Abstract
BACKGROUND AND OBJECTIVES
The exact antenatal prevalence of congenital anomalies in Saudi society is unknown. Early antenatal diagnosis of congenital anomalies is crucial for early counselling, intervention and possible fetal therapy. The objective of this study was to evaluate the antenatal frequency of major congenital anomalies and malformation patterns in our hospital population and to evaluate the outcome and perinatal mortality rates for major congenital anomalies.
PATIENTS AND METHODS
This was a prospective study of the antenatal diagnosis of major fetal congenital anomalies conducted in the Ultrasound Department of the Women’s Specialized Hospital at King Fahad Medical City from March 2005 to February 2007. During this period, 16 639 obstetrical ultrasound examinations were performed for 7762 patients and 5379 babies delivered in our institution.
RESULTS
We diagnosed 217 cases of fetal anomalies. The antenatal prevalence of congenital anomalies was 27.96 per 1000. The median maternal age at diagnosis was 27.5 years. The median gestational age at diagnosis was 31 weeks. Genitourinary and cranial anomalies were the commonest; for 186 patients delivered in our institution, the birth prevalence was 34.57 per 1000 births. The median gestational age at delivery was 38 weeks. The perinatal mortality rate was 34.9% (65/186), including all cases of intrauterine fetal and neonatal deaths.
CONCLUSION
The prevalence of major congenital anomalies in our population appears to be similar to international figures. Major congenital anomalies are a major cause of perinatal mortality.
A congenital anomaly is an abnormality of structure, function or body metabolism that is present at birth and results in physical or mental disability, or is fatal. Each year, eight million children are born worldwide with congenital anomalies, of which 3.3 million die before the age of five; 3.2 million of the survivors may be mentally and/or physically disabled.1 The role of environmental pollutants, drugs, and infectious agents in the causation of congenital defects is a major global concern.2 However, the underlying causes for most congenital anomalies remain obscure and multifactorial inheritance is believed to be the underlying etiology of most of the common congenital anomalies. It has been estimated that about 15% to 25% of congenital anomalies are due to recognized genetic conditions, 8% to 12% to environmental factors, and 20% to 25% to multifactorial inheritance. The majority of congenital anomalies, 40% to 60%, are unexplained.3
The registration and monitoring of the type and number of congenital anomalies is vital to identify possible clusters and trends, and to address concerns about recognized environmental teratogens. Early prenatal diagnosis of congenital anomalies is crucial for early counselling, intervention and possible fetal therapy.
The prevalence of birth defects is comparable all over the world; about 3% in the United States,4 2.5% in India,5 and 2% to 3% in the United Kingdom.6 The most prevalent conditions include congenital heart defects,7 orofacial clefts, Down syndrome,8 and neural tube defects. 9 By routine prenatal ultrasonography during the antenatal period, approximately half of the diagnosed fetal malformations were abnormalities of the urinary tract.10 Congenital anomalies in the United States are a leading cause of infant morbidity and mortality, as well as fetal mortality.4 In India, congenital anomalies are responsible for 15% of perinatal mortality and for 10% to 15% of neonatal deaths.5 In the United Kingdom congenital anomalies account for 21% of perinatal and infant deaths.6
In Saudi Arabia, a recent study estimated the incidence of major and minor congenital malformations among live born infants to be 27.1/1000 live births and the highest incidence was for cardiovascular (7.1/1000 live births), and musculoskeletal/limb malformations (4.1/1000 live births).11 Another study found the incidence of congenital abnormalities to be 23/1000 live births.12 The incidence for congenital malformation of the gastrointestinal tract was 1.3 per 1000 live births,13 for neural tube defects (NTD) 1.9/1000 live births,14 and for Down’s syndrome 1.8 per 1000 live births.15 The exact antenatal prevalence of major congenital anomalies in Saudi Arabia and the prognosis of those anomalies have not been reported. To our knowledge, this is the first study reporting on the antenatal prevalence of major congenital anomalies in Saudi Arabia. The primary outcome of interest in our study was to evaluate the antenatal prevalence of major congenital anomalies in our hospital population. The secondary outcomes were to determine the types of malformations and the perinatal mortality rate.
PATIENTS AND METHODS
This was a hospital-based prospective study conducted at the Women’s Specialized Hospital in King Fahad Medical City (WSH-KFMC). WSH-KFMC is a tertiary care referral center in Riyadh, Saudi Arabia. The study was conducted over a 2-year period, from the first of March 2005 to the end of February 2007.
All pregnant women followed in WSH-KFMC had a routine anatomy ultrasound examination between 18 and 20 weeks of gestation or later at a scheduled visit. All women with pregnancies complicated by major congenital anomalies having either an antenatal diagnosis at or referred to WSH-KFMC with congenital anomalies were included in this study. Nearly all of the patients had antenatal follow up with the Fetal Developmental Clinic (FDC). FDC, which is staffed exclusively by maternal-fetal medicine (MFM) specialists, is a specialized clinic created to follow only women with an antenatal diagnosis of major congenital anomalies. All the patients were scanned, diagnosed, and counseled by MFM specialists. The majority of the patients had two or more ultrasound examinations. All ultrasound scans were performed using Voluson 730 expert series, BT 2005 (GE Kretz, Austria). A data sheet created for her at the time of diagnosis was used to record all general maternal information, fetal congenital anomalies in detail, delivery details, neonatal outcomes, and recommendations.
Data collection and follow up were performed in the ultrasound unit, the FDC, the maternity wards, and the labor ward. Information was also collected from neonatal intensive care unit (NICU), cytogenetic laboratories, departments of medical genetics and birth certificates. A miscarry was recorded if a pregnancy loss occurred before 22 weeks of gestation. Stillbirths were registered at a gestational age of 22 weeks or older. A neonatal death was recorded up to 28 days of infant life. Perinatal mortality was defined as fetal or neonatal loss if it occurred from 22 weeks of gestation to the 28th day of infant life. Parental consanguinity was defined as first or second cousin marriage.
Major congenital anomalies were divided according to the system involved (cranial, neural tube defect [NTD], face and neck, thoracic, cardiac, ventral wall defects, abdominal, genitourinary system [GUS], or skeletal). The fetuses were diagnosed as having either isolated anomalies (only one system involved) or complex anomalies (two or more systems involved). Minor anomalies or soft markers (pyelectasis, echogenic bowel, choroid plexus cyst, echogenic intracardiac focus, and short femur) were excluded from the study.
After initial diagnosis, cases were presented at multidisciplinary perinatal committee meetings for management planning, and were again presented after delivery for final diagnosis and future planning. The newborns were followed in the NICU or the nursery and the diagnoses were confirmed in all the cases by clinical examination and imaging studies.
During the 24-month study period, 16 639 ultrasound examinations were performed for 7762 obstetrics patients and for a total of 5379 patients delivered in WSH-KFMC. The remainder of the patients were either referred back to deliver in their referring centers after management or lost follow up. Antenatal prevalence was calculated from the total number of obstetrics patients, while birth prevalence and the perinatal mortality rates were calculated from the number of delivered patients only.
RESULTS
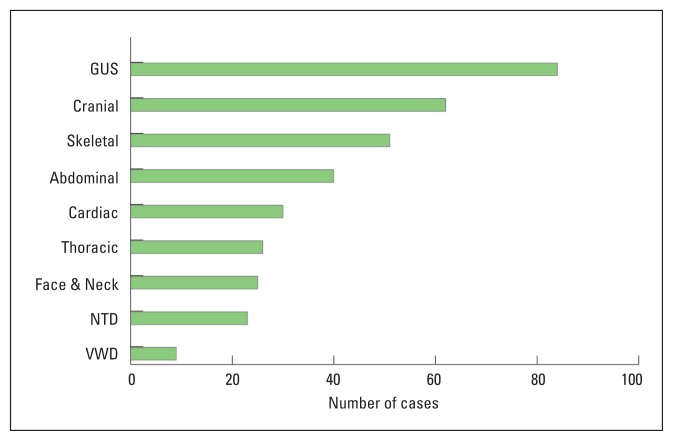
217 cases of major fetal congenital anomalies were diagnosed. The antenatal prevalence of major congenital anomalies was 27.96 per 1000 pregnancies. GUS and cranial anomalies were the most common (Figure 1). Of the 217 patients, 71 (32.7%) had fetuses with complex anomalies and 146 (67.3%) had fetuses with isolated anomalies. Maternal characteristics by bodily system are shown in Table 1. Parent consanguinity was reported in 78 patients (35.9%). Thirty-five patients (16.1%) reported a history of previously affected children or other family members with similar anomalies. Six of the women had a miscarriage, 25 were lost to follow up, and 186 were delivered in WSH-KFMC. The birth prevalence of congenital anomalies was 34.57 per 1000 births. Fetal characteristics by bodily system are shown in Table 2. The newborns were 104 (55.9%) males, 78 (41.9%) females, and 4 (2.2%) were of an unrecognized sex. Of the 186 delivered, 129 (69.35%) newborns had isolated anomalies and 57 (30.7%) newborns had complex anomalies. Only 39 genetic studies were performed during the antenatal period (amniocentesis or cordocentesis), which revealed 8 cases of aneuploidy.
Figure 1.
The number of cases of congenital anomalies by bodily system. (GUS: genitourinary system, NTD: neutral tube defects, VWD: ventral wall defects).
Table 1.
Maternal characteristics by bodily system.
| System involvement | Number (%) | Median maternal age | Median parity | Consanguinity (%) | Family history (%) |
|---|---|---|---|---|---|
| Cranial | 62 (28.6%) | 28 | 2 | 24 (38.7%) | 11 (17.7%) |
| Neural tube defects | 23 (10.6%) | 29 | 3 | 5 (21.7%) | 2 (8.7%) |
| Face | 25 (11.5%) | 28 | 2 | 6 (24%) | 4 (16%) |
| Thoracic | 26 (12%) | 26 | 2 | 11 (42.3%) | 4 (14.3%) |
| Cardiac | 30 (13.8%) | 28 | 2 | 12 (40%) | 3 (10%) |
| Ventral wall defects | 9 (4.1%) | 35 | 3 | 1 (11.1%) | 1 (11.1%) |
| Abdominal | 40 (18.4%) | 28 | 2 | 15 (37.5%) | 6 (15%) |
| Genitourinary system | 84 (38.6%) | 27.5 | 1.5 | 32 (38.1%) | 16 (19%) |
| Skeletal | 51 (23.5%) | 26 | 2 | 14 (27.4%) | 14 (27.5%) |
| Total | 217 | 27.5 | 2 | 78/217 (35.9%) | 35 (16.1%) |
Table 2.
Fetal characteristics by bodily system.
| System involvement | Number (%) | Prevalence per 1000 pregnancies | Median gestational age at diagnosis (weeks) | Median gestational age at delivery (weeks) | Number of complex anomalies (%) | Mortality rate (%) |
|---|---|---|---|---|---|---|
| Cranial | 62 (28.6%) | 7.98 | 33 | 38 | 26 (41.9 %) | 13/56 (23.2 %) |
| Neural tube defects | 23 (10.6%) | 2.96 | 26 | 31 | 14 (60.9 %) | 8/15 (53.3 %) |
| Face | 25 (11.5%) | 3.22 | 27 | 38 | 14 (56 %) | 7/20 (35 %) |
| Thoracic | 26 (12%) | 3.35 | 25.5 | 32 | 21 (80.8 %) | 16/21 (76.2 %) |
| Cardiac | 30 (13.8%) | 3.87 | 31 | 36 | 22 (73.3 %) | 11/25 (44 %) |
| Ventral wall defects | 9 (4.1%) | 1.16 | 29 | 35 | 6 (66.7 %) | 5/7 (71.4 %) |
| Abdominal | 40 (18.4%) | 5.15 | 30.5 | 37 | 28 (70 %) | 21/34 (61.8 %) |
| Genitourinary system | 84 (38.6%) | 10.82 | 31 | 38 | 31 (36.9 %) | 27/73 (37 %) |
| Skeletal | 51 (23.5%) | 6.57 | 25 | 35 | 42 (82.3 %) | 29/43 (67.4 %) |
| Total | 217 | 27.96 | 31 | 38 | 71 (32.7 %) |
65/186 (34.9%) |
The perinatal mortality rate was 34.9% (65 stillborn and neonatal deaths out of 186 deliveries). The perinatal mortality rate with complex anomalies was 61.4% (35 out of 57 patients) and with isolated anomalies was 23.3% (30 of 129 patients). Perinatal mortality was strongly related to the gestational age at delivery; 14 patients delivered at or before 30 weeks of gestation had a 100% perinatal mortality rate. Survival rates by gestational age at delivery are shown in Figure 2.
Figure 2.
Perinatal survival rates per gestational age at delivery.
Cranial anomalies were diagnosed in 62 cases. The commonest anomalies were hydrocephalus and ventriculomegaly in 21 cases and the Dandy-Walker syndrome in 5 cases. The GUS was the most commonly involved system. The commonest anomalies were hydronephrosis in 23 cases, multicystic kidneys in 12 cases, and bilateral renal agenesis in 9 cases. Twentythree patients were diagnosed with renal anomalies in association with anhydramnios; 20 of them delivered in WSH-KFMC, with the median gestational age at delivery of 35 weeks, with a 100% (20 of 20) perinatal mortality rate. Fifteen cases were isolated renal anomalies. The male to female ratio was 1:0.42. Of 9 cases with bilateral renal agenesis, 8 were males to one female.
NTD was diagnosed in 23 cases. The diagnoses were spina bifida in 12 cases, anencephaly in 6 cases, and encephalocele in 5 cases. NTDs were more common in female fetuses, with a male-to-female ratio of 1:2.5. Skeletal anomalies were diagnosed in 51 fetuses. The commonest anomaly was clubfoot in 14 fetuses. Skeletal anomalies were associated with other systems in 82.3% of the cases, which had a high perinatal mortality rate of 67.4% (Table 2). The male-to-female ratio was 1:1.2.
We diagnosed 18 cases of hydrops fetalis; in 6 fetuses hydrops was associated with other major anomalies. Twelve patients were diagnosed as non-immune hydrops. Of those 12 patients, 6 (50%) gave a history of consanguinity and 4 patients (33.3%) had previously affected children. Three patients were lost to follow up and of the remaining patients, 13 delivered live or stillborn babies and 2 had miscarriages. The perinatal mortality rate was 77% (10 of 13).
DISCUSSION
This is the first study in Saudi Arabia to examine the antenatal prevalence of major congenital anomalies and report perinatal mortality rates. Only patients booked for delivery in WSH-KFMC were included in this study. Many women who were not booked and delivered stillborn or fetuses with a postnatal diagnosis of possible birth defects were not included because the diagnosis and the abnormalities were not confirmed in the postnatal examination in many stillborn fetuses. If those patients had an antenatal diagnosis, the prevalence and the mortality rates in this study would be different.
The late gestational age at diagnoses is a major factor affecting proper antenatal diagnosis and outcome. Only 39 patients (17.9%) had genetic studies. The lack of genetic studies can be explained by the late gestational age at diagnosis due to late booking or late referral, and many patients refuse invasive testing. The limited number of abortions is due to the late antenatal diagnosis and to the fact that termination of pregnancy is not legal in Saudi Arabia. Thus, most women carry their pregnancies to term even with the diagnosis of lethal anomalies such as anencephaly or bilateral renal agenesis.
Cardiac anomalies are one of the most common anomalies worldwide and at the same time the most commonly missed anomalies in antenatal ultrasound examination. This was the case in our study, but we believe many cases were missed. Because of the unavailability of a pediatric cardiac surgeon at our hospital during the study period, cases of cardiac anomalies, diagnosed by a detailed ultrasound scan and fetal-echocardiography in WSH-KFMC, that needed cardiac surgery were referred to centers where pediatric cardiac surgery services were available. The perinatal mortality rate was higher with major congenital anomalies, and was strongly related to gestational age at delivery and the complexity of the anomalies. The association of GUS anomalies and anhydramnios carries a very poor prognosis.
Our study was hospital-based and does not represent the actual prevalence and outcome in Saudi Arabia. This data should stimulate future research and collaboration for more accurate and complete reporting of major congenital anomalies in Saudi Arabia. A multicenter study can more accurately assess the prevalence of various congenital anomalies. We suggest establishing a national registry of birth defects to monitor the epidemiology (occurrence, etiology, morbidity, mortality, clusters, etc) of birth defects. In conclusion, the prevalence of major congenital anomalies in the WSH-KFMC population appears to be similar to international data. Major congenital anomalies are a major cause of perinatal mortality.
REFERENCES
- 1.March of Dimes Resource Center. Birth Defects. 1998. Available from: www.modimes.org.
- 2.Taffel S. Congenital anomalies and birth injuries among live births: United States, 1973--74. Vital Health Stat 21. 1978 Nov;(31):i–vi. 1–58. [PubMed] [Google Scholar]
- 3.Nelson K, Holmes LB. Malformations due to presumed spontaneous mutations in newborn infants. N Eng J Med. 1989 Jan 5;320(1):19–23. doi: 10.1056/NEJM198901053200104. [DOI] [PubMed] [Google Scholar]
- 4.Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, Devine O, Petrini J, Ramadhani TA, Hobbs CA, Kirby RS. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth defects Res A Clin Mol Teratol. 2006 Nov;76(11):747–756. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- 5.Patel ZM, Adhia RA. Birth defects surveillance study, year 2005. Indian J Pediatr. 2005 Jun;72(6):489–491. doi: 10.1007/BF02724426. [DOI] [PubMed] [Google Scholar]
- 6.Boyd PA, Armstrong B, Dolk H, Botting B, Pattenden S, Abramsky L, Rankin J, Vrijheid M, Wellesley D. Congenital anomaly surveillance in England-ascertainment deficiencies in the national system. BMJ. 2005 Jan 1;330(7481):27–31. doi: 10.1136/bmj.38300.665301.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin AE, Herring AH, Amstutz KS, Westgate MN, Lacro RV, Al-Jufan M, Ryan L, Holmes LB. Cardiovascular Malformations: changes in prevalence and birth status, 1972–1990. Am J Med Genet. 1999 May 21;84(2):102–110. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Improved national prevalence estimates for 18 selected major birth defects-United States, 1999–2001. MMWR Morb Mortal Wkly Rep. 2006 Jan 6;54(51):1301–1305. [PubMed] [Google Scholar]
- 9.Wen SW, Liu S, Joseph KS, Rouleau J, Allen A. Patterns of infant mortality caused by major congenital anomalies. Teratology. 2000 May;6(5):342–346. doi: 10.1002/(SICI)1096-9926(200005)61:5<342::AID-TERA5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 10.Helin I, Per-Hakan P. Prenatal diagnosis of urinary tract abnormalities by ultrasound. Pediatr. 1986 Nov;78(5):879–883. [PubMed] [Google Scholar]
- 11.Fida NM, Al-Aama J, Nichols W, Alqahtani M. A prospective study of congenital malformations among live born neonates at a University Hospital in Western Saudi Arabia. Saudi Med J. 2007 Sep;28(9):1367–73. [PubMed] [Google Scholar]
- 12.Khorshid EA, Dokhan AL, Turkistani AF, Shadi SM, Hassab MH. Five year experience in prenatal ultrasound diagnosis of esophageal atresia in Saudi Arabia. Ann Saudi Med. 2003 May-Jul;23(3–4):132–4. doi: 10.5144/0256-4947.2003.132. [DOI] [PubMed] [Google Scholar]
- 13.Asindi AA, Al-Daama SA, Zayed MS, Fatinni YA. Congenital malformation of the gastrointestinal tract in Aseer region, Saudi Arabia. Saudi Med J. 2002 Sep;23(9):1078–82. [PubMed] [Google Scholar]
- 14.Safdar OY, Al-Dabbagh AA, Abuekieneen WA, Kari JA. Decline in the incidence of neural tube defects after the national fortification of flour (1997–2005) Saudi Med J. 2007 Aug;28(8):1227–9. [PubMed] [Google Scholar]
- 15.Niazi MA, Al-Mazyad AS, Al-Husain MA, Al-Mofada SM, Al-Zamil FA, Khashoggi TY, Al-Eissa YA. Down’s syndrome in Saudi Arabia: incidence and cytogenetics. Hum Hered. 1995 Mar-Apr;45(2):65–9. doi: 10.1159/000154261. [DOI] [PubMed] [Google Scholar]