Congenital duodenal obstruction is one of the relatively common surgical conditions in infants and children. Over the years, the prognosis of infants and children with this condition has improved markedly, which is attributed to several factors such as early diagnosis, improved surgical techniques, and improved perioperative management, including availability of total parenteral nutrition. Several factors, however, still affect the overall outcome including pre-maturity, a high incidence of associated anomalies and reoperations.1,2 The high incidence of associated anomalies, mainly severe congenital heart disease, contributes to the increased morbidity and sometimes mortality.3 The overall long-term outcome is also affected by the high incidence of Down’s syndrome.2 This report is an analysis of our experience with 35 cases of intrinsic congenital duodenal obstruction with an emphasis on factors affecting the final outcome.
PATIENTS AND METHODS
Over a period of 12 years from 1993 to 2005, a total of 35 infants and children with intrinsic congenital duodenal obstruction were treated at our hospital. Their medical records were retrospectively reviewed for age at diagnosis, sex, gestation, and birth weight, history of polyhydramnios, symptomatology, associated anomalies, and method of diagnosis, treatment and outcome. Extrinsic causes of duodenal obstruction were excluded.
RESULTS
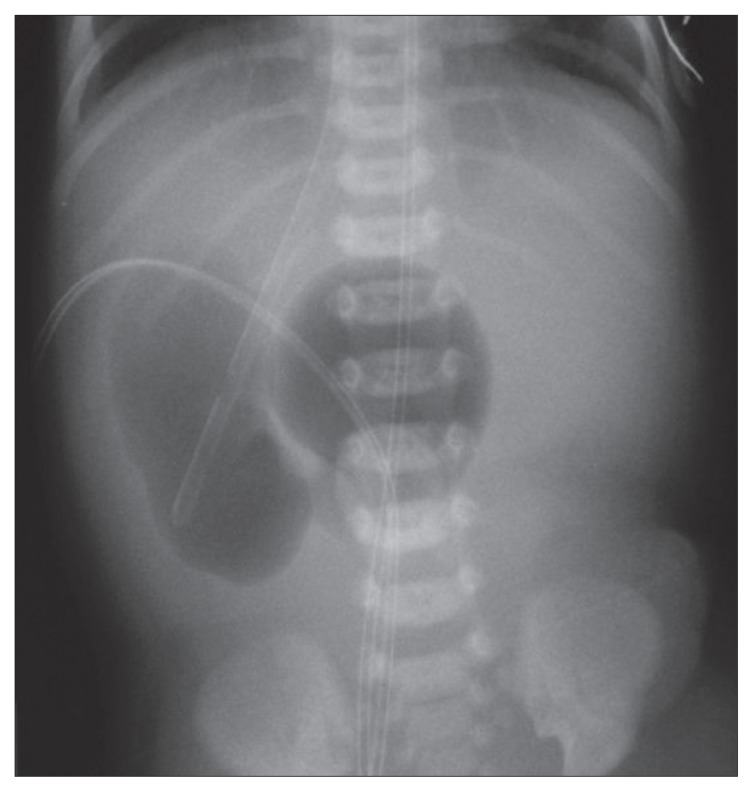
Of the 35 cases of congenital intrinsic duodenal obstruction treated at our hospital, 19 were males and 16 females. Their birth weight ranged from 1.4 kg to 3.8 kg (mean, 2.5 kg). Twelve (34.3%) were premature. The mean maternal age was 23 years (range, 18–34 years). All our patients presented or were referred to our hospital within two weeks of life except for five who presented at 5 months, 3.5 years, 1.8 years, and 1.5 years, and 2 months of age. All five had duodenal diaphragm with a hole, which was the reason for the delayed presentation. Two of them presented with complete duodenal obstruction following the eating of dates with seeds, which resulted in occlusion of the hole in the diaphragm. The remaining patients presented with bile-stained vomiting. Upper abdominal distension was seen in 19 (54.3%) and polyhydramnios was reported in 12 patients (34.3%). Associated anomalies were seen in 23 patients (65.7%) (Table 1). Ten had Down’s syndrome (28.6%) and 7 (20%) had congenital heart disease. Six (17%) had rotational abnormalities of the gut including two patients with situs inversus. These two patients presented with bile-stained vomiting immediately after birth and abdominal x-ray showed the classic double- bubble sign, but the stomach was on the right side (Figure 1). The diagnosis of situs inversus was confirmed by preoperative abdominal ultrasound. Echocardiography was normal in one and in the other there were features of Fallot’s tetralogy. In the remaining four patients, the diagnosis of associated malrotation was made intraoperatively. All had malrotation with Ladd’s bands, and in addition, they had intrinsic duodenal obstruction, which was diagnosed intraoperatively. Two had duodenal atresia and four had duodenal diaphragm with a hole. Interestingly, two of our patients had congenital pyloric atresia associated with duodenal atresia at the fourth part of the duodenum. This resulted in a closed duodenal loop with pyloric atresia at one end and duodenal atresia at the other end with accumulation of biliary and pancreatic secretions. This resulted in duodenal perforation in one patient. In both, the diagnosis of associated duodenal atresia was made only intraoperatively. One of our patients had dysmorphic features, esophageal atresia with tracheoesophageal fistula and hydronephrosis. Intraoperatively, this patient had duodenal atresia, annular pancreas and a preduodenal portal vein. One of our patients had eventration of the right diaphragm.
Table 1.
Associated anomalies.
| Associated anomaly | No. | % |
|---|---|---|
| Down’s syndrome | 10 | 28.6 |
| Congenital heart disease | 7 | 20 |
| Malrotation and situs inversus | 6 | 17.14 |
| Congenital pyloric atresia | 2 | 5.7 |
| Duplication cyst | 2 | 5.7 |
| Anorectal malformation | 2 | 5.7 |
| Pelvi-ureteric junction obstruction | 2 | 5.7 |
| Arterio-venous malformation | 1 | 2.9 |
| Congenital leukemia | 1 | 2.9 |
| Esophageal atresia an tracheoesphageal fistula | 1 | 2.9 |
| Cystic left kidney | 1 | 2.9 |
| Syndactly | 1 | 2.9 |
| Hypoplastic left thumb | 1 | 2.9 |
| Meckel’s diverticulum | 1 | 2.9 |
| Eventration of right diaphragm | 1 | 2.9 |
Figure 1.
Plain abdominal x-ray showing classic double-bubble sign in a patient with situs inversus and congenital duodenal obstruction.
All our patients were operated on and intraoperatively the site of duodenal obstruction was located in the second part of duodenum in 32 patients (91.4%). In two, the site of obstruction was at the fourth part of the duodenum while in the third it was located at the third part of duodenum. The causes of obstruction were duodenal atresia without a gap in 12 (34.3%), duodenal atresia with a gap in 6 (17.14%), duodenal stenosis in 4 (11.43%) and duodenal diaphragm in 13 (37.14%). In 7 patients (20%), there was an associated annular pancreas. The different operative procedures, which are shown in Table 2, were used initially in the series. Subsequently, none of our patients had gastrostomy or a transanastomotic feeding tube as part of their operative management. Fourteen (40%) required total parenteral nutrition. Two of our patients had reduction duodenoplasty to decrease the size of the duodenum. Two of our patients required reoperations, one because of an anastomotic leak and another because of duodenal dysfunction. This patient underwent reduction duodenoplasty and subsequently did well. Four of our patients died giving an overall mortality of 11.4%. In all 4, associated anomalies were the cause of death (Table 3).
Table 2.
The operative procedures.
| Operative procedure | No. of patients |
|---|---|
| Duodeno-duodenostomy | 13 |
| Duodeno-duodenostomy and appendecectomy | 5 |
| Excision of duodenal diaphragm and duodenoplasty | 7 |
| Ladd’s procedure + excision of duodenal diaphragm and duodenoplasty + appendecectomy | 4 |
| Duodeno-jejunostomy | 6 |
Table 3.
Causes of death in four patients.
| Age | Sex | Associated anomalies | Cause of death |
|---|---|---|---|
| 5 days | M | Congenital pyloric atresia, duplication cyst, jejunal atresia. | Immunodeficiency and sepsis |
| 2 days | M | Congenital pyloric atresia. | Immunodeficiency and sepsis |
| 3 hours | M | Esophageal atresia and tracheoesophageal fistula, non-rotation, preduodenal portal vein, annular pancreas, dysmorphic features, intra-uterine growth retardation, hydronephrosis. | Associated anomalies and cardiorespiratory arrest. |
| 5 days | F | Down’s syndrome, severe congenital heart disease, prematurity. | Congenital heart disease, prematurity, septicemia |
DISCUSSION
Congenital duodenal obstruction is an interesting anomaly, both embryologically and clinically. During the fourth to the sixth week of intrauterine life, the duodenal lumen is obliterated by the rapidly growing epithelium. The duodenal lumen than recanalyzes by the twelfth week. Intrinsic duodenal obstruction is thought to result from failure of recanalization or arrest of duodenal growth.4 This differentiates congenital duodenal atresia from atresia in the rest of the intestines, which result from an intrauterine vascular accident. 5 Congenital duodenal obstruction is divided into intrinsic and extrinsic depending on the etiology.6 Intrinsic causes include atresia, stenosis and duodenal diaphragm with or without a hole. Extrinsic causes include malrotation with congenital bands, duplication, annular pancreas and preduodenal portal vein. It is not uncommon to find combined intrinsic and extrinsic causes of congenital duodenal obstruction in the same patient.1 This was the case in four of our patients who had malrotation with congenital bands as well as intrinsic duodenal obstruction. The importance of this needs to be emphasized to obviate subsequent morbidity and reoperations, which are not uncommon in patients with congenital duodenal obstruction. Bailey et al, in a large series of 138 infants and children with congenital duodenal obstruction, reported reoperations in 14% for technical reasons, for other atresias that were missed initially and for other unrelated lesions.1 There was also a surprisingly high incidence (20%) of combined anatomic abnormalities in their series, including a number of infants with both malrotation with bands and duodenal atresia, stenosis, or web.1 Intraoperatively, it is important to check the patency of the gastrointestinal tract even in the presence of an extrinsic cause of duodenal obstruction. Seven of our patients had associated annular pancreas and two had associated duplication but in all, there was an associated intrinsic duodenal obstruction.
The incidence of associated anomalies with congenital duodenal obstruction is variable.1–3,7–9 Down’s syndrome and congenital heart disease continue to be the commonest anomalies associated with congenital duodenal obstruction. The incidence of Down’s syndrome in our series was 28.6%, which is lower than that reported by Akhtar and Guiney.2 One reason for this is the high proportion of younger mothers in our series (mean maternal age was 23 years) when compared to the Akhtar and Guieny series (mean maternal age was 33 years). Two of our patients had situs inversus in association with congenital intrinsic duodenal obstruction. Situs inversus is a very rare condition with an estimated frequency ranging from 1 in 4000 to 1 in 20 000 live births.10 It is commonly associated with other serious cardiac and splenic malformation.11,12 This was the case in one of our two patients who had Fallot’s tetralogy. The diagnosis of congenital duodenal obstruction in association with situs inversus can be made on plain erect abdominal x-ray when a reverse double-bubble sign is seen. This can be confirmed further by barium meal and follow-through.
The treatment of congenital duodenal obstruction is standardized. Duodeno-duodenostomy continues to be the treatment of choice. Kimura et al in 1977 described the diamond-shaped duodeno-duodenostomy.13 None of our patients had the diamond-shaped anastomosis, but there are several reports supporting this type of anastomosis as these patients had earlier oral feeds when compared with the side-to-side duodeno-duodenostomy. 2,13 For those with duodenal diaphragm, we continue to do excision of the duodenal diaphragm and duodenoplasty.14 We resort to duodeno-duodenostomy only when there are technical difficulties because of the close proximity of the duodenal diaphragm to the ampulla of Vater. Six of our patients were treated with duodeno-jejunostomy. In three patients, this was because of the site of obstruction in the third and fourth parts of the duodenum while in the other three patients it was due to technical operative difficulties. Duodeno-jejunostomy is considered a less physiological procedure when compared to duodeno-duodenostomy, but when the obstruction is located in the third or fourth parts of the duodenum, duodeno-jejunostomy is a more feasible procedure. Gastrostomy was used as part of the operative treatment of congenital duodenal obstruction in four of our patients in the initial series. We agree with others that gastrostomy should no longer be used as part of the management of congenital duodenal obstruction. 2 A transanastomotic tube was used in six of our patients, and like others we no longer use transanastomotic tubes.2 They tend to recoil back and cause anastomotic disruption.15 Two of our patients had reduction duodenoplasty due to megaduodenum during the initial operation in one and secondary to duodenal dysfunction postoperatively in the other. We advocate reduction duodeoplasty during the initial operation in patients with megaduodenum.
Our overall survival of 88.6% compares favorably to reports from Western countries.1,2 This survival rate was in spite of a high rate of prematurity (34.3%) in our series. This is attributed to improved surgical techniques and improved perioperative management, including total parenteral nutrition. Severe associated anomalies, however, continue to contribute to a high mortality. Four of our patients died and associated anomalies were the cause of death. Two of them had associated congenital pyloric atresia and both died because of sepsis secondary to immunodeficiency. When congenital pyloric atresia forms part of the hereditary multiple intestinal atresias it is known to be associated with combined immunodeficiency, which contributes to mortality.16–18
REFERENCES
- 1.Bailey PV, Tracy TF, Connors TR, Monney DP, Lewis JE, Weber TR. Congenital duodenal obstruction: A 32-year review. J Pediatr Surg. 1993;28(1):92–95. doi: 10.1016/s0022-3468(05)80364-1. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar J, Guiney EJ. Congenital duodenal obstruction. Br J Surg. 1992;79:133–135. doi: 10.1002/bjs.1800790212. [DOI] [PubMed] [Google Scholar]
- 3.Young DG, Wilkinson AW. Abnormalities associated with neonatal duodenal obstruction. Surgery. 1968;63:832–836. [PubMed] [Google Scholar]
- 4.Gray SW, Skandalakis JH. The embryological basis for treatment of congenital defects. Philadelphia, PA: Saunders; 1972. Embryology for surgeons; pp. 177–178. [Google Scholar]
- 5.Louw JH, Barnard CN. Congenital intestinal atresia: observation on its origin. Lancet. 1955;2:1065. doi: 10.1016/s0140-6736(55)92852-x. [DOI] [PubMed] [Google Scholar]
- 6.Ladd WE. Congenital duodenal obstruction. Surgery. 1973;1:878–885. [Google Scholar]
- 7.Atwell JD, Klidjian AM. Vertebral anomalies and duodenal atresia. J Pediatr Surg. 1982;17:237–240. doi: 10.1016/s0022-3468(82)80004-3. [DOI] [PubMed] [Google Scholar]
- 8.Adeyemi SD. Duodenal obstruction in Nigerian newborns and infants. Scan J Gastroenterol. 1986;124:157–161. doi: 10.3109/00365528609093799. [DOI] [PubMed] [Google Scholar]
- 9.Gavopoulos S, Limas CH, Avtzoglou P, et al. Operative and postoperative management of congenital duodenal obstruction: a 10 year experience. Pediatr Surg Int. 1993;8:122–124. [Google Scholar]
- 10.Ruben DG, Templeton My, Ziegler MM. Situs inversus: the complex inducing neonatal intestinal obstruction. J Pediatr Surg. 1983;18:751–756. doi: 10.1016/s0022-3468(83)80018-9. [DOI] [PubMed] [Google Scholar]
- 11.Akel S, Halabi J, Shawis R. Abdominal situs inversus with congenital duodenal stenosis: rare association. Eur J Pediatr Surg. 1998;8:55–57. doi: 10.1055/s-2008-1071120. [DOI] [PubMed] [Google Scholar]
- 12.Mordehi J, Cohen Z, Kurzbart E, Mares AJ. Preduodenal portal vein causing duodenal obstruction associated with situs inversus, intestinal malrotation and polysplenia. J Pediatr Surg. 2002;27:1–3. doi: 10.1053/jpsu.2002.31643. [DOI] [PubMed] [Google Scholar]
- 13.Kimura K, Tsugawa C, Ogawa K, Matsumoto Y, Asada S. Diamond-shaped anastomosis for congenital duodenal obstruction. Arch Surg. 1977;112:1262–1263. doi: 10.1001/archsurg.1977.01370100116026. [DOI] [PubMed] [Google Scholar]
- 14.Nawaz A, Matta H, Jacobsz A, Trad O, Al-Salem AH. Congenital duodenal diaphragm in eight children. Ann Saudi Med. 2004;24(3):193–197. doi: 10.5144/0256-4947.2004.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Salem AH, Khawaja S, Grant C, Dawodu A. Congenital duodenal obstruction: Problems in diagnosis and management. J Pediatr Surg. 1989;24:1247–1249. doi: 10.1016/s0022-3468(89)80560-3. [DOI] [PubMed] [Google Scholar]
- 16.Al-Salem AH, Qaissaruddin S, Varma KK. Pyloric atresia associated with intestinal atresia. J Pediatr Surg. 1997;32:1262–1263. doi: 10.1016/s0022-3468(97)90700-4. [DOI] [PubMed] [Google Scholar]
- 17.Rothenberg ME, White FV, Chilmonezyk B, Chatila T. A syndrome involving immunodeficiency and multiple intestinal atresias. Immunodeficiency. 1995;5:171–178. [PubMed] [Google Scholar]
- 18.Walker MW, Lovel MA, Kelly TE, Golden W, Saulsbury FT. Multiple areas of intestinal atresias associated with immunodeficiency and post-transfusion graft-versus-host disease. J Pediatr. 1993;123:93–95. doi: 10.1016/s0022-3476(05)81547-1. [DOI] [PubMed] [Google Scholar]