Abstract
BACKGROUND
Current guidelines for massive pulmonary embolism (PE) treatment recommend primary reperfusion therapy and the option of extracorporeal membrane oxygenation (ECMO). However, these recommendations might not be optimal for patients with poor prognoses who are in cardiogenic shock (CS) or require cardiopulmonary resuscitation (CPR).
OBJECTIVE
Evaluate the impact of ECMO support on the clinical outcome of patients with massive PE complicated by CPR or CS.
DESIGN
Retrospective review of medical records.
SETTING
A university hospital, South Korea.
PATIENTS AND METHODS
We collected data on patients from 2004 through 2009 (stage 1) and from 2010 through June 2017 (stage 2). Patients with confirmed massive PE received medical therapy (stage 1) or medical therapy that included extracorporeal membrane oxygenation (ECMO) support (stage 2).
MAIN OUTCOME MEASURES
All-cause mortality at 90 days after therapy.
SAMPLE SIZE
9 patients with confirmed massive PE that received medical therapy (stage 1); 14 patients with confirmed massive PE that received medical therapy with ECMO support (stage 2).
RESULTS
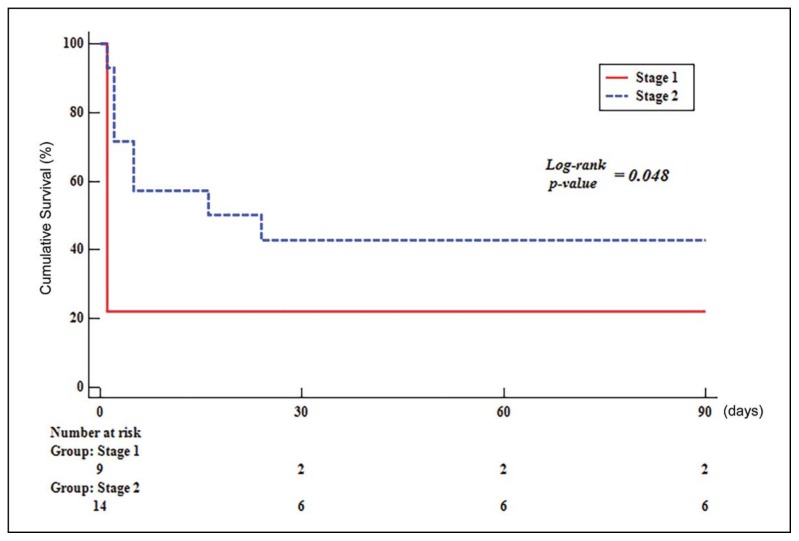
In stage 1, 5 of 9 patients received systemic thrombolysis and 4 patients received anticoagulation. Thirteen of the 14 stage 2 patients received anticoagulation with ECMO support and one patient received systemic thrombolysis with ECMO support. Tricuspid annular plane systolic excursion in stage 1 was lower than in stage 2. Proximal PE in chest CT was more common in stage 2. Survival was significantly improved at 90 days for patients in stage 2 (log-rank, P=.048). There were no differences in baseline characteristics, ECMO complications and transfusion between survivors and nonsurvivors in stage 2.
CONCLUSIONS
Anticoagulation with ECMO support is associated with good survival rate outcomes compared with medical therapy alone.
LIMITATIONS
Relatively small number of patients and retrospective design.
Pulmonary embolism (PE) is a major cause of morbidity and mortality in the general population. In particular, massive PE is rare and critical. Overall, in-hospital mortality rates for massive PE ranged from 25% for patients with cardiogenic shock (CS), to 65% for those that required cardiopulmonary resuscitation (CPR), while in-hospital mortality rate in stable patients with PE was 8.1%.1,2 Primary reperfusion therapy is the treatment of choice for patients with unstable PE with shock or hypotension, based on the European Society of Cardiology (2014) guidelines. Primary reperfusion therapy includes thrombolysis (class IB), surgical pulmonary embolectomy (class IC) and percutaneous catheter-directed embolectomy (class IIaC).3 However, in clinical practice, relatively large numbers of patients cannot receive thrombolysis or embolectomy.2 Major contraindications to thrombolytic therapy include recent major surgery or trauma and prolonged CPR. The clinical course of massive PE can rapidly progress before surgical or catheter-directed embolectomy. Based on the International Cooperative Pulmonary Embolism Registry, two-thirds of patients with massive PE did not receive thrombolysis or embolectomy,4 and the cases of untreated massive PE remain problematic.
Anticoagulation with peripheral extracorporeal membrane oxygenation (ECMO) support can be a a rapid, effective option for patients with massive PE.5–7 Veno-arterial(VA) ECMO is one of the most reliable and quickest ways to decrease right ventricle (RV) overload, improve RV function and hemodynamic status, and restore tissue oxygenation. The impacts of ECMO on survival have not been investigated in massive PE, compared to the medical therapy alone. This study investigated the impact of ECMO on survival in patients with massive PE.
PATIENTS AND METHODS
This was a retrospective study conducted between January 2004 and June 2017. ECMO was introduced into our hospital in May 2009. Prior to 2010, medical therapy including primary reperfusion therapy was the most commonly used therapy in our hospital for massive PE. Patients with confirmed massive PE, complicated by profound CS or that required CPR (n=23), were enrolled in the study to analyze clinical outcomes. PE was diagnosed using the diagnostic strategy tools of the most recent European Society of Cardiology guidelines.3 In the present study, the most useful initial test in suspected PE with CS or requiring CPR is bedside transthoracic echocardiography according to the guidelines. In an unstable patient, echocardiographic evidence of RV dysfunction is sufficient to prompt reperfusion without further testing. When the patient was stabilized, CT angiography was performed. CS was defined as follows: Chest radiograph that showed pulmonary edema or end-organ failure with systolic blood pressure less than 90 mm Hg that did not respond to fluid supply and required a vasopressor agent. Of the predisposing factors, orthopedic surgery includes total knee replacement arthroplasty, total hip replacement arthroplasty, carpal tunnel release, and surgical treatment of wrist fracture. Bleeding complications were reported using the Global Utilization of Streptokinase and TPA for Occluded arteries (GUSTO) classification.8,9 Briefly, severe life-threatening bleeding was defined as intracerebral bleeding or bleeding that caused substantial hemodynamic compromise and required treatment (GUSTO 1). GUSTO 2 defined moderate bleeding that required blood transfusion, whereas GUSTO 3 referred to other bleeding that did not require transfusion or cause hemodynamic compromise. Data obtained from medical records, clinical case histories, and laboratory investigations were retrospectively reviewed. Follow-up data were collected from outpatient clinic records or via telephone. The composite outcome was all-cause mortality at 90 days. This study was approved by the Institutional Review Board of the Catholic University of Korea, St. Vincent’s Hospital (IRB approval number: VC17RESI0146). The study was exempted from requirements for written informed consent because the medical records were retrospectively reviewed. All data records were de-identified and analyzed anonymously.
ECMO and management
The ECMO team consisted of interventional cardiologists, cardiovascular surgeons, and perfusionists. A Capiox emergency bypass system (Terumo, Tokyo, Japan) was used in all cases. VA-ECMO indications included acute refractory cardiac failure, defined as evidence of tissue hypoxia concomitant with adequate intravascular volume status, severely diminished right ventricular or left ventricular ejection fraction (RV/LVEF), low cardiac index (cardiac output from left ventricle in one minute to body surface area, <2.2 L/min/m2), and sustained hypotension despite high-dose catecholamine infusion. ECMO exclusion criteria were as follows: Malignancies with fatal prognosis within 5 years or irreversible neurological pathologies and decisions to limit therapeutic interventions. VA-ECMO cannulas were inserted by trained cardiovascular surgeons with a femoral-femoral approach. The tip of the arterial sheath (15–16F catheter) was placed in the common iliac artery, and the tip of the venous sheath (22–23F catheter) reached the junction between the right atrium and inferior vena cava. The remaining test method was described in detail in a previous paper.10
Statistical analysis
Continuous variables are presented as the median and interquartile ranges or the means and standard deviation (SD), and compared using the Mann-Whitney U test or t test. Categorical variables are presented as numbers and percentages, and compared using the chi-squared or Fisher’s exact tests. The survival of stage 1 and stage 2 was determined using the Kaplan-Meier method and compared using a log rank test. A P value <.05 was considered statistically significant. Analyses were performed using Statistical Analysis Software (SAS, version 9.2, SAS Institute, Cary, NC, USA).
RESULTS
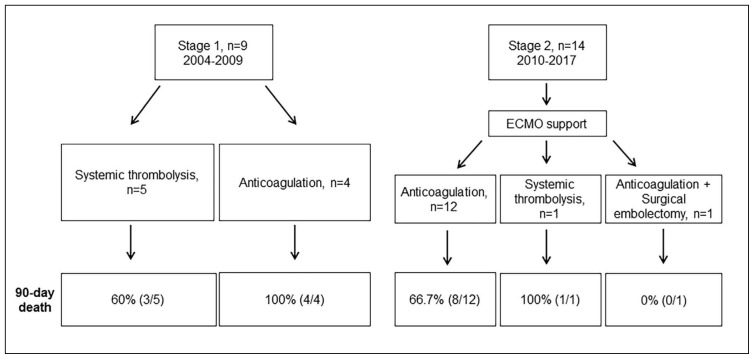
From 2004 through 2009, 9 patients presented at our medical center with confirmed massive PE and these patients received medical therapy alone without ECMO; this was defined as stage 1. However, only five of these patients received systemic thrombolysis and four patients received anticoagulation due to recent major surgery and prolonged CPR. From 2010 through June 2017, 14 patients presented at our medical center with confirmed massive PE, this was defined as stage 2. All patients received ECMO support. Thirteen of the 14 stage 2 patients received anticoagulation due to recent major surgery and prolonged CPR and one of these patients received the additional surgical embolectomy due to unresolved thrombus burden. One patient received systemic thrombolysis (Figure 1). Tricuspid annular plane systolic excursion (TAPSE) in stage 1 was lower than in stage 2. Proximal PE in chest CT was also diagnosed more frequently in stage 2 (Table 1). There was no difference in predisposing factors and laboratory findings for cardiogenic shock between those two groups. The stage 2 group had a higher survival rate at 90 days after therapy (Figure 2) (log-rank, P=.048). There were no differences in baseline characteristics between survivors and non-survivors in the stage 2 group (Table 2). Moreover, there was no differences in ECMO complications between those two groups (Table 3). Seven (50%) patients had a moderate to severe hemorrhage classified as GUSTO ≤2, such as cannula site bleeding (n=4), pulmonary hemorrhage (n=2) and ulcer bleeding (n=1). Compartment syndrome, pseudoaneurysm, and cannula site infection occurred in one patient, respectively. In addition, there were three patients with pneumonia, seven patients with acute kidney injury, four patients with multiple organ failure, two patients with leg ischemia, five patients with hypoxic brain injury, and two patients with neuropathy. There were no differences in transfusion, mechanical ventilation duration, and ECMO duration. However, hospital duration was longer in survivors than in non-survivors.
Figure 1.
Study flow chart.
Table 1.
Baseline characteristics.
| Stage 1 Without ECMO (n=9) | Stage 2 With ECMO (n=14) | P | |
|---|---|---|---|
|
| |||
| Male | 0 (0.0) | 4 (28.6) | .127 |
| Mean age (years) | 65.4 (14.8) | 53.6 (17.7) | .110 |
| BMI | 25.1 (3.1) | 26.0 (4.4) | .577 |
| Smoking | 0 (0.0) | 4 (28.6) | .127 |
| DM | 2 (22.2) | 2 (14.3) | >.999 |
| Hypertension | 4 (44.4) | 3 (21.4) | .363 |
| Cancer | 2 (22.2) | 0 (0.0) | .142 |
| Predisposing factor | |||
| Post orthopedic surgery | 6 (66.7) | 3 (21.4) | .077 |
| Immobility | 3 (33.3) | 5 (35.7) | >.999 |
| Previous DVT | 1 (11.1) | 0 (0.0) | .391 |
| Trauma | 0 (0.0) | 2 (14.3) | .502 |
| Cancer | 2 (22.2) | 0 (0.0) | .142 |
| Unknown | 0 (0.0) | 3(21.4) | .253 |
| TTE parameter | |||
| RVSP | 35.1 (28.4) | 42.9 (13.3) | .455 |
| TAPSE | 6.3 (6.2) | 11.9 (4.3) | .027 |
| RV dilation | 7 (77.8) | 13 (92.9) | .538 |
| Chest CT finding | |||
| Proximal PE | 3 (33.3) | 12 (85.7) | .023 |
| Cardiac arrest | |||
| IHCA | 7 (77.8) | 7 (50.0) | .228 |
| OHCA | 1 (11.1) | 4 (28.6) | .611 |
| Cardiogenic shock | 1 (11.1) | 3 (21.4) | >.999 |
| Treatment | |||
| Heparin | 2 (22.2) | 13 (92.9) | .001 |
| LMWH | 2 (22.2) | 0 (0.0) | .142 |
| Urokinase | 4 (44.4) | 1 (7.1) | .056 |
| tPA | 1 (11.1) | 0 (0.0) | .391 |
| Surgical embolectomy | 0 (0.0) | 1 (7.1) | >.999 |
| ECG finding | |||
| Asystole | 2 (22.2) | 4 (28.6) | >.999 |
| PEA | 5 (55.6) | 5 (35.7) | .417 |
| VT/V.fib | 1 (11.1) | 2 (14.3) | >.999 |
| Sinus tachycardia | 1 (11.1) | 3 (21.4) | >.999 |
| GCS | 11.1 (5.6) | 6.3 (4.1) | .056 |
| APACHE II score | 22.1 (10.8) | 22.4 (6.9) | .932 |
| Laboratory finding at cardiogenic shock | |||
| pH | 7.2 (0.3) | 7.2 (0.2) | .972 |
| HCO3 (mmol/L) | 14.7 (5.7) | 12.6 (3.1) | .281 |
| Potassium (mEq/L) | 4.3 (0.9) | 4.0 (0.9) | .466 |
| Creatinine (mg/dL) | 1.6 (1.3) | 1.3 (0.7) | .526 |
| Platelet (×109/L) | 183.0 (86.9) | 175.4 (83.7) | .841 |
| D-dimer | 3.4 (1.7) | 11.6 (12.9) | .148 |
| CK-MB (ng/mL) | 13.1 (14.8) | 6.5 (5.9) | .125 |
| Death in hospital | 7 (77.8) | 8 (57.1) | .400 |
Data are expressed as mean(standard deviation) or N (%); ECMO, extracorporeal membrane oxygenation; BMI, body mass index; DM, diabetes mellitus; DVT, deep vein thrombosis; TTE, trans-thoracic echocardiography; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricle; CT, computed tomography; PE, pulmonary embolism; IHCA, in-hospital cardiac arrest; OHCA, out of hospital cardiac arrest; LMWH, low molecular weight heparin; tPA, tissue plasminogen activator; ECG, electrocardiography; PEA, pulseless electrical activity; VT, ventricular tachycardia; V.fib, ventricular fibrillation; GCS, Glasgow Coma Scale; APACHE II, Acute Physiology and Chronic Health Evaluation II; CK-MB, creatine kinase MB.
Figure 2.
90 day Kaplan-Meier survival curves by treatment. Kaplan-Meier analysis showing the survival rate of stage 2 (n=14) compared with stage 1 (n=9). (Stage 1, medical therapy alone; stage 2, medical therapy with ECMO support).
Table 2.
Survivors versus non-survivors in ECMO-treated massive PE patients.
| Survivor (n=6) | Non-survivor (n=8) | P | |
|---|---|---|---|
|
| |||
| Male | 2 (33.3) | 2 (25.0) | >.999 |
| Mean age (years) | 49.8 (8.9) | 56.4 (22.5) | .516 |
| BMI | 24.2 (0.9) | 27.4 (5.5) | .152 |
| Smoking | 2 (33.3) | 2 (25.0) | >.999 |
| DM | 1 (16.7) | 1 (12.5) | >.999 |
| Hypertension | 0 (0.0) | 3 (37.5) | .209 |
| Cancer | 0 (0.0) | 0 (0.0) | - |
| Predisposing factor | |||
| Post orthopedic surgery | 1 (16.7) | 2 (25.0) | >.999 |
| Immobility | 1 (16.7) | 4 (50.0) | .301 |
| Trauma | 2 (33.3) | 0 (0.0) | .165 |
| Unknown | 1 (16.7) | 2 (25.0) | >.999 |
| TTE parameter | |||
| RVSP | 45.4 (6.4) | 41.1 (17.0) | .524 |
| TAPSE | 12.1 (3.8) | 11.7 (4.8) | .877 |
| RV dilation | 6(100.0) | 7 (87.5) | >.999 |
| Chest CT finding | |||
| Proximal PE | 6 (100.0) | 6 (75.0) | .473 |
| Cardiac arrest | |||
| IHCA | 3 (50.0) | 4 (50.0) | >.999 |
| OHCA | 2 (33.3) | 2 (25.0) | >.999 |
| Cardiogenic shock | 1 (16.7) | 2 (25.0) | >.999 |
| Treatment | |||
| Heparin | 6 (100.0) | 7 (87.5) | >.999 |
| Urokinase | 0 (0.0) | 1 (12.5) | >.999 |
| Surgical embolectomy | 1 (16.7) | 0 (0.0) | .429 |
| ECG finding | |||
| Asystole | 2 (33.3) | 2 (25.0) | >.999 |
| PEA | 3 (50.0) | 2 (25.0) | .580 |
| VT/V.fib | 0 (0.0) | 2 (25.0) | .473 |
| Sinus tachycardia | 1 (16.7) | 2 (25.0) | >.999 |
| GCS | 5.8 (3.1) | 6.6 (4.9) | .737 |
| APACHE II score | 19.0 (4.5) | 25.0 (7.6) | .111 |
| Pre-ECMO laboratory findings | |||
| pH | 7.3 (0.1) | 7.2 (0.2) | .133 |
| HCO3 (mmol/L) | 14.4 (2.6) | 11.3(2.9) | .056 |
| Potassium (mEq/L) | 3.8 (0.4) | 4.1 (1.2) | .534 |
| Creatinine (mg/dL) | 1.0 (0.1) | 1.6 (0.8) | .080 |
| Platelet (×109/L) | 197.3 (81.3) | 158.9 (86.9) | .417 |
| D-dimer | 21.3 (16.8) | 5.6 (4.0) | .106 |
| CK-MB (ng/mL) | 4.0 (2.9) | 8.4 (7.1) | .178 |
| CA-ECMO time | 22.5 (15.5) | 53.6 (68.0) | .246 |
Data are expressed as mean(standard deviation) or N (%); ECMO, extracorporeal membrane oxygenation; BMI, body mass index; DM, diabetes mellitus; DVT, deep vein thrombosis; TTE, trans-thoracic echocardiography; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricle; CT, computed tomography; PE, pulmonary embolism; IHCA, in-hospital cardiac arrest; OHCA, out of hospital cardiac arrest; LMWH, low molecular weight heparin; tPA, tissue plasminogen activator; ECG, electrocardiography; PEA, pulseless electrical activity; VT, ventricular tachycardia; V.fib, ventricular fibrillation; GCS, Glasgow Coma Scale; APACHE II, Acute Physiology and Chronic Health Evaluation II; CK-MB, creatine kinase MB.
Table 3.
Complications and outcomes of ECMO-treated massive PE patients.
| Survivor (n=6) | Non-survivor (n=8) | P | |
|---|---|---|---|
|
| |||
| Complications | |||
| Cannula site bleeding | 3 (50.0) | 1 (12.5) | .245 |
| Pulmonary hemorrhage | 0 (0.0) | 2 (25.0) | .473 |
| Ulcer bleeding | 0 (0.0) | 1 (12.5) | >.999 |
| Compartment syn. | 1 (16.7) | 0 (0.0) | .429 |
| Pseudoaneurysm | 1 (16.7) | 0 (0.0) | .429 |
| Cannula site infection | 1 (16.7) | 0 (0.0) | .429 |
| Pneumonia | 2 (33.3) | 1 (12.5) | .539 |
| AKI | 3 (50.0) | 4 (50.0) | >.999 |
| MOF | 0 (0.0) | 4 (50.0) | .085 |
| Leg ischemia | 1 (16.7) | 1 (12.5) | >.999 |
| Hypoxic brain injury | 2 (33.3) | 3 (37.5) | >.999 |
| Neuropathy | 2 (33.3) | 0 (0.0) | .165 |
| Transfusion | |||
| PRC (mL/kg) | 138.4 (121.1) | 81.0 (81.1) | .308 |
| FFP (mL/kg) | 32.2 (57.7) | 48.2 (63.0) | .635 |
| PC (mL/kg) | 143.4 (147.1) | 345.0 (451.6) | .268 |
| Ventilator duration, days | 11.5 (5.5) | 7.1 (7.8) | .267 |
| Hospital duration, days | 41.5 (15.1) | 13.9 (15.0) | .005 |
| ECMO duration, days | 7.8 (3.3) | 8.0 (8.1) | .963 |
Data are expressed as mean( standard deviation) or N (%); ECMO, extracorporeal membrane oxygenation; PE, pulmonary embolism; Syn, syndrome; AKI, acute kidney injury; MOF, multiple organ failure; PRC, packed red blood cell; FFP, fresh frozen plasma; PC, platelet concentrate.
DISCUSSION
We investigated the impact of ECMO on survival in patients with massive PE. Medical therapy was the primary treatment approach in stage 1, while anticoagulation with ECMO support was the primary treatment in stage 2. ECMO support was used as a bridge therapy. ECMO helps to decrease right ventricle overload and stabilize the hemodynamic status. Anticoagulation therapy was used as a definite therapy in stage 2 after stabilizing the status with ECMO support. The stage 2 group showed a higher survival rate at 90 days. There were no differences in baseline characteristics, ECMO complications, and transfusion between survivors and non-survivors in the stage 2 group.
Based on current guidelines, primary reperfusion therapy with systemic thrombolytic agents or surgical pulmonary embolectomy is the treatment of choice for patients with unstable PE with shock or hypotension.3 However, those recommendations may not be optimal for patients with poor prognoses in CS or CPR. In real clinical practice, a relatively large number of patients do not receive thrombolysis or embolectomy because thrombolysis takes time to be effective and surgery is not immediately available. In addition, the clinical course of massive PE can rapidly advance before surgical or catheter-directed embolectomy can be achieved.
Anticoagulation with ECMO support can be a rapid, effective option for patients with massive PE to stabilize hemodynamic instability. Many studies have reported that ECMO support is also effective for patients with life-threatening massive PE.6,11–16 However, these reports were only based on ECMO applied for managing massive PE. The reports did not investigate the impact of ECMO on survival in patients with massive PE, compared to the medical therapy alone. Hashiba et al have reported that the ECMO survival rate at discharge was 83.3%. The outcome could be attributable to lower proportions of patients with cardiac arrest, compared with our study population.11 Maggio et al have found that the survival rate of ECMO for massive PE was 13 of 21 or 62%. However, only 8 of 21 patients experienced cardiac arrest, which was a smaller percentage than in our study. Surprisingly, Maggio et al reported cases of emboli resolved with anticoagulation in 10 of 13 survivors and required no additional therapy.12 Those results are similar to the results of our study. Munakata et al reported that the 30-day mortality of ECMO for massive PE was 30%. All patients had a diagnosis of PE, which was confirmed by pulmonary angiography. Systemic or catheter-based thrombolysis was administered to all of the patients in that study.13 A French study group recently reported that VA-ECMO could be applied as a lifesaving rescue therapy for patients with high risk and massive PE. The 90-day survival was 47%, although 15 of 17 patients suffered cardiac arrest.6
Cho et al reported that surgical embolectomy is also associated with lower cardiac mortality risk than thrombolysis and is a viable option after cardiac arrest.15,17 Compared to other studies, the number of patients in our study that received surgical or catheter-directed embolectomy was small. This could have impacted the low survival rate observed in the present study. Surgical or catheter directed embolectomy should be actively considered if RV failure does not improve.
Moreover, 7 (50%) of 14 patients had a moderate to severe hemorrhage. Four of these patients had cannula site bleeding. In our study, ECMO cannulation was performed without imaging modalities because all cases were urgent. In addition, development of vascular complications is associated with worse survival outcome.18,19 That is one of the reasons for the low survival rate in our study, compared to other studies. Imaging modalities, including ultrasound and fluoroscopy can reduce vascular complications.20 Therefore, we should actively utilize imaging modalities to reduce bleeding events for ECMO cannulation. In our study, there were no differences in baseline characteristics, ECMO complications, and transfusion between survivors and non-survivors in the stage 2 group. These results are likely attributable to the small sample size.
In our study, the survival rates in patients with massive PE that were treated with anticoagulation with ECMO, compared to the medical therapy alone, were a notable outcome. However, the present study has several limitations. First, the present study was the retrospective observational study and a small sample size. Second, the period was different in stage 1 and 2. Therefore it might be possible that the improved outcome in stage 2 is associated with improved management for massive PE. Third, TAPSE scores were higher in stage 2. This situation might have affected the improved outcomes in stage 2. Finally, the differences in medical therapy between stage 1 and 2 might be a confounding factor. Because of these limitations, bias and confounding parameters could not be eliminated. Moreover, it would be difficult to perform a randomized study because the condition of patients with massive PE is often critical.
In conclusion, ECMO can provide lifesaving hemodynamic support in patients with massive PE who are too unstable to tolerate primary reperfusion therapy. Anticoagulation with ECMO support is associated with good survival rate outcomes, compared with medical therapy alone. However, further randomized, multicenter, controlled, and larger studies are needed to identify the role of ECMO in massive PE.
Footnotes
Funding: None.
CONFLICT OF INTEREST: None.
REFERENCES
- 1.Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363(3):266–74. doi: 10.1056/NEJMra0907731. [DOI] [PubMed] [Google Scholar]
- 2.Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30(5):1165–71. doi: 10.1016/s0735-1097(97)00319-7. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–69. 69a–69k. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 4.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113(4):577–82. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 5.Malekan R, Saunders PC, Yu CJ, Brown KA, Gass AL, Spielvogel D, et al. Peripheral extracorporeal membrane oxygenation: comprehensive therapy for high-risk massive pulmonary embolism. Ann Thorac Surg. 2012;94(1):104–8. doi: 10.1016/j.athoracsur.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 6.Corsi F, Lebreton G, Brechot N, Hekimian G, Nieszkowska A, Trouillet JL, et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care. 2017;21(1):76. doi: 10.1186/s13054-017-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuff HO, Zochios V, Vuylsteke A. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a systematic review. Perfusion. 2015;30(8):611–6. doi: 10.1177/0267659115583377. [DOI] [PubMed] [Google Scholar]
- 8.The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med. 1993;329(22):1615–22. doi: 10.1056/NEJM199311253292204. [DOI] [PubMed] [Google Scholar]
- 9.Mehran R, Steg PG, White HD, Rao SV. Letter by Mehran et al regarding article, “Bleeding academic research consortium consensus report: the food and drug administration perspective”. Circulation. 2012;125(10):e460. doi: 10.1161/CIRCULATIONAHA.111.065888. [DOI] [PubMed] [Google Scholar]
- 10.Lee SN, Jo MS, Yoo KD. Impact of age on extracorporeal membrane oxygenation survival of patients with cardiac failure. Clin Interv Aging. 2017;12:1347–53. doi: 10.2147/CIA.S142994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashiba K, Okuda J, Maejima N, Iwahashi N, Tsukahara K, Tahara Y, et al. Percutaneous cardiopulmonary support in pulmonary embolism with cardiac arrest. Resuscitation. 2012;83(2):183–7. doi: 10.1016/j.resuscitation.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007;62(3):570–6. doi: 10.1097/TA.0b013e318031cd0c. [DOI] [PubMed] [Google Scholar]
- 13.Munakata R, Yamamoto T, Hosokawa Y, Tokita Y, Akutsu K, Sato N, et al. Massive pulmonary embolism requiring extracorporeal life support treated with catheter-based interventions. Int Heart J. 2012;53(6):370–4. doi: 10.1536/ihj.53.370. [DOI] [PubMed] [Google Scholar]
- 14.Pavlovic G, Banfi C, Tassaux D, Peter RE, Licker MJ, Bendjelid K, et al. Peri-operative massive pulmonary embolism management: is veno-arterial ECMO a therapeutic option? Acta Anaesthesiol Scand. 2014;58(10):1280–6. doi: 10.1111/aas.12411. [DOI] [PubMed] [Google Scholar]
- 15.Cho YH, Kim WS, Sung K, Jeong DS, Lee YT, Park PW, et al. Management of cardiac arrest caused by acute massive pulmonary thromboembolism: importance of percutaneous cardiopulmonary support. Asaio j. 2014;60(3):280–3. doi: 10.1097/MAT.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 16.Dolmatova EV, Moazzami K, Cocke TP, Elmann E, Vaidya P, Ng AF, et al. Extracorporeal Membrane Oxygenation in Massive Pulmonary Embolism. Heart Lung. 2017;46(2):106–9. doi: 10.1016/j.hrtlng.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Cho YH, Sung K, Kim WS, Jeong DS, Lee YT, Park PW, et al. Management of acute massive pulmonary embolism: Is surgical embolectomy inferior to thrombolysis? Int J Cardiol. 2016;203:579–83. doi: 10.1016/j.ijcard.2015.10.223. [DOI] [PubMed] [Google Scholar]
- 18.Aziz F, Brehm CE, El-Banyosy A, Han DC, Atnip RG, Reed AB. Arterial complications in patients undergoing extracorporeal membrane oxygenation via femoral cannulation. Ann Vasc Surg. 2014;28(1):178–83. doi: 10.1016/j.avsg.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Bisdas T, Beutel G, Warnecke G, Hoeper MM, Kuehn C, Haverich A, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg. 2011;92(2):626–31. doi: 10.1016/j.athoracsur.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Kashiura M, Sugiyama K, Tanabe T, Akashi A, Hamabe Y. Effect of ultrasonography and fluoroscopic guidance on the incidence of complications of cannulation in extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a retrospective observational study. BMC Anesthesiol. 2017;17(1):4. doi: 10.1186/s12871-016-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]