Abstract
BACKGROUND
Congenital glaucoma appears in the first months of life, eventually at birth. Isolated congenital glaucoma is characterized by minor malformations of the irido-corneal angle of the anterior chamber of the eye. Clinical manifestations include tearing, photophobia and enlargement of the globe appearing in the first months of life. Imaging technology such as optical coherence tomography and measurement of central corneal thickness may play an important role in the assessment of children with suspected or known glaucoma. However, no MRI findings of the CNS in patients with primary congenital glaucoma (PCG) were reported in the literature. The purpose of this study was to investigate MRI findings of the brain in infants with PCG.
METHODS
We reviewed the radiological, histopathological and clinical characteristics of infants with primary congenital glaucoma. The records of 17 patients with PCG were reviewed and the MRIs of the brain and associated manifestations were analyzed.
RESULTS
Three patients with PCG had abnormal MRI findings suggesting agenesis of the corpus callosum. Two infants had delayed myelinization of the brain.
DISCUSSION
Significant abnormal optic nerve excavation and increased corneal diameters in 2 patients with delayed myelinization may suggest that intraocular pressure can be more striking and more severe, revealing a close relationship with PCG and abnormal myelinization in the white matter. Studies with more patients are needed to confirm these results.
Congenital glaucoma appears in the first months of life, sometimes at birth. Isolated congenital glaucoma is characterized by minor malformations of the irido-corneal angle of the anterior chamber of the eye. The cause of congenital glaucoma is the presence of an obstacle to aqueous humor outflow and the treatment is primarily surgical. Congenital glaucoma occurs in 1 of 10 000 births in Westem countries and the frequency is higher in some countries (especially in the Middle East). Heredity is autosomal recessive, and the genes involved are CYP1B1, GLC3A and GLC3B.1
Clinical manifestations include tearing, photophobia and enlargement of the globe, which appear in the first months of life. Primary congenital glaucoma (PCG) is characterized by elevated intraocular pressure (IOP), enlargement of the globe (buphthalmus), edema, and opacification of the cornea with rupture of Descemet’s membrane, thinning of the anterior sclera and atrophy of the iris, an anomalously deep anterior chamber, structurally normal posterior segment except for progressive optic atrophy, and photophobia, blepharospasm, and excessive tearing.2 Typically, the diagnosis is made in the first year of life. Depending on when treatment is instituted, visual acuity may be reduced and/or visual fields may be restricted. In untreated cases, blindness invariably occurs.3
The treatment of congenital glaucoma is primarily surgical and appears as an emergency, since the corneal opacity can dramatically increase in a few days or hours. Interventions aim to facilitate the aqueous humor outflow in the irido-corneal angle towards the Schlemm’s canal and the subconjunctival space. Goniotomy consists of introducing a needle in the anterior chamber to open the opposite side of the angle. Trabeculotomy creates a communication between Schlemm’s canal and the anterior chamber. Trabeculectomy consists of opening the anterior chamber, under a scleral flap, to produce an aqueous humor outflow towards the subconjunctival space.4
Imaging technology such as optical coherence tomography and measurement of central corneal thickness may play an important role in the assessment of children with suspected or known glaucoma.5 However, no MRI findings of the CNS in patients with PCG were reported in the literature. Based on this information, we decided to evaluate our experiences in a group of infants with PCG in a multicenter study. In the present study, we retrospectively reviewed MRI findings of the brain in the infants with primary congenital glaucoma. We focused on the signs and symptoms of clinical manifestations in patients with PCG who had abnormal MRI findings. To the best of our knowledge, this is the first patient series reporting MRI findings of the brain in patients with PCG.
METHODS
The authors reviewed the medical records of 17 infants with PCG seen between 2003 to 2006. We identified a cohort of infants who were diagnosed with PCG in the fırst month of life and presented to the pediatric neurology and/or opthalmology clinic within the fırst 6 months of life. We reviewed the clinical records over a 3-year span for all infants who were diagnosed with glaucoma in the Pediatric Neurology Clinic in the Department of Pediatrics, School of Medicine, Gaziantep University, Gaziantep, Turkey and the Pediatric Department of the Eye Clinic in Bielefeld, Germany.
Complete ophthalmic examination was done, including assessment of the intraocular pressure, gonioscopic examination of the filtration angle and examination of the cornea under magnification. Traditional comprehensive evaluations were conducted under anesthesia, and some patients were also safely sedated with chlorohydrate without influencing the IOP. We usually obtained pressure readings with the Perkins applanation tonometers (Keeler, London, UK), the Tono Pen, and the Schiotz tonometers. After a complete neurological evaluation, brain images were requested for all patients.
Seventeen infants diagnosed with PCG had been ascertained and enrolled in the present study. The medical history of all participants was taken in the form of a questionnaire. A comprehensive eye examination by an experienced ophthalmologist, and/or a complete neurological examination by pediatric neurologist were done. Glaucoma was diagnosed and classified as primary infantile, aphakic, syndrome-related, and secondary glaucoma. Patients with IOP associated with other ocular or systemic anomalies such as megalocornea, high myopia, congenital idiopathic edema of the cornea, corneal dystrophic conditions, and corneal opacification secondary to corneal dystrophies or abnormalities of metabolism were excluded from the study.
A diagnosis of glaucoma was established by having at least 2 of the following criteria: 1) repeated IOP measurement over 21 mm Hg, 2) cupping of the optic nerve consistent with glaucoma, 3) increasing corneal diameter, and 4) an excessive myopic shift in the refractive error. The IOP was considered to be in “good control” if it was 19 mm Hg or less and was considered to be in “poor control” if it was 20 mm Hg or more.
Patients with glaucoma were excluded if they had 1) a history of cataract surgery, 2) various conditions that might predipose to development of glaucoma such as rubella, anterior segment dysgenesis or persistent hyperplastic primary vitreous, 3) a syndrome associated with glaucoma, or 4) glaucoma related to systemic problems.
RESULTS
Five of the 17 patients with PCG had abnormal MRI findings. We identified 3 infants with agenesis of the corpus callosum and 2 infants with delayed myelinization.
DISCUSSION
Primary congenital glaucoma is present at birth, but its manifestations may not be recognized until infancy or early childhood. It is characterized by improper development of the eye’s aqueous outflow system, leading to increased intraocular pressure, with consequent damage to ocular structures, resulting in loss of vision. Although the disease is relatively rare, the impact on visual development can be extreme.6 Early recognition and appropriate therapy of glaucoma can significantly improve the child’s visual future. The classic triad of manifestations, any one of which should arouse suspicion of glaucoma in an infant or young child, includes epiphora, photophobia, and blepharospasm.7
The purpose of this study was to investigate MRI findings of the brain in infants with PCG. We reviewed the radiological, histopathological and clinical characteristics of infants with primary congenital glaucoma. The records of 17 patients with PCG were reviewed and MRIs of the brain and associated manifestations were analyzed. Three of them with PCG had abnormal MRI findings suggesting agenesis of corpus callosum. Two infants had delayed myelinization of the brain.
Three infants with agenesis of the corpus callosum had PCG and agenesis of the corpus callosum, and significant decreased vacuoles adjacent to the wall of the Schlemm’s canal. This may be one of the primary causes of increased IOP.8 One of these patients had abnormal pupillary enlargement, which was easily detected, and suggested aniridic pupils on initial examination. One infant with a corneal haze for a week had resolution of corneal cloudiness.
MRI findings in 2 patients showed delayed myelinization. In these 2 infants with PCG, corneal diameters were large (11.5 and 12 cm, respectively). When glaucoma is diagnosed in an infant, the optic nerve head is usually abnormal. Variable cupping is present, usually annular in form, with nasalization of vessels and preservation of the well-vascularized rim. It is believed that increased intraocular pressure affects the optic nerve head much more rapidly in the pediatric population.9,10 In this study, optic nerve excavation is even more significant in an infant with delayed myelinization of the brain.
The significant abnormal cup/disc ratio (optic nerve excavation) and increased corneal diameters in these 2 patients with delayed myelinization may suggest that intraocular pressure can be more striking and more severe, revealing a close relationship between PCG and abnormal myelinization in the white matter.
An abnormal MRI finding, such as delayed myelinization of the white matter, may also contribute important clinical findings in PCG, including the degree of intraocular pressure, which can determine the prognosis of the infant’s vision. We believe there appears to be a relationship between glacuoma and delayed myelinization, although this study had few patients and the findings are not statistically significant due to the limited number of patients. Nevertheless, in the medical literature, this is the first retrospective chart review to report the presence of abnormal MRI findings in infants with PCG. Further studies with larger numbers of patients are needed to confirm these results.
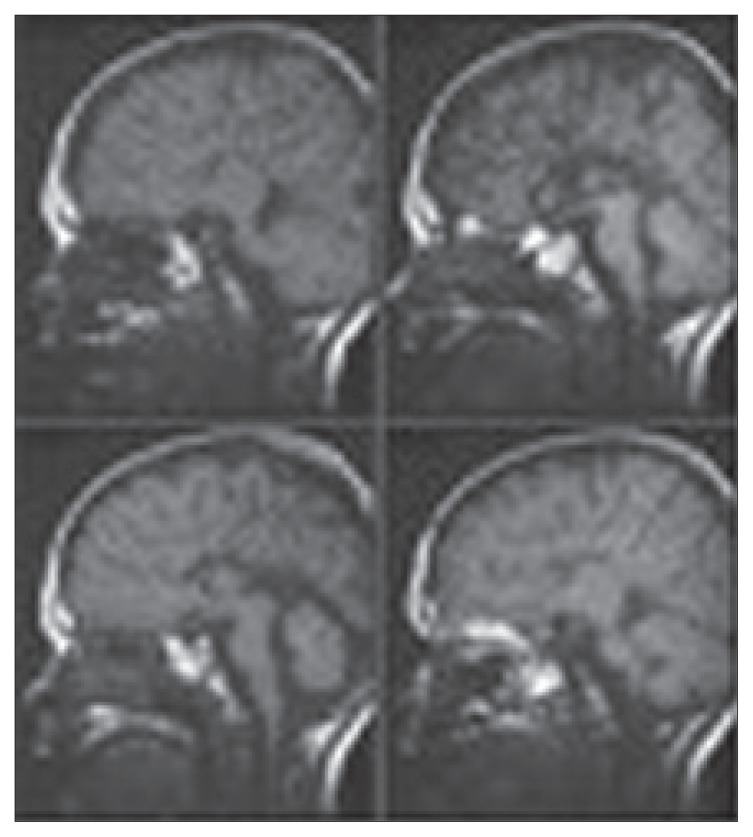
Figure 1.
Midline saggittal T-1 weighted MR images showing the medial hemispheric sulci coursing all the way in a radial fashion. The trigones, occipital horns and temporal horns of the lateral ventricles are variably dilated as a result of the agenesis of the corpus callosum.
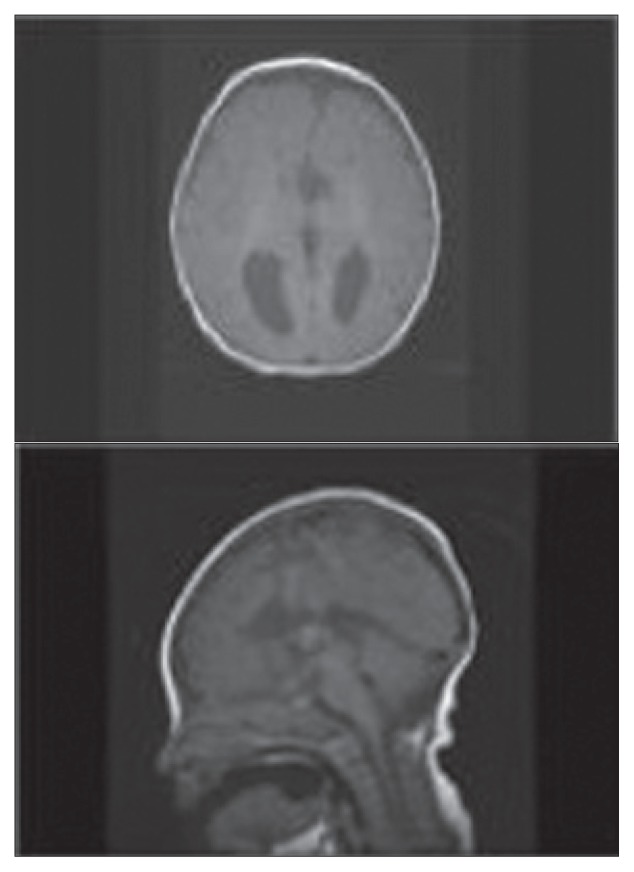
Figure 2.
Axial T-1 MRI demonstrating parallel prominent lateral ventricles, which are a typical finding for agenesis of the corpus callosum. The midline saggital T-1 weighted images show that the frontal horns are vertically oriented. The third ventricle is continuous with the interhemispheric fissure and extends somewhat higher than normal between the lateral ventricle.
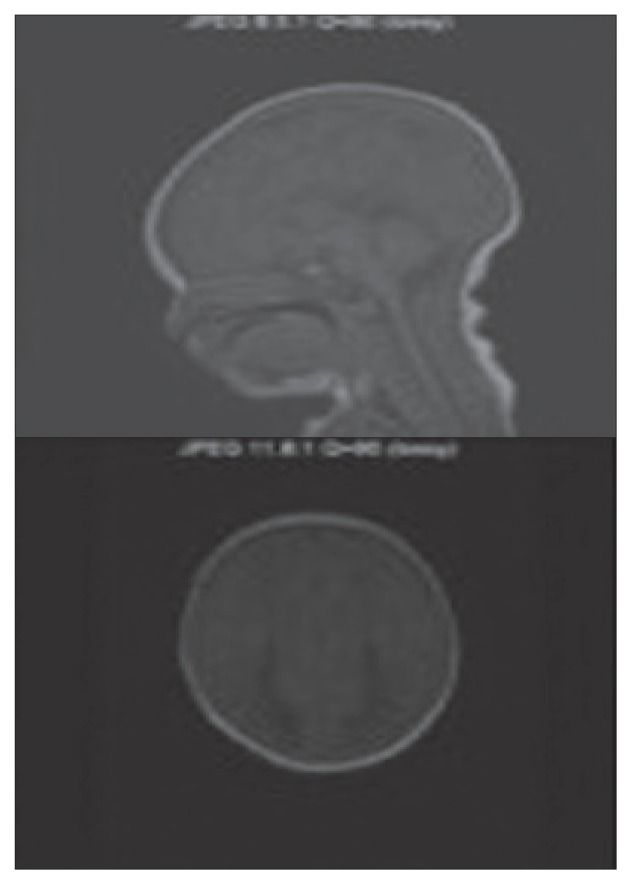
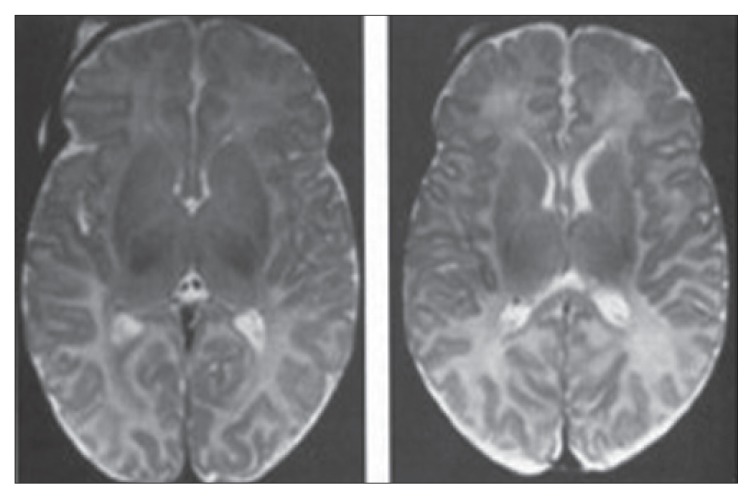
Figure 3.
The midline saggittal T-1 weighted MR image is quite characteristic in patients with agenesis of the corpus callosum. Axial T-1 demonstrates enlarged posterior horns and lateral ventricles in parallel alignment. The posterior horns have a characteristic shape that has been described as being similar to the wings of a bird. The third ventricle is continuous with the interhemisperic fissure both superiorly and anteriorly. The lateral ventricles tend to be parallel instead of showing their normal lateral position
Figure 4.
T-2 weighted MRI of 6 month old infant shows rostral progression of the maturation of the internal capsule. The anterior limbs of the anterior capsule are not well myelinated. Isointensity of the cortical gray matter and subcortical white matter, resulting in difficulty in the identification of structural abnormalities at this age suggest delayed myelinization of the white matter.
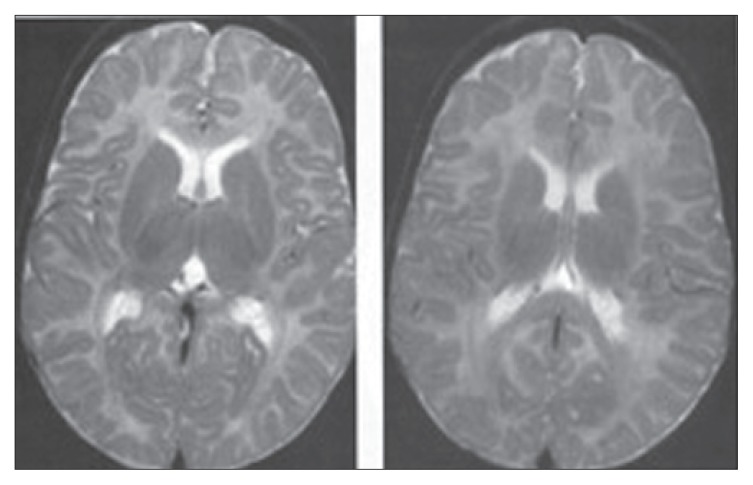
Figure 5.
Axial T-2 weighted MRI of a 3-month-old infant showing low signal intensity in the posterior aspect of the posterior limb of the internal capsule, the ventrolateral thalamus and the perirolandic gyri of the cortex. Some low signal peripheral to the frontal horns of the lateral ventricle represent residual germinal matrix. The T-2 weighted images correspond more closely to the MRI of a 1-month-old infant.
REFERENCES
- 1.Ho CL, Walton DS. Primary congenital glaucoma: 2004 update. J Pediatr Ophthalmol Strabısmus. 2004;41:271–88. doi: 10.3928/01913913-20040901-11. [DOI] [PubMed] [Google Scholar]
- 2.Mandal AK, Gothwal VK, Bagga H, Nutheti R. Outcome of Surgery on Infants Younger than 1 Month with Congenital Glaucoma. Ophthalmology. 2003;110:1909–15. doi: 10.1016/S0161-6420(03)00671-7. [DOI] [PubMed] [Google Scholar]
- 3.Akpek EK, Jun AS, Goodman DF, Green WR, Gottsch JD. Clinical and Ultrastructural Features of a Novel Hereditary Anterior Segment Dysgenesis. Ophthalmology. 2002;109:513–519. doi: 10.1016/s0161-6420(01)00975-7. [DOI] [PubMed] [Google Scholar]
- 4.The AGIS investigators. The advanced glaucoma intervention study (AGIS). The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 5.Heijl MC, Leske B, Bengtsson L, Hyman B, Bengtsson M Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression. Results from the early manifest glaucoma trial, Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The ocular hypertension treatment study. Baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb RN, Friedman DS, Fechner RD, Cioffi GA, Coleman AL, Girkin CA, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458–467. doi: 10.1016/j.ajo.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 8.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltne JL, et al. Ocular Hypertension Treatment Study Group. The ocular hypertension treatment study. A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 9.Lichter PR. Expectation from clinical trials, Results of the early manifest glaucoma trial. Arch Ophthalmol. 2002;120:1371–1372. doi: 10.1001/archopht.120.10.1371. [DOI] [PubMed] [Google Scholar]
- 10.Leske MC, Heijl A, Hussein M, Bengtwwon B, Hyman L, Komaroff E Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment. The early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]