of neutrophil CD64 expression for the diagnosis and monitoring of sepsis in critically ill patients. Clin Infect Dis. 2014;58(6):820–9. doi: 10.1093/cid/cit936. [DOI] [PubMed] [Google Scholar]
- 11.Roussel M, Gros A, Sauvadet E, Gacouin A, Marqué S, Chimot L, et al. CD64, a reliable biomarker of bacterial infection in intensive care units? Am J Respir Crit Care Med. 2012;186(10):1058. doi: 10.1164/ajrccm.186.10.1058. [DOI] [PubMed] [Google Scholar]
- 12.Gámez-Díaz LY, Enriquez LE, Matute JD, Velásquez S, Gómez ID, Toro F, et al. Diagnostic accuracy of HMGB-1, sTREM-1, and CD64 as markers of sepsis in patients recently admitted to the emergency department. Acad Emerg Med. 2011;18(8):807–15. doi: 10.1111/j.1553-2712.2011.01113.x. [DOI] [PubMed] [Google Scholar]
- 13.Farias MG, de Lucena NP, Dal Bó S, de Castro SM. Neutrophil CD64 expression as an important diagnostic marker of infection and sepsis in hospital patients. J Immunol Methods. 2014;414(12):65–8. doi: 10.1016/j.jim.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Lynema S, Marmer D, Hall ES, Meinzen-Derr J, Kingma PS. Neutrophil CD64 as a Diagnostic Marker of Sepsis: Impact on Neonatal Care. Am J Perinatol. 2014 Jul 31; doi: 10.1055/s-0034-1384644. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du J, Li L, Dou Y, Li P, Chen R, Liu H. Diagnostic utility of neutrophil CD64 as a marker for early-onset sepsis in preterm neonates. PLoS One. 2014;9(7):e102647. doi: 10.1371/journal.pone.0102647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elawady S, Botros SK, Sorour AE, Ghany EA, Elbatran G, Ali R. Neutrophil CD64 as a diagnostic marker of sepsis in neonates. J Investig Med. 2014;62(3):644–9. doi: 10.2310/JIM.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Huang X, Chen Z, Zhong H, Peng Q, Deng Y, et al. Neutrophil CD64 expression as a biomarker in the early diagnosis of bacterial infection; a meta-analysis. Int J Infect Dis. 2013;17(1):e12–23. doi: 10.1016/j.ijid.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Ling XL, Wu YY, Lü MH, Guo H, Zhang PB, et al. CD64 expression is increased in patients with severe acute pancreatitis; clinical significance. Gut Liver. 2014;8(4):445–51. doi: 10.5009/gnl.2014.8.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagella F, De Amici M, Matti E, Pusateri A, Benazzo M, Ciprandi G. CD64 expression on monocytes in children with adenoid hypertrophy. Asian Pac J Allergy Immunol. 2013;31(2):132–7. doi: 10.12932/AP0294.31.2.2013. [DOI] [PubMed] [Google Scholar]
- 20.Katoh N, Nishino J, Nishimura K, Kawabata C, Hotta Y, Matsui T, et al. Normal sequential changes in neutrophil CD64 expression after total joint arthroplasty. J Orthop Sci. 2013;18(6):949–54. doi: 10.1007/s00776-013-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogina P, Stubljar D, TLZ, Osredkar J, Skvarc M. Expression of CD64 on neutrophils (CD64 index): diagnostic accuracy of CD64 index to predict sepsis in critically ill patients is better than of procalcitonin C-reactive protein, research note. Clin Chem Lab Med. 2015 Mar;53(4):e89–91. doi: 10.1515/cclm-2014-0814. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann JJ. Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin Chem Lab Med. 2009;47(8):903–916. doi: 10.1515/CCLM.2009.224. [DOI] [PubMed] [Google Scholar]