Abstract
BACKGROUND
Miliary tuberculosis (TB) usually presents with atypical clinical manifestations; thus it is often recognized only at autopsy.
OBJECTIVES
Our objectives were to study the frequency of MT diagnosed at autopsy and determine clinical diagnoses that masked TB, as well as causes of death and comorbidities.
DESIGN
Retrospective study of all autopsies performed between 2008 and 2014.
SETTING
Institute of Pathology, Belgrade, Serbia.
SUBJECTS AND METHODS
In subjects where autopsy showed the presence of MT that was not recognized clinically, we recorded the clinical diagnoses (presumed causes of death) as reported in autopsy request forms, as well as actual cause of death and comorbidities as determined at autopsy.
MAIN OUTCOME MEASURES
Clinically unrecognized MT.
RESULTS
The total number of autopsies in this period was 6206. Thirty-five individuals showed clinically unrecognized MT (0.56% of all autopsies, age: 62.2 [17.2] years, M:F=2:3). Common clinical diagnoses masking pulmonary MT were exacerbation of COPD (25%) and pulmonary thromboembolism (25%), with common radiological presentation of diffuse pulmonary infiltrates (56.3%). Dominant clinical diagnoses in patients with generalized MT were adult respiratory distress syndrome, sepsis, gastrointestinal bleeding and meningoencephalitis. Disseminated MT was often associated with secondary anemia or thrombocytopenia (15.8%) and recent surgery (15.8%). Frequent comorbidities included chronic renal failure and malignancies, whereas MT was a dominant cause of death.
CONCLUSION
Greater awareness of MT is needed to improve recognition in clinical settings. In particular, MT should be considered in patients with atypical clinical presentation and diffuse pulmonary infiltrates on chest X-ray, particularly if they have chronic renal failure, malignancy, hematological disorders or a history of recent surgery.
LIMITATIONS
None.
Tuberculosis (TB) is a chronic infectious disease that represents an important cause of death in undeveloped and developing countries. According to the latest WHO data, 9 million people acquired TB and 1.5 million died in 2013 worldwide.1 Countries with immigrants and workers from TB endemic countries face an increasing TB burden even though the incidence may have been decreasing.2 For instance, in the period between 1991 and 2010 in Saudi Arabia, the annual TB rate was in the range 8.6–12.2 per 100 000 persons for the original Saudi population, while non-Saudis had 2 to 3 times higher incidence.2 The incidence of TB in Serbia has been declining since 1990 and current data of the national Institute of Public Health in Belgrade report the actual incidence rate for TB to be 15.61 per 100 000 persons.3 Efforts are underway to control the disease, but new strategies are necessary to eliminate it at a global level.4
TB is caused by the human type of Mycobacterium tuberculosis, which is usually transmitted via inhalation of infective droplets. Reservoirs of infection are usually people with the active pulmonary form of TB. Classification of TB is based on characteristics of its development and course, and encompassing primary, postprimary or secondary, and miliary TB. Miliary TB is a complication occurring either during primary or postprimary TB when M tuberculosis gets into the bloodstream and is disseminated within the lungs or other organs.5
Even in 1700, John Jacob Manget described disseminated TB as numerous small nodules in lung parenchyma, and termed it “miliary tuberculosis” (lat. “millet”, grain of millet) and suggested it was a fatal form of the disease.6 Although the incidence of TB is declining, miliary TB still displays a notable and stable incidence. If not recognized and treated in time, miliary TB is potentially lethal.5 Unfortunately, diagnosis of miliary TB can be complicated even for the most experienced clinicians. Given that it has an atypical clinical presentation and can resemble many other diseases, diagnosis of miliary TB is often made at autopsy. Miliary TB can manifest acutely as multiorgan dysfunction,7 septic shock syndrome8 or as adult respiratory distress syndrome.9,10 Patients with more chronic form of disease can show a delay in growth and development,11 fever of unknown origin12 or dysfunction of one or more organs.13 Predisposing factors and conditions associated with development of miliary TB are malnutrition, HIV infection, diabetes mellitus, chronic renal failure, dialysis, organ transplantation, malignant tumors, and silicosis.5 However, the pathogenetic mechanism is not clear.
Given the great importance of clinically unrecognized forms of TB both in specialized lung hospitals and in general hospitals,14 the aim of this study was to analyze autopsy cases of clinically unrecognized cases of miliary TB, to determine the clinical diagnoses that most frequently mask the TB, as well as comorbidities that contibute to development and a lethal outcome.
SUBJECTS AND METHODS
This was a retrospective study of all autopsy protocols and reports conducted at the Institute of Pathology, Faculty of Medicine, University of Belgrade in a 7-year period (January 1st 2008-December 31st 2014).
Autopsy request forms (the forms that had been sent to the Institute of Pathology by the clinicians to require autopsy of the deceased patient) were analyzed. From these forms, we recorded patient sex and age, and the main clinical diagnoses, i.e., presumed causes of death of each individual as reported by the responsible clinicians. In addition, we noted the type of hospital (general hospitals vs. specialized pulmonary hospitals) from which the deceased were sent to the Institute of Pathology for autopsy.
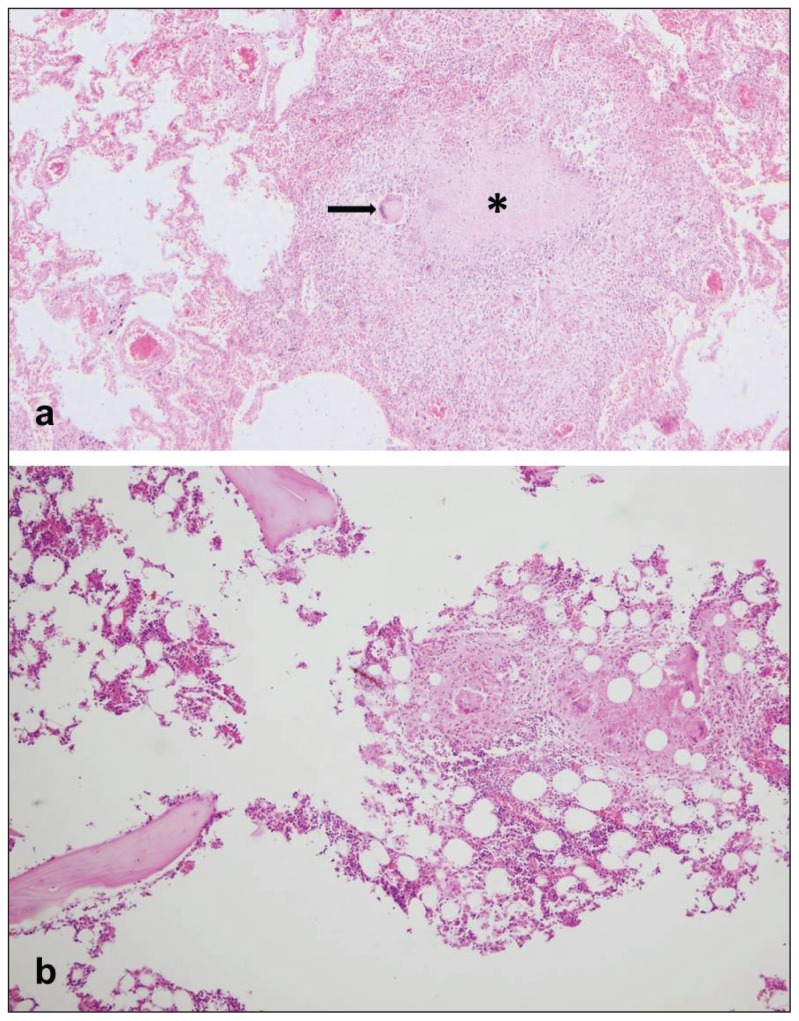
Based on the information from autopsy request forms as well as autopsy findings, the study group was composed of autopsied individuals with no mention of clinical diagnosis of TB or antemortem suspicion of TB, but in whom the miliary TB was ascertained at autopsy. These individuals were referred to as “clinically unrecognized TB”. They were classified based on autopsy diagnosis to those with miliary pulmonary TB and those with miliary generalized (disseminated) TB. In the individuals with miliary generalized form of TB, we recorded the distribution of TB granulomas throughout the body. TB granulomas were recognized as small whitish nodular formations with typical histological features of agglomeration of epitheloid cells, giant cells of Langhans type and lymphocytes, with centrally located caseous necrosis (Figure 1). Based on autopsy findings, the actual causes of death were noted, as well as main comorbidities.
Figure 1.
a) Histopathological findings of tuberculous granuloma in lung parenchyma (H&E staining, ×40) (Asterisk denotes the area of caseous necrosis, and the black arrow points to a giant cell of Langhans); b) Histopathological findings of numerous tuberculous granulomas in bone marrow of the autopsied individual with clinical diagnosis of thrombocytopenia and rectorrhage (H&E staining, ×100).”
Statistical analysis was performed with the software package SPSS v.15.0 (SPSS Inc. Chicago, IL). Differences were considered significant if the P value was less than .05. Chi square or the t test were applied for comparisons.
RESULTS
The number of autopsies in the reported 7-year-period was 6206, of which 35 (0.56%) were of individuals with clinically unrecognized miliary TB. Of these, 16 autopsied individuals (45.7%) had miliary pulmonary TB, while 19 individuals (54.3%) had miliary disseminated TB. Mean age of the individuals expressing miliary TB was 62.2 (17.2) years. Females predominated (n=21, 60%) over males (n=14, 40%). Mean age (standard deviation) was 56.5 (16.8) years in men and 66.1 (16.9) in women (P=.11). Fewer autopsied persons with clinically unrecognized miliary TB were sent from specialized pulmonary clinics (n=12, 34.29%) in comparison with deceased patients (n=23, 65.71%) sent from general hospitals (P=.06). The mean age of individuals with unrecognized miliary TB who died in specialized pulmonary clinics was 66.2 (4.1) years, while the mean age of those who died in general hospitals was 60.6 (3.9) (P=.46).
Correlation of autopsy findings with the data from autopsy request forms filled out by clinicians revealed a number of diseases for which TB was mistaken (i.e., diagnoses that “masked” the actual TB). Most common clinical diagnoses that had been reported in the autopsy request forms for the individuals in whom miliary pulmonary TB was discovered in autopsy material were chronic obstructive pulmonary disease in exacerbation (25%) and pulmonary thromboembolism (25%) (Table 1a, b, c).
Table 1a.
Clinical diagnoses (presumed causes of death) as reported in autopsy request forms, in individuals where autopsy showed miliary pulmonary tuberculosis: sex distribution.
| Clinical diagnoses | N | Percentage (%) | ||
|---|---|---|---|---|
| Male | Female | Total | ||
|
| ||||
| Chronic obstructive pulmonary disease in exacerbation | 1 | 3 | 4 | 25 |
| Pulmonary thromboembolism | 1 | 3 | 4 | 25 |
| Pleural effusion | 3 | 0 | 3 | 18.75 |
| Bronchopneumonia | 0 | 2 | 2 | 12.5 |
| Pulmonary edema | 0 | 2 | 2 | 12.5 |
| Status febrilis | 0 | 1 | 1 | 6.25 |
| Total | 5 | 11 | 16 | 100 |
Table 1b.
Clinical diagnoses (presumed causes of death) as reported in autopsy request forms, in the individuals where autopsy showed miliary pulmonary tuberculosis: age distribution.
| Clinical diagnoses | Age (years) | Total | |||
|---|---|---|---|---|---|
| <40 | 40–59 | 60–79 | ≥80 | ||
|
| |||||
| Chronic obstructive pulmonary disease in exacerbation | 0 | 0 | 2 | 2 | 4 |
| Pulmonary thromboembolism | 0 | 1 | 3 | 0 | 4 |
| Pleural effusion | 0 | 0 | 2 | 1 | 3 |
| Bronchopneumonia | 0 | 0 | 1 | 1 | 2 |
| Pulmonary edema | 0 | 1 | 0 | 1 | 2 |
| Status febrilis | 0 | 0 | 0 | 1 | 1 |
Table 1c.
Clinical diagnoses (presumed causes of death) as reported in autopsy request forms, in the individuals where autopsy showed miliary pulmonary tuberculosis: distribution by year.
| Clinical diagnoses | Year | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | ||
|
| ||||||||
| Chronic obstructive pulmonary disease in exacerbation | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 4 |
| Pulmonary thromboembolism | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 4 |
| Pleural effusion | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 3 |
| Bronchopneumonia | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| Pulmonary edema | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Status febrilis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
Most frequent clinical diagnoses reported in the autopsy request forms for the individuals with miliary disseminated TB revealed on autopsy were sepsis (10.52%), adult respiratory distress syndrome (ARDS) (10.52%), gastrointestinal bleeding (10.52%) and meningoencephalitis (10.52%) (Table 2a, b, c).
Table 2a.
Clinical diagnoses (presumed causes of death) as reported in autopsy request forms, in the individuals where autopsy showed miliary disseminated tuberculosis: sex distribution.
| Clinical diagnoses | N | Percentage (%) | ||
|---|---|---|---|---|
| Male | Female | Total | ||
|
| ||||
| Sepsis | 1 | 1 | 2 | 10.52 |
| ARDS | 1 | 1 | 2 | 10.52 |
| Gastrointestinal bleeding | 1 | 1 | 2 | 10.52 |
| Meningoencephalitis | 1 | 1 | 2 | 10.52 |
| Suspected metastatic deposits in lung parenchyma | 1 | 0 | 1 | 5.26 |
| Suspected metastatic deposits in liver | 0 | 1 | 1 | 5.26 |
| Bronchial hemorrhage with suffocation | 0 | 1 | 1 | 5.26 |
| Status febrilis | 0 | 1 | 1 | 5.26 |
| Crohn’s disease | 0 | 1 | 1 | 5.26 |
| Ileus | 0 | 1 | 1 | 5.26 |
| Syndroma Moszkowicz | 0 | 1 | 1 | 5.26 |
| Hydrocephalus | 1 | 0 | 1 | 5.26 |
| Respiratory failure | 2 | 1 | 3 | 15.84 |
| Total | 8 | 11 | 19 | 100 |
Table 2b.
Clinical diagnoses (presumed causes of death) as reported in autopsy request forms, in the individuals where autopsy showed miliary disseminated tuberculosis: age distribution
| Clinical diagnoses | Age (years) | Total | |||
|---|---|---|---|---|---|
| <40 | 40–59 | 60–79 | ≥80 | ||
|
| |||||
| Sepsis | 0 | 1 | 1 | 0 | 2 |
| ARDS | 1 | 1 | 0 | 0 | 2 |
| Gastrointestinal bleeding | 0 | 0 | 1 | 1 | 2 |
| Meningoencephalitis | 0 | 1 | 1 | 0 | 2 |
| Suspected metastatic deposits in lung parenchyma | 0 | 0 | 1 | 0 | 1 |
| Suspected metastatic deposits in liver | 0 | 0 | 1 | 0 | 1 |
| Bronchial hemorrhage with suffocation | 0 | 0 | 1 | 0 | 1 |
| Status febrilis | 0 | 0 | 1 | 0 | 1 |
| Crohn’s disease | 0 | 1 | 0 | 0 | 1 |
| Ileus | 0 | 0 | 1 | 0 | 1 |
| Syndroma Moszkowicz | 1 | 0 | 0 | 0 | 1 |
| Hydrocephalus | 1 | 0 | 0 | 0 | 1 |
| Respiratory failure | 0 | 0 | 3 | 0 | 3 |
Table 2c.
Clinical diagnoses (presumed causes of death) as reported in autopsy request forms, in the individuals where autopsy showed miliary disseminated tuberculosis: distribution by year.
| Clinical diagnoses | Year | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | ||
|
| ||||||||
| Sepsis | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| ARDS | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Gastrointestinal bleeding | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
| Meningoencephalitis | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| Suspected metastatic deposits in lung parenchyma | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Suspected metastatic deposits in liver | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Bronchial hemorrhage with suffocation | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Status febrilis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Crohn’s disease | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Ileus | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Syndroma Moszkowicz | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hydrocephalus | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Respiratory failure | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 3 |
Based on the information from autopsy request forms, chest X-ray showed diffuse pulmonary infiltrates in 56.3% of individuals with miliary pulmonary TB and 21.1% of those with miliary disseminated TB. Hematological abnormalities (secondary anemia, thrombocytopenia) dominated in three cases of miliary disseminated TB (15.8%). Moreover, three patients (15.8%) with postmortem diagnosed miliary disseminated TB had been subjected to recent surgery for kidney transplantation, hysterectomy with adnexectomy, and intestinal subocclusion.
Table 3 shows actual causes of death in all individuals with clinically unrecognized miliary TB according to sex, age and year of the study (Table 3a, b, c). The autopsied individuals with miliary TB mostly died of miliary pulmonary TB (n=9, 25.7%), miliary disseminated TB (n=8, 22.9%), acute myocardial infarction (n=4, 11.4%) and bronchopneumonia (n=4, 11.4%) (Table 3a, b, c). When separately analyzing the individuals with miliary pulmonary TB, it was obvious that they mostly died of miliary pulmonary TB (n=9, 56.25%), followed by myocardial infarction (n=4, 25%), pseudomembranous colitis (n=2, 12.5%) and pulmonary thromboembolism (n=1, 6.25%). The autopsied individuals with pathological findings of miliary disseminated TB mostly died of miliary TB (n=8, 42.11%), followed by bronchopneumonia (n=4, 21.05%), ARDS (n=3, 15.79%), tuberculous meningoencephalitis (n=3, 15.79%) and pulmonary thromboembolism (n=1, 5.26%).
Table 3a.
Causes of death as determined during autopsy: sex distribution.
| Causes of death | N | Percentage (%) | ||
|---|---|---|---|---|
| Males | Females | Total | ||
|
| ||||
| Miliary pulmonary tuberculosis | 4 | 5 | 9 | 25.7 |
| Miliary disseminated tuberculosis | 1 | 7 | 8 | 22.9 |
| Adult respiratory distress syndrome (ARDS) | 2 | 1 | 3 | 8.6 |
| Bronchopneumonia | 2 | 2 | 4 | 11.4 |
| Pseudomembranous colitis | 1 | 1 | 2 | 5.7 |
| Tuberculous meningoencephalitis | 3 | 0 | 3 | 8.6 |
| Acute myocardial infarction | 1 | 3 | 4 | 11.4 |
| Pulmonary thromboembolism | 0 | 2 | 2 | 5.7 |
| Total | 14 | 21 | 35 | 100 |
Table 3b.
Causes of death as determined during autopsy: age distribution.
| Cause of death | Age (years) | Total | |||
|---|---|---|---|---|---|
| <40 | 40–59 | 60–79 | ≥80 | ||
|
| |||||
| Miliary pulmonary tuberculosis | 0 | 3 | 3 | 3 | 9 |
| Miliary disseminated tuberculosis | 0 | 1 | 6 | 1 | 8 |
| Adult respiratory distress syndrome (ARDS) | 1 | 0 | 2 | 0 | 3 |
| Bronchopneumonia | 2 | 1 | 0 | 1 | 4 |
| Pseudomembranous colitis | 0 | 0 | 2 | 0 | 2 |
| Tuberculous meningoencephalitis | 1 | 1 | 1 | 0 | 3 |
| Acute myocardial infarction | 1 | 1 | 1 | 1 | 4 |
| Pulmonary thromboembolism | 0 | 0 | 1 | 1 | 2 |
Table 3c.
Causes of death as determined during autopsy: distribution by year.
| Causes of death | Year | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | ||
|
| ||||||||
| Miliary pulmonary tuberculosis | 1 | 2 | 2 | 1 | 2 | 1 | 0 | 9 |
| Miliary disseminated tuberculosis | 0 | 0 | 2 | 3 | 2 | 1 | 0 | 8 |
| Adult respiratory distress syndrome | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 3 |
| Bronchopneumonia | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 4 |
| Pseudomembranous colitis | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| Tuberculous meningoencephalitis | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 3 |
| Acute myocardial infarction | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 4 |
| Pulmonary thromboembolism | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
Extrapulmonary granulomas in miliary disseminated TB were most commonly found in the liver (68.4%), the spleen (63.2%) and kidneys (42.1%) (Table 4a, b, c; Figure 1b). In all cases of miliary pulmonary and miliary disseminated TB, the lungs were affected by disseminated granulomas. The most frequent comorbidities in patients with miliary pulmonary TB and miliary disseminated TB were hypertensive heart disease (17.1%), chronic renal failure (11.4%), malignancies (11.4%), diabetes mellitus type 2 (8.6%), alcoholism (2.9%) and liver cirrhosis (2.9%).
Table 4a.
Distribution of TB granulomas in patients with disseminated miliary TB, according to sex.
| Organ | N | Percentage* (%) | ||
|---|---|---|---|---|
| Males | Females | Total | ||
|
| ||||
| Lungs | 8 | 11 | 19 | 100 |
| Liver | 4 | 9 | 13 | 68.42 |
| Spleen | 4 | 8 | 12 | 63.15 |
| Kidney | 4 | 4 | 8 | 42.10 |
| Adrenal glands | 2 | 2 | 4 | 21.05 |
| Bone marrow | 2 | 2 | 4 | 21.05 |
| Brain | 3 | 0 | 3 | 15.78 |
| Small and large intestines | 1 | 3 | 4 | 21.05 |
| Prostate | 1 | 0 | 1 | 5.26 |
| Urinary bladder | 0 | 1 | 1 | 5.26 |
| Ovary | 0 | 1 | 1 | 5.26 |
| Fallopian tube | 0 | 1 | 1 | 5.26 |
| Uterus | 0 | 1 | 1 | 5.26 |
Percentage of individuals showing TB granuloma in a particular organ.
Table 4b.
Distribution of TB granulomas in patients with miliary disseminated TB, according to age.
| Organ | Age (years) | Total | |||
|---|---|---|---|---|---|
| <40 | 40–59 | 60–79 | ≥80 | ||
|
| |||||
| Lungs | 4 | 3 | 10 | 2 | 19 |
| Liver | 3 | 2 | 7 | 1 | 13 |
| Spleen | 1 | 3 | 7 | 1 | 12 |
| Kidney | 1 | 2 | 5 | 0 | 8 |
| Adrenal glands | 1 | 1 | 2 | 0 | 4 |
| Bone marrow | 0 | 0 | 4 | 0 | 4 |
| Brain | 1 | 1 | 1 | 0 | 3 |
| Small and large intestines | 2 | 1 | 0 | 1 | 4 |
| Prostate | 0 | 0 | 1 | 0 | 1 |
| Urinary bladder | 0 | 0 | 1 | 0 | 1 |
| Ovary | 1 | 0 | 0 | 0 | 1 |
| Fallopian tube | 1 | 0 | 0 | 0 | 1 |
| Uterus | 1 | 0 | 0 | 0 | 1 |
DISCUSSION
In this 7-year study, 0.56% of all autopsies revealed signs of miliary TB that were not recognized clinically. Different autopsy studies have reported that miliary TB has accounted for from 0.3% to 13.3% of all autopsies, while miliary TB represented 11.9% to 40.5% of all cases with TB.15 We found a female predominance in clinically unrecognized TB, which is in agreement with Vasankari et al.16 A mean age above 60 years corresponds to the findings from the study of clinically unrecognized miliary TB by Rosenthal et al.17 Immunocompromised and older patients are most commonly affected.18
Our findings revealed that the most common clinical diagnoses for which miliary pulmonary TB was mistaken were exacerbation of chronic obstructive pulmonary disease and pulmonary thromboembolism, whereas miliary generalized TB clinically presented mostly as sepsis, ARDS, gastrointestinal bleeding and meningoencephalitis. They also showed hematological disturbances and were associated with different postoperative conditions. Both types of miliary TB on chest radiographs most frequently showed diffuse bilateral infiltrates of unclear etiology.
Miliary TB usually has an atypical clinical presentation, which poses a significant diagnostic challenge even for experienced clinicians. Indeed, our study demonstrated that even in specialized pulmonary hospitals miliary TB may be unrecognized; still, there was a notably higher proportion of clinically unrecognized TB in general hospitals. This finding may be due to the common belief that TB incidence is drastically declining, so clinicians do not consider it in the differential diagnosis. In particular, miliary TB is usually overlooked in countries with a low incidence of TB,19 and a lack of experience with this disease may be the reason for inadequate diagnosis.20 Moreover, miliary TB may remain undiagnosed as the symptoms may be masked with previously administrated antibiotics,21 or due to atypical X-ray findings22 and an increased prevalence of extrapulmonary TB.23 However, clinicians should pay more attention to this condition, considering that individuals with unrecognized TB pose a serious risk for other patients, physicians and pathologists.
This study showed the liver as the most common site of TB dissemination following the lungs in the case of miliary generalized TB. According to published data, clinical presentation of disseminated TB in the liver may be accompanied by jaundice and fever, while disseminated intravascular coagulation and multiorgan dysfunction syndrome lead to a lethal outcome in these patients.24 The spleen was the second most frequent localization of TB granulomas in our study, although published data suggests they are quite rare, especially in immunocompetent persons. They can be associated with hematological disturbances,25 as shown in our study.
TB of the kidneys and urinary tract is easily overlooked given that symptoms may resemble bacterial cystitis; however, if the usual antibiotics do not help, miliary disseminated TB should be considered. High temperature, lumbar pain, hematuria and chest X-ray findings are not specific enough,26 but could suggest TB.
Patients who suffer from TB often have comorbidities. For instance, patients with chronic renal failure have a 10 to 15 times higher risk of TB, predominantly due to deficiencies in the cellular immune system.27 These patients rarely show fever, while laboratory findings indicate uremia with lymphopenia.27 Uremia may be the cause of delayed hypersensitivity, decreased proliferation of Th cells and reduced phagocytic capacity of macrophages. Extrapulmonary TB is about 50% more frequent in the dialysis patients compared to general population.27
Bukhary et al previously showed that diabetes mellitus type 2 is a risk factor of TB development. Namely, patients with diabetes mellitus and TB had significantly more bacteria in sputum, positive bacterial cultures even after administration of adequate therapy, as well as more frequent resistance to anti-TB antibiotics.28
Resemblance in clinical and radiological presentation of TB and malignant tumors may lead to frequent erroneous diagnosis of these conditions. However, TB and malignancies can even coexist, as it was shown in our study. TB may be associated with an increased risk of various cancers, due to chronic inflammation and possible common risk factors, including immunosuppression and smoking.29
In conclusion, miliary TB has an atypical clinical presentation. In the case of etiologically unclear diffuse pulmonary infiltrates on chest X-ray, particularly if the patients have chronic renal failure, malignancy, hematological abnormalities (anemia, thrombocytopenia) or a history of recent surgical intervention, miliary TB should be considered. Better understanding of clinical presentation of miliary TB as well as common misdiagnoses in clinical contexts is essential for improving disease control and elimination of TB in countries facing an increased TB burden due to migrations, as well as for disease control at a global level.
REFERENCES
- 1.World Health Organization. Global tuberculosis report 2014. Geneva, Switzerland: World Health Organization; 2014. p. 17. [Google Scholar]
- 2.Al-Orainey I, Alhedaithy MA, Alanazi AR, Barry MA, Almajid FM. Tuberculosis incidence trends in Saudi Arabia over 20 years: 1991–2010. Ann Thorac Med. 2013;8:148–152. doi: 10.4103/1817-1737.114303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zdravstveno statistički godišnjak Republike Srbije, Institut za javno zdravlje “Dr Milan Jovanović Batut”. 2012. [accessed on July 30th 2015]. http://www.batut.org.rs/download/publikacije/pub2012.pdf.
- 4.Enarson DA, Seita A, Fujiwara P. Global elimination of tuberculosis: implementation, innovation, investigation. Int J Tuberc Lung Dis. 2003;7(S3):S328–S332. [PubMed] [Google Scholar]
- 5.Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res. 2012;135(5):703–730. [PMC free article] [PubMed] [Google Scholar]
- 6.Manget JJ. Sepulchretumsive anatomica practica. Vol. 1. London: Cramer and Perachon; 1700. Observatio XLVII (3 vols) [Google Scholar]
- 7.Sydow M, Schauer A, Crozier TA, Burchardi H. Multiple organ failure in generalized disseminated tuberculosis. Respir Med. 1992;86(6):517–519. doi: 10.1016/s0954-6111(96)80014-x. [DOI] [PubMed] [Google Scholar]
- 8.Ahuja SS, Ahuja SK, Phelps KR, Thelmo W, Hill AR. Hemodynamic confirmation of septic shock in disseminated tuberculosis. Crit Care Med. 1992;20(6):901–903. doi: 10.1097/00003246-199206000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Piqueras AR, Marruecos L, Artigas A, Rodriguez C. Miliary tuberculosis and adult respiratory distress syndrome. Intensive Care Med. 1987;13(3):175–182. doi: 10.1007/BF00254701. [DOI] [PubMed] [Google Scholar]
- 10.Mohan A, Sharma SK, Pande JN. Acute respiratory distress syndrome (ARDS) in miliary tuberculosis: a twelve year experience. Indian J Chest Dis Allied Sci. 1996;38(3):157–162. [PubMed] [Google Scholar]
- 11.Proudfoot AT, Akhtar AJ, Douglas AC, Horne NW. Miliary tuberculosis in adults. Br Med J. 1969;2(5652):273–276. doi: 10.1136/bmj.2.5652.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JH, Langston AA, Gallis HA. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis. 1990;12(4):583–590. doi: 10.1093/clinids/12.4.583. [DOI] [PubMed] [Google Scholar]
- 13.Asada Y, Hayashi T, Sumiyoshi A, Aburaya M, Shishime E. Miliary tuberculosis presenting as fever and jaundice with hepatic failure. Hum Pathol. 1991;22(1):92–94. doi: 10.1016/0046-8177(91)90068-z. [DOI] [PubMed] [Google Scholar]
- 14.Ashba JK, Boyce JM. Undiagnosed tuberculosis in a general hospital. Chest. 1972;61:447–451. doi: 10.1378/chest.61.5.447. [DOI] [PubMed] [Google Scholar]
- 15.Jagirdar J, Zagzag D. Pathology and insights into pathogenesis of tuberculosis. In: Rom WN, Garay SM, editors. Tuberculosis. Philadelphia, USA: Lippincott Williams & Wilkins; 2004. pp. 323–344. [Google Scholar]
- 16.Vasankari T, Liippo K, Tala E. Overt and cryptic miliary tuberculosis misdiagnosed until autopsy. Scand J Infect Dis. 2003;35(11–12):794–796. doi: 10.1080/00365540310016961. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal T, Pitlik S, Michaeli D. Fatal undiagnosed tuberculosis in hospitalized patients. J Infect Dis. 1975;131(Suppl):S51–S56. doi: 10.1093/infdis/131.supplement.s51. [DOI] [PubMed] [Google Scholar]
- 18.Teale C, Goldman JM, Pearson SB. The association of age with the presentation and outcome of tuberculosis: a five year survey. Age Ageing. 1993;22:289–293. doi: 10.1093/ageing/22.4.289. [DOI] [PubMed] [Google Scholar]
- 19.Naalsund A, Heldal E, Johansen B, et al. Deaths from pulmonary tuberculosis in a low-incidence country. J Intern Med. 1994;236:137–142. doi: 10.1111/j.1365-2796.1994.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldman KP. Tuberculosis in hospital doctors. Tubercle. 1988;69:237–240. doi: 10.1016/0041-3879(88)90046-3. [DOI] [PubMed] [Google Scholar]
- 21.Slavin RE, Walsh TJ, Pollack AD. Late generalised tuberculosis: a clinical pathologic analysis and comparison of 100 cases in the preantibiotic and antibiotic eras. Medicine (Baltimore) 1980;59:352–366. [PubMed] [Google Scholar]
- 22.Selby C, Thomson D, Leitch AG. Death in notified cases of tuberculosis in Edinburgh: 1983–1992. Respir Med. 1995;89:369–371. doi: 10.1016/0954-6111(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee JK, Ng TH. Undiagnosed tuberculosis in hospitalised patients—an autopsy survey. J R Soc Health. 1990;110:141–143. doi: 10.1177/146642409011000411. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SK, Shamim SQ, Bannerjee CK, Sharma BK. Disseminated tuberculosis presenting as massive hepatosplenomegaly and hepatic failure. Am J Gastroenterol. 1981;76(2):153–156. [PubMed] [Google Scholar]
- 25.Gupta PP, Fotedar S, Agarwal D, Sansanwal P. Tuberculosis of spleen presenting with pyrexia of unknown origin in a non-immunocompromised woman. Lung India. 2008;25(1):22–24. doi: 10.4103/0970-2113.44134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eastwood JB, Corbishley MC, Grange J. Tuberculosis and the Kidney. J Am Soc Nephrol. 2001;12(6):1307–1314. doi: 10.1681/ASN.V1261307. [DOI] [PubMed] [Google Scholar]
- 27.Mimi N, Medregoniu D, Olteanu M, et al. Tuberculosis and Chronic Renal Failure. Curr Health Sci J. 2011;37(2):106–108. [Google Scholar]
- 28.Bukhary ZA. Rediscovering the Association between Tuberculosis and Diabetes Mellitus: A Perspective. J Taibah Uni Med Sci. 2014;(9):257–344. [Google Scholar]
- 29.Simonsen DF, Farkas DK, Søgaard M, Horsburgh CR, Sørensen HT, Thomsen RW. Tuberculosis and risk of cancer: a Danish nationwide cohort study. Int J Tuberc Lung Dis. 2014;18(10):1211–1219. doi: 10.5588/ijtld.14.0161. [DOI] [PubMed] [Google Scholar]