The effective control of human immunodeficiency virus type 1 (HIV-1) using antiretroviral therapy (ART) results in prolonged survival.1 However, because of high rates of viral replication, along with other factors, resistance to antiretroviral drugs has become an important reason for therapy failure and progression to acquired immunodeficiency syndrome (AIDS).2 The prevalence of drug-resistant strains is variable depending on location and use of ART; it is very high in treatment-experienced patients.3,4 Even in treatment-naïve patients, resistance rates can be up to 19%.5 Alternate therapies for extensively drug-resistant HIV are limited to recently introduced antiretroviral agents: darunavir, etravirine, maraviroc, and raltegravir. We report on a patient with multidrug-resistant HIV who had a remarkable response to a snake venom preparation. The patient signed a written informed consent to publish his case.
CASE
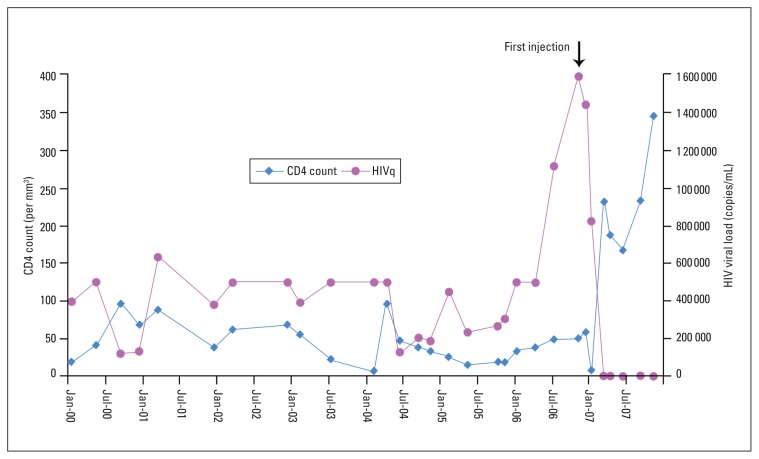
The patient was a hemophiliac male born in 1978 who was confirmed to be asymptomatic HIV positive in 1987. Zidovudine was started in 1990 for a CD4+ T-lymphocyte (CD4) count of less than 200/mm3. In 1997, the CD4 count was 24/mm3 and didanosine was added to his ART. In 2000, he was transferred to our facility. The initial viral load at that time was more than 500 000 copies/mL (Bayer Quantiplex bDNA System, detection range 50–500 000 copies/mL) and the CD4 count was 19/mm3. Highly active antiretroviral combination therapy, including a protease inhibitor, was started. Because of a persistently low CD4 count and high viral load, the ART was changed based on resistance genotyping (Roche Molecular Systems, Inc.) in 2002. Genotyping-based salvage therapy including enfuvirtide was started in 2004. However, the CD4 count continued to be low and viral load was more than 500 000 copies/mL. In March 2006, a new regimen of didanosine, tenofovir, and efaverinz was started based on genotyping. Nevertheless, in November 2006, viral load remained high at 1 580 000 copies/mL (Abbott RealTime HIV assay, range 40–10 000 000 copies/mL). Repeat genotyping revealed resistance to all agents except tenofovir. The patient decided to take a preparation with the trade name Samayz, given to him by a friend. Samayz (formerly known as Bioven, and later Autoimmune-5; Equimune Research Corp., Coral Springs, FL, USA) is described in advertising material as “a combination of various components derived from snake venom”. He took 0.1 mL subcutaneous injection daily for one month. Preinjection viral load was 1 580 000 copies/mL and the CD4 count was 52/mm3. ART was unchanged. In March 2007, viral load was 3279 copies/mL and the CD4 count increased to 232/mm3. In April, efaverinz was changed to lopinavir/ritonavir, and didanosine was changed to zidovudine due to adverse effects. The virus remained resistant to all these antiretroviral agents. He self-repeated the venom preparation course in April and July 2007. In November 2007, the viral load was 1981 copies/mL, and the CD4 count was 345/mm3 (Figure 1). Repeat genotyping revealed the same resistance pattern. The specific mutations found were L74V, L100I, K103N, M184V, T215Y, K103N, I13V, K20R, D30N, L33I, M36I, I54L/V, I54V, L63P, A71T, N88D, L90M (Trugene HIV-1, Bayer HealthCare LLC). He continued to take zidovudine, tenofovir, and lopinavir/ritonavir as ART. The patient did not report any significant adverse effects related to the injections.
Figure 1.
HIV viral load and CD4+ T-lymphocyte count over 8 years.
DISCUSSION
The in vitro anti-HIV activity of snake venom has been reported.6,7 We believe this is the first case of an apparent in vivo effect of a snake venom preparation in controlling HIV. The peak viral load assay was not at a time of intercurrent illness. In fact, the preceding viral loads of 500 000 copies/mL were the maximum by the assay available at that time. When better assay tools became available, the viral load was more than a million. Although the patient was still using ART and genotyping revealed multidrug resistance, the dramatic drop in viral load and increase in CD4 count occurred only after use of the venom preparation. The evident change in viral load and CD4 count cannot be explained by the ART alone as the patient has been on the same regimen. Compliance with ART was suboptimal initially and probably improved after taking the venom preparation. Possibly, the interaction between the venom preparation and current ART worked to his advantage.8 Another possible explanation is the high mutation rate of HIV, which may render the virus less fit, either through susceptibility to antiretroviral agents or an intrinsic defect.9,10 We believe that the response was related to the injectable snake venom preparation in the background of maintenance ART that the virus was resistant to on genotyping. Further research to confirm the effect and the exact mechanism of antiretroviral activity of the snake venom preparation is required. Phase II and III clinical studies using the preparation would hopefully establish its role as an adjuvant salvage therapy for multidrug-resistant HIV.
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.DeGruttola V, Dix L, D’Aquila R, et al. The relation between baseline HIV drug resistance and response to antiretroviral therapy: re-analysis of retrospective and prospective studies using a standardized data analysis plan. Antivir Ther. 2000;5:41–8. doi: 10.1177/135965350000500112. [DOI] [PubMed] [Google Scholar]
- 3.Richman DD. Antiviral drug resistance. Antiviral Research. 2006;71:117–21. doi: 10.1016/j.antiviral.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Sen S, Tripathy SP, Patil AA, Chimanpure VM, Paranjape RS. High prevalence of human immunodeficiency virus type 1 drug resistance mutations in antiretroviral treatment-experienced patients from Pune, India. AIDS Res Hum Retroviruses. 2007;23:1303–8. doi: 10.1089/aid.2007.0090. [DOI] [PubMed] [Google Scholar]
- 5.Cane P, Chrystie I, Dunn D, et al. Time trends in primary resistance to HIV drugs in the United Kingdom: multicentre observational study. BMJ. 2005;331:1368. doi: 10.1136/bmj.38665.534595.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenard D, Lambeau G, Valentin E, Lefebvre JC, Lazdunski M, Doglio A. Secreted phospholipases A(2), a new class of HIV inhibitors that block virus entry into host cells. J Clin Invest. 1999;104:611–8. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YJ, Wang JH, Lee WH, et al. Molecular characterization of Trimeresurus stejnegeri venom L-amino acid oxidase with potential anti-HIV activity. Biochem Biophys Res Commun. 2003;309:598–604. doi: 10.1016/j.bbrc.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Sillero MA, Madrid O, Zaera E, Sillero A. 2′,3′-dideoxynucleoside triphosphates (ddNTP) and di-2′,3′-dideoxynucleoside tetraphosphates (ddNp4ddN) behave differently to the corresponding NTP and Np4N counterparts as substrates of firefly luciferase, dinucleoside tetraphosphatase and phosphodiesterases. Biochim Biophys Acta. 1997;1334:191–9. doi: 10.1016/s0304-4165(96)00092-x. [DOI] [PubMed] [Google Scholar]
- 9.Hanna Z, Priceputu E, Hu C, Vincent P, Jolicoeur P. HIV-1 Nef mutations abrogating downregulation of CD4 affect other Nef functions and show reduced pathogenicity in transgenic mice. Virology. 2006;346:40–52. doi: 10.1016/j.virol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Tang S, Murakami T, Cheng N, Steven AC, Freed EO, Levin JG. Human immunodeficiency virus type 1 N-terminal capsid mutants containing cores with abnormally high levels of capsid protein and virtually no reverse transcriptase. J Virology. 2003;77:12592–602. doi: 10.1128/JVI.77.23.12592-12602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]