Abstract
BACKGROUND
Myiasis complication of diabetic foot ulcer has only been presented in a few case reports. Therefore, there is a need for additional data on this infestation.
OBJECTIVE
Evaluate clinical characteristics of human myiasis in patients with diabetic foot.
DESIGN
Case series.
SETTINGS
A tertiary referral healthcare institution and a diabetic foot center.
PATIENTS AND METHODS
Patients with diabetic foot infection complicated by myiasis who were admitted between June 2012 and July 2017.
MAIN OUTCOME MEASURES
Bacterial infection rate, accompanying bacterial agents, amputation (morbidity) and mortality rate.
SAMPLE SIZE
18.
RESULTS
Eight (44.4%) of the patients were female. Sixteen (88.9%) had moderate-to-severe infections; 15 (83.3%) had necrotic tissue. Larval debridement therapy was performed on all patients at the bedside in consecutive sessions. A third-stage larva of Calliphora was detected in one case (5.6%). Second- and third-stage larvae of Lucilia sericata were detected in 5 (27.8%) and 7 (38.9%) patients, respectively. All the patients had a bacterial infection with myiasis. Twelve (66.7%) patients underwent amputation. Three (16.7%) patients died. Myiasis was more frequent in the months of May, June and July.
CONCLUSION
To our knowledge, this is the largest reported series of cases of diabetic foot with myiasis. The most common parasitic agent was Lucilia sericata. Bacterial soft tissue infections were observed in all cases. Poor hygienic conditions were noteworthy and all patients were in need of radical surgery. Myiasis complication of diabetic foot is more frequently seen in the spring and summer.
LIMITATIONS
Insufficient follow-up time for analysis of possible confounding factors.
Myiasis, an infestation of fly larvae, was first described in 1840. The larvae are deposited as eggs into living mammals where they feed and complete their life cycle.1 The clinical findings of myiasis vary according to the fly species, the number of larvae and the location of the invaded area. The most common type of myiasis involves the skin. Cutaneous myiasis can manifest as furuncular, inflamed skin, wounded skin, myiasis linearis (larvae migrans) and traumatic myiasis. Myiasis in diabetic patients falls into the wounded skin group. In this group, myiasis can be caused by the larvae of Cochliomyia hominivorax, Chrysomya bezziana, Lucilia sericata, Phormia regina, Sarcophaga, Calliphora and Stomoxys.2,3 Wound myiasis often occurs as an infestation of a wound with fly larvae. In neglected and mistreated wounds, the first stage larvae initially penetrate into the exfoliating part and go deeper, initially damaging the tissue.1,3 The incidence of myiasis increases in elderly patients living in conditions of poor environmental hygiene and of low socioeconomic status, with mental retardation, diabetes and vascular problems. Myiasis is especially common but not strictly confined to the tropics..1,2,4 Although diabetes mellitus is a risk factor for myiasis, diabetic foot is quite rare among myiasis cases.5,6 This observational retrospective study was designed to identify the characteristics of myiasis in patients with diabetic foot.
PATIENTS AND METHODS
Cases with diabetic foot infection complicated by myiasis that were admitted to the Diabetic Foot Council of Ege University and/or the Diabetic Foot Clinic of Buca SD State Hospital between June 2012 and July 2017 were included in the study. We recorded patient age, gender, duration of diabetes, coexisting bacterial infections on the wound site, infectious agents causing infestations, socioeconomic status, presence of mental retardation, confinement to bed and additional comorbid diseases. The necessity for amputation, presence of necrosis, and presence of sepsis were recorded.7 Polymicrobial infection was recorded in cases of a positive culture of a sample of the abscess, bone, tissue or blood with two or more different microorganisms at the same time.8,9
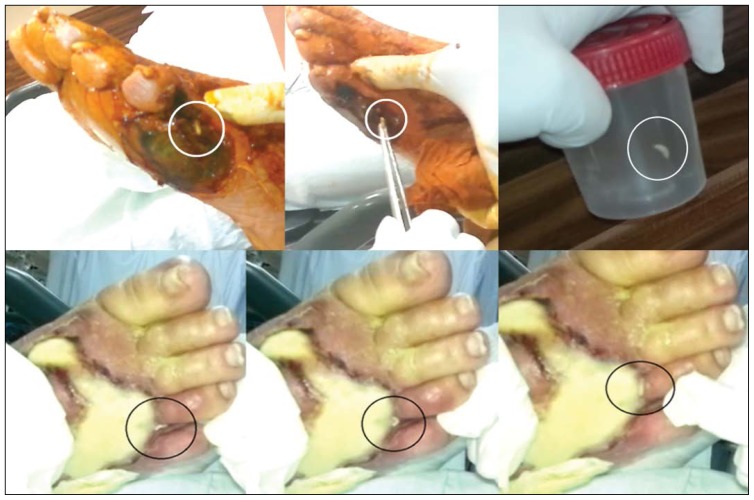
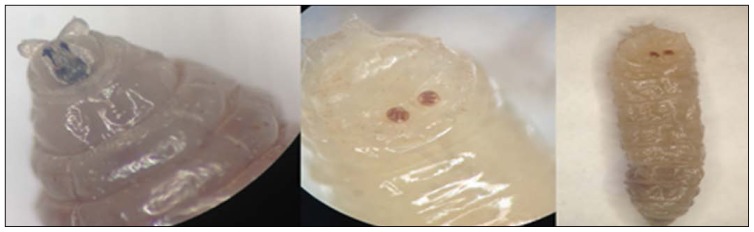
In all cases, larval debridement therapy was performed at the bedside in consecutive sessions. The entire wound was cleaned with 10% polyvinyl chloride-iodine. After a minute, all larvae were collected one by one with sterile tweezers (Figure 1). Lastly, the wounds were covered with sterile dressings. This method was repeated daily. After larval debridement therapy, the wounds were washed with saline, and superficial necrotic tissues were debrided. Deep infected tissues were collected using another sterile surgical blade and sterile tissue scissors. These deep tissue samples were used for bacterial and fungal cultures. Both larvae and tissue samples were placed in a sterile container and quickly delivered to the laboratory. Macroscopic and microscopic examinations of the debrided larvae were performed. In addition, species were identified by distinctive morphological features such as the state of peritreme, and by identification of stigmata (Figure 2).10
Figure 1.
Larvae were collected with a sterile surgical tweezers.
Figure 2.
Larvae were identified macro- and microscopically.
During admission, all cases (or their relatives) were informed and signed the informed consent form. During the first larval debridement therapy, a video was filmed to record the characteristics of the larvae (Online Resource 1). The patients were reported using the guidelines of the PROCESS Statement.11
RESULTS
Eighteen cases of diabetic foot infections complicated by myiasis were included in the study. Eight (44.4 %) of the cases were female. According to the Infectious Diseases Society of America score, ten (55.6%) were severe, six (33.4%) moderate and two (11.1%) were mild diabetic foot infections. In all cases, larval debridement treatment was performed at the bedside in consecutive sessions. All the wounds were cleaned of larvae on the second day. However, the wounds of the 11th and 14th patients were completed on the third day (11.1%) (Table 1). The wounds of all cases were followed up for at least four days. Larval structures were examined macroscopically and microscopically, and a third stage larva of Calliphora was detected in one case (5.56%). In five (27.8%) patients, a second stage Lucilia sericata larva-was found, and in seven (38.9%) patients, a third stage Lucilia sericata larva was found. Because of inappropriate transport conditions, the stage of the larva was not identified correctly in five cases (27.8%). Myiasis was more frequent in the months of May, June and July.
Table 1.
Demographic and clinical features of the cases.
| No | Age (years) | Gender | DM duration (years) | Wound duration (days) | Necrosis | Sepsis | PEDIS-IDSA infection score | Addition diseases | Confined to bed | Living in nursing home | Homeless or living alone | Poor hygienic conditions | Bacterial growth in tissue culture | Date of infestation (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| 1 | 87 | Female | 30 | 15 | Yes | Yes | Severe | Alzheimer | Yes | Yes | No | Yes | Acinetobacter baumannii | August 2014 |
| 2 | 64 | Female | 13 | 60 | No | Yes | Severe | CVE | Yes | No | No | Yes | Pseudomonas aeruginosa | June 2013 |
| 3 | 67 | Male | 12 | 40 | Yes | Yes | Severe | CRF, HT | No | No | No | Yes | Serratia liquefaciens, Escherichia coli | February 2013 |
| 4 | 74 | Male | 25 | 180 | No | No | Moderate | – | No | No | No | Yes | Negative | July 2013 |
| 5 | 48 | Male | 15 | 180 | Yes | No | Moderate | CRF, HT | No | No | Yes | No | Negative | June 2013 |
| 6 | 83 | Female | 27 | 30 | Yes | Yes | Severe | HT, Alzheimer | No | Yes | No | No | Klebsiella oxytoca, Enterobacter aerogenes | March 2016 |
| 7 | 62 | Male | 10 | 150 | No | No | Moderate | RA, HT, CVE, PAD | No | No | No | Yes | Pseudomonas aeruginosa, Proteus mirabilis ESBL(+) | May 2017 |
| 8 | 78 | Male | 11 | 30 | Yes | Yes | Severe | HT, CHF, CVE | Yes | Yes | No | Yes | Morganella morganii | May 2017 |
| 9 | 82 | Female | 20 | 12 | Yes | No | Mild | HT | No | No | No | Yes | Negative | May 2017 |
| 10 | 64 | Female | 14 | 7 | Yes | Yes | Severe | HT | No | No | No | Yes | Staphylococcus aureus | July 2016 |
| 11 | 77 | Female | 25 | 60 | Yes | Yes | Severe | HT | No | No | No | Yes | Pseudomonas aeruginosa | May 2017 |
| 12 | 63 | Male | 12 | 85 | Yes | No | Moderate | PAD, HT | No | No | No | Yes | Negative | May 2017 |
| 13 | 59 | Male | 10 | 20 | Yes | Yes | Severe | CAD | No | No | Yes | Yes | Negative | May 2017 |
| 14 | 70 | Female | 10 | 30 | Yes | No | Moderate | CAD, PAD | No | No | Yes | Yes | Morganella morganii ESBL(+) | June 2017 |
| 15 | 66 | Male | 15 | 40 | Yes | No | Mild | – | No | No | No | Yes | Negative | June 2017 |
| 16 | 74 | Male | 27 | 80 | Yes | No | Moderate | HT, CAD, PAD | No | No | No | Yes | Stenotrophomonas maltophilia, Morganella morganii ESBL(+) | June 2017 |
| 17 | 64 | Female | 4 | 30 | Yes | Yes | Severe | CRF, HT, Cataract | No | No | Yes | Yes | Pseudomonas aeruginosa | July 2017 |
| 18 | 53 | Male | 10 | 15 | Yes | Yes | Severe | CRF, PAD, HT | No | No | Yes | Yes | Negative | July 2017 |
CAD, coronary artery disease; CRF, chronic renal failure; CVE, central vascular event; DM, diabetes mellitus; ESBL, extended-spectrum beta lactamase; HT, hypertension; IDSA, Infectious Diseases Society of America score; PEDIS, Perfusion, Extent, Depth, Infection And Sensation Score; RA, rheumatoid arthritis; PAD, peripheral artery disease.
Fourteen (77.8%) patients underwent surgery: four (22.2%) received below-knee amputations, four (22.2%) had metatarsophalangeal joint disarticulations, three (16.7%) ray amputations, two (11.1%) deep surgical debridement therapies, and one (5.56%) a syme amputation. Four (22.2%) of the patients could not undergo major surgery. Two (11.1%) of these cases refused amputation treatment after myiasis debridement therapy and were discharged upon their request. The other two (11.1%) died before surgery. One (5.56%) patient died within 48 hours after surgery. All patients had bacterial infections with myiasis. Wound cultures were taken from all as shown in Table 1. In seven (38.9%) patients who had no bacterial growth, we detected leukocytosis and left shift accompanied by edema or discharge around the wound.
DISCUSSION
Diabetic foot infections (DFIs) rarely include fungal infections, but when they do amputation rates are increased by ten times.8 Even if fungal DFIs are rare, their presence cannot be neglected since it may be a prognostic factor in DFIs. Findings in myiasis vary depending on the affected body region. Among our patients with DFI, myiasis was frequently seen in the months of May, June and July, but myiasis is often seen in rural areas in autumn and summer.1 Myiasis can cause psychological disorders due to functional health problems and appearance.1,6 Although not a tropical climate zone like Africa and America, Turkey is at risk for myiasis, which could be endemic due to reasons such as a high population in rural areas and animal husbandry.6,12,13
Foot, wound and skin care is an essential practice that needs to be performed carefully and systematically in patients with chronic wounds such as diabetic foot ulcers. The probability of developing neuropathy for all diabetic diseases is 30% to 50%.14 The most common type of neuropathy is distal symmetrical polyneuropathy that can cause serious sensory loss in the affected areas. Diabetic polyneuropathy is the major cause of amputation with infection and ischemia.15 This makes patients with myiasis infestation feel little or no pain. Thus, it is difficult to be aware of the situation and take precautions. This is also the reason why diabetic foot ulcers are high-risk ulcers for myiasis. The risk is also high in paralyzed patients who are bedridden and unable to care for themselves,1,2,4,6 which is why the wounds should not be left open for a long time in patients with these conditions. The wound site may need to be aerated to avoid conditions like maceration. If the wound is left open for aeration, it must be covered with a thin layer of sterile gauze. The pores of the gauze must be small enough to prevent flies from penetrating while allowing airflow. In addition, chronic wounds should be treated at least once a day and should be closed well.16 The wound site should be inspected and cleaned regularly, so that formation of myiasis again is less likely.
The recommended treatment of myiasis is to collect all visible larvae and screwworms directly from the wound and to perform active debridement and daily dressing with antiseptic solutions; if possible, the infested area should be removed completely.17,18 If necessary, excisions can also be made to reach the larvae.12 First, larvae and eggs are forced to the surface by triggering regional hypoxia with a toxic substance; then, the larvae on the surface are mechanically cleaned.2,19 These procedures are the only active treatment protocols.20 The most important recommendation is related to the prevention of myiasis. Therefore, the care and protection of chronic and untreated wounds is highly important.20
In our study, active sequential debridement was applied to all patients. By sequential debridement of the wound site, we aimed to find all multi-stage larvae, which might have been overlooked and subsequently matured. Skin myiasis patients have been reported as “rare cases” since 2000. Three cases reported from Germany involved elderly and alcohol-dependent patients of low socioeconomic status and/or with diabetes mellitus, which constitute a high-risk group.21 A case of Sarcophaga in diabetics was reported from Saudi Arabia16 and a similar case was reported from Italy.22 In addition, a notable case was reported from Turkey.6 A case from Argentina reported larvae of the Calliphoridae family, which we also found in one of our patients.23
Samples taken from the wounds of myiasis patients should be sent to the nearest parasitology laboratory of a training and research or university hospital.21 In this study, the larval specimens taken from the patients were studied in the Direct Diagnosis Laboratory in the Department of Parasitology at University Medical Faculty, Ege University. In addition, early admission to a tertiary care hospital improves the prognosis due to the experienced diabetic foot consultants located in these centers. This is also true for cases of diabetic foot complicated by myiasis.24
In conclusion, myiasis has only been reported in single case reports in patients with diabetic foot infection. To the best of our knowledge, our study presents the largest series of myiasis cases in the literature. The increased incidence of myiasis in Turkey could be due to the self-care deficit in diabetic patients.25
Acknowledgments
We would like to thank all the officials and health care team at Ege University Diabetic Foot Council who per formed or helped the study, and all the participants in the study. Special thanks to Prof. Dr. Sevki Cetinkalp, Prof. Dr. Ilgen Ertam, Prof. Dr. Halit Ozyalçın, Dr. Idil Unal, Dr. Deniz Akyol, Dr. Ugur Onal, Dr. Mehmet Karakus and Dr. Hilmi Gungor for their efforts in the study.
Footnotes
Funding: None.
CONFLICT OF INTEREST: None.
REFERENCES
- 1.Daldal N, Atambay M. Ozcel’s Medical Parasitic Diseases. 1st. Ed. Izmir: Turkish Society for Parasitology; 2007. Myiasis (miyaz) pp. 867–881. [Google Scholar]
- 2.Francesconi F, Lupi O. Myiasis. Clinical microbiology reviews. 2012;25(1):79–105. doi: 10.1128/CMR.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safdar N, Young DK, Andes D. Autochthonous furuncular myiasis in the united states: Case report and literature review. Clin Infect Dis. 2003;36(7):e73–80. doi: 10.1086/368183. [DOI] [PubMed] [Google Scholar]
- 4.Zumpt F. Myiasis in man and animals in the old world. 1 Ed. London: Butterworths; 1965. [Google Scholar]
- 5.Chan JC, Lee JS, Dai DL, Woo J. Unusual cases of human myiasis due to old world screwworm fly acquired indoors in Hong Kong. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99(12):914–8. doi: 10.1016/j.trstmh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Kaya FD, Orkun O, Cakmak A, Inkayan AC, Erguven S. Cutaneous myiasis caused by sarcophaga spp. Larvae in a diabetic patient. Mikrobiyol Bul. 2014;48(2):356–61. doi: 10.5578/mb.7107. [DOI] [PubMed] [Google Scholar]
- 7.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uysal S, Arda B, Tasbakan MI, Cetinkalp S, Simsir IY, Ozturk AM, et al. Risk factors for amputation in patients with diabetic foot infection: A prospective study. Int Wound J. 2017 doi: 10.1111/iwj.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinojosa CA, Boyer-Duck E, Anaya-Ayala JE, Nunez-Salgado A, Laparra-Escareno H, Torres-Machorro A, et al. Impact of the bacteriology of diabetic foot ulcers in limb loss. Wound Repair Regen. 2016;24(5):923–927. doi: 10.1111/wrr.12462. [DOI] [PubMed] [Google Scholar]
- 10.Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clinical microbiology reviews. 2014;27(1):48–67. doi: 10.1128/CMR.00008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agha RA, Fowler AJ, Rajmohan S, Barai I, Orgill DP, Afifi R, et al. Preferred reporting of case series in surgery; the process guidelines. International Journal of Surgery. 2016;36:319–323. doi: 10.1016/j.ijsu.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Millikan LE. Myiasis. Clinics in dermatology. 1999;17(2):191–5. doi: 10.1016/s0738-081x(99)00011-5. discussion 105–6. [DOI] [PubMed] [Google Scholar]
- 13.Kuk S, Yildirim S, Ozden M, Erensoy A, Saki CE. Ophthalmomyiasis is not only a problem for rural regions of eastern anatolia of Turkey. Med Sci Monit. 2009;15(11):Cs166–8. [PubMed] [Google Scholar]
- 14.Maser RE, Steenkiste AR, Dorman JS, Nielsen VK, Bass EB, Manjoo Q, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh epidemiology of diabetes complications study. Diabetes. 1989;38(11):1456–61. doi: 10.2337/diab.38.11.1456. [DOI] [PubMed] [Google Scholar]
- 15.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–34. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaglool DA, Tayeb K, Khodari YA, Farooq MU. First case report of human myiasis with sarcophaga species in makkah city in the wound of a diabetic patient. Journal of natural science, biology, and medicine. 2013;4(1):225–8. doi: 10.4103/0976-9668.107301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sesterhenn AM, Pfutzner W, Braulke DM, Wiegand S, Werner JA, Taubert A. Cutaneous manifestation of myiasis in malignant wounds of the head and neck. European journal of dermatology: EJD. 2009;19(1):64–8. doi: 10.1684/ejd.2008.0568. [DOI] [PubMed] [Google Scholar]
- 18.Ergun S, Akinci O, Sirekbasan S, Kocael A. Postoperative wound myiasis caused by sarcophaga carnaria. Turkiye Parazitol Derg. 2016;40(3):172–175. doi: 10.5152/tpd.2016.4621. [DOI] [PubMed] [Google Scholar]
- 19.Francesconi LO. Tropical dermatology. Philadelphia, PA: Elsevier; 2006. Myiasis; pp. 232–239. [Google Scholar]
- 20.Centers for Disease Control and Prevention-CDC. Parasites-myiasis. https://www.cdc.gov/parasites/myiasis/. [cited in August 1, 2017, English version]
- 21.Public Health Institution-Ministry of Health, Turkey. Field guide for laboratory diagnosis of infectious diseases: Myiasis. http://mikrobiyoloji.thsk.saglik.gov.tr/ums/M-N/Miyazlar.pdf. [cited in June 03, 2017]
- 22.Dutto M, Pellegrino M, Vanin S. Nosocomial myiasis in a patient with diabetes. J Hosp Infect. 2013;83(1):74–6. doi: 10.1016/j.jhin.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Olea MS, Centeno N, Aybar CA, Ortega ES, Galante GB, Olea L, et al. First report of myiasis caused by Cochliomyia hominivorax (diptera: Calliphoridae) in a diabetic foot ulcer patient in Argentina. The Korean Journal of Parasitology. 2014;52(1):89–92. doi: 10.3347/kjp.2014.52.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleem S, Hayat N, Ahmed I, Ahmed T, Rehan AG. Risk factors associated with poor outcome in diabetic foot ulcer patients. Turk J Med Sci. 2017;47(3):826–831. doi: 10.3906/sag-1602-119. [DOI] [PubMed] [Google Scholar]
- 25.Tarkun I, Ozgoksu SD., [14][15] Attitudes, wishes, and needs of diabetes patients and their relatives: Turkish data from the dawn2 study. Turk J Med Sci. 2017;47(2):447–454. doi: 10.3906/sag-1509-67. [DOI] [PubMed] [Google Scholar]