Abstract
BACKGROUND
Improper prescription of antibiotics for treatment of acute pharyngitis predisposes to emergence of a carrier state and antibiotic-resistant strains of group A streptococci (GAS). We sought to identify the frequency and antimicrobial susceptibility patterns of group A streptococci among Egyptian children with acute pharyngitis compared with asymptomatic children.
DESIGN AND SETTING
Case-control study conducted from September 2013 to August 2014 at a pediatric outpatient clinic in Egypt.
PATIENTS AND METHODS
Throat swabs were collected from children with acute pharyngitis and from asymptomatic children. We evaluated the accuracy of McIsaac scores and the rapid antigen detection test (RADT) for diagnosis of GAS pharyngitis with throat culture as a reference test. Antimicrobial susceptibility testing of GAS isolates was done by the disc diffusion method.
RESULTS
Of 142 children with acute pharyngitis (cases) and 300 asymptomatic children (controls) (age range, 4–16 years), GAS pharyngitis was diagnosed in 60/142 children (42.2%); 48/300 (16%) were found to be carriers. All GAS isolates in the case group were sensitive to penicillin; however, an MIC90 (0.12 μg/mL) for penicillin is high and an alarming sign. The resistance rate to macrolides was 70% with the cMLSB phenotype in 65.1%. The sensitivities and specificities were 78.3% and 73.2% for McIsaac score of ≥4 and 81.1% and 93.9% for RADT, respectively. GAS isolates in the control group were 100% sensitive to penicillin, while 12.5% and 37.5% were resistant to macrolides and tetracycline, respectively.
CONCLUSION
An increased MIC90 for GAS isolates to penicillin is an alarming sign. A high frequency of resistance to macrolides was also observed.
Group A streptococcus (GAS) is the most common bacterial cause of acute pharyngitis, which is a great burden on school-aged children.1 Beta-hemolytic streptococci can colonize the throat of healthy carriers, serving as a reservoir for pathogen transmission.2 Antibiotic-resistant GAS isolated from asymptomatic school children are a public health concern. When screened and appropriately treated with antibiotics, carriers can be prevented from spreading GAS infections in the school environment and the community.3 Penicillin is still the most commonly used drug for treatment of acute streptococcal pharyngitis and tonsillitis. 2 However, use of antibiotics without prescription increases the rate of strains of GAS resistant to commonly used antibiotics, and fosters emergence of a carrier state.2,3 Clinical scores such as the Modified McIsaac score can be used to help clinicians in the diagnosis of GAS pharyngotonsillitis and determine which patients should undergo diagnostic testing.4 Rapid GAS antigen detection tests (RADTs) can provide results in 10–15 min, and most have a specificity of 90% and sensitivity of >90%.5
Knowledge of the antibiotic resistance patterns in a certain geographical region is important in the choice of the most effective antibiotic for treatment of GAS infections.2 This study was designed to estimate the frequency of GAS acute pharyngotonsillitis, as well as the carrier state among asymptomatic healthy Egyptian children, to identify the antimicrobial susceptibility pattern of GAS, and to evaluate the effectiveness of the McIsaac score and the rapid antigen detection test for patient diagnosis.
PATIENTS AND METHODS
This case-control study was conducted at the Pediatric Outpatient Clinic of Ain Shams University, Children’s Hospital, Cairo, Egypt, during the period from September 2013 to August 2014. Children ranging in age from range 4–16 years who attended the outpatient clinic and complained of fever, sore throat and unequivocal erythema of the pharynx were classified as cases; asymptomatic healthy children, matched for age and gender, with no history of recurrent pharyngitis were classified as controls. Written informed consent was obtained from parents or legal guardians after approval of the study by the Local Ethical Committee, Ain Shams University. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki 1975.
All children were subjected to a full history taking (age, sex, number of days ill before the visit, sore throat, runny nose, cough, swollen neck glands, general aches, rash, gastrointestinal discomfort, history of a temperature greater than 38/history of recurrent attacks of tonsillitis and multiple treatment courses (>7 episodes in the past year, >5 episodes per year for 2 years, or >3 episodes per year for 3 years).6 A complete physical examination (red throat, tonsillar swelling, tonsillar exudates, tender anterior cervical lymph node, a rash typical of scarlet fever, abnormal tympanic membrane, and lung findings), and clinical data were used to calculate the McIsaac score 7 for each patient. The McIsaac score comprises the following criteria: temperature >38°C; absence of cough; tender anterior cervical adenopathy; tonsillar swelling or exudate; age 3–14 years scored +1 each, age 15–44 years scored 0, age 3 45 years scored −1. Exclusion criteria were previous oral antibiotics within the last two weeks, intramuscular benzathine penicillin in the last month, history of rheumatic heart disease, poststreptococcal glomerulonephritis, immunosuppression or obvious alternate diagnosis (such as herpes gingivostomatitis).
Throat sample collection and testing
A Dacron swab collection-transport system (COPAN Diagnostics Inc., Corona, CA) was used for all sampling. Double samples were collected from the patient’s posterior pharynx and tonsillar surfaces as recommended by the Infectious Disease Society of America.8 Immediately after sample collection, one swab from each patient was used for rapid antigen detection testing (Signify Strep A; Abbott Laboratories, Abbott Park). The remaining swab was transported to the Clinical Microbiology Laboratory within 2 hours. Culture of the swab was performed on blood agar with an SXT disc (Oxoid Microbiology Products, http://www.oxoid.com/), and the plate was incubated in 5% CO2 at 35°C for 48 h.
Selection and identification of GAS isolates
Beta-hemolytic streptococci were phenotypically identified by standard methods: beta-hemolysis on blood agar, colony morphology, Gram stain, catalase reaction, and susceptibility to 0.04U bacitracin. GAS identification was performed with a positive latex agglutination test containing group A specific antisera (Slidex, Bio Mrieux, Paris, France, http://www.biomerieux.fr/).8
Antibiotic susceptibility testing
Susceptibility to penicillin G (10 units), erythromycin (15 μg), clindamycin (2 μg), tetracycline (30 μg), ceftriaxone (30 μg), clarithromycin (15 μg), azithromycin (15 μg) and vancomycin (30 μg) (Microbiology Products, http://www.oxoid.com/) was tested by the disc diffusion method according to CLSI recommendations.9
Minimum inhibitory concentrations (MICs) for penicillin and erythromycin were determined by a reference E test method (OxoidMicrobiology Products, http://www.oxoid.com/) in Mueller-Hinton agar supplemented with 10% RBC(Microbiology Products, http://www.oxoid.com/).9 The MIC interpretive criteria was as follows: for penicillin 20.12 μg/mL (sensitive), for erythromycin 20.25 μg/mL (sensitive), for both 0.5 (intermediate), and 31 (resistant).
Phenotypic detection of macrolide resistance
The resistance phenotypes of erythromycin-resistant (intermediate and resistant) isolates were determined by the double-disk test with erythromycin (15 μg) and clindamycin (2 μg) disks to detect inducible macrolide-lincosamide-streptogramin B (iMLSB) resistance phenotype. The MLSB phenotype was defined as resistance to both erythromycin and clindamycin, the iMLS phenotype was defined as resistance to erythromycin, susceptibility to clindamycin, and a positive D test; the M phenotype was defined as resistance to erythromycin, susceptibility to clindamycin, and a negative D test.10
Statistical methods
Data were analysed by SPSS version 20. Data are presented as numbers and percentages. Comparison between qualitative data are by the chi-square test. The Fisher exact test was used instead of the chi-square test when the expected count in any cell was less than 5. The receiver operating characteristic curve (ROC) was used to assess the sensitivity and specificity of the McIsaac score and RADT with their negative and positive predictive values. The confidence interval was set to 95%, and P value <.05 was considered significant.
RESULTS
Of 450 children enrolled in this study, 8 children among in the case group were excluded because they had been taking antibiotics in the previous two weeks. The case group included 142 children; 68 boys and 74 girls with a mean age 9.7 (3.5) years. The control group included 300 children; 120 boys and 180 girls with a mean age 10.1 (3.2) years. Table 1 shows the demographic and clinical characteristics. There was no statistically significant difference between both groups in mean age (P=.23) or sex distribution (P=.12). The McIsaac score for patients in the case group showed that 48.6% (69/142) had a score 34, 30.3% (43/142) had score 3, 15.5% (22/142) had score 2 and 5.6% (8/142) had score 1. Among children in the case group, 38% (54/142) had a positive RADT; 81.1% (44/54) of children with positive RADT had a McIsaac score 34 (Table 2). Throat culture of cases found that 67 (47.2%) yielded no growth of pathogenic bacteria, 15 (10.6%) yielded growth of β hemolytic streptococci identified as non-group A streptococci (they were bacitracin and sutrim resistant) mostly group C (Streptococcus dysagalatiae), and 60 (42.2%) showed growth of β-hemolytic streptococci confirmed to be GAS on the basis of a battery of tests. They exhibited beta-hemolysis on blood agar. Gram stain showed gram positive cocci arranged in chains. They were catalase and latex agglutination test positive and bile esculin negative. They were sensitive to bacitracin and resistant to sutrim. A high percentage of children (78.3%, 47/60) with positive throat culture had a McIsaac score of 34 (Table 2).
Table 1.
Demographic and clinical characteristics of children with acute tonsillitis and healthy control children.
| Cases n=142 | Controls n=300 | |
|---|---|---|
|
| ||
| Age (years) | 9.7 (3.5) | 10.1 (3.2) |
| Mean (SD) | ||
| Sex, n (%) | ||
| Males | 68 (47.9) | 120 (40) |
| Females | 74 (52.1) | 180 (60) |
| History of fever, n (%) | 126 (88.7) | - |
| Absence of cough, n (%) | 86 (60.6) | - |
| Anterior cervical adenopathy, n (%) | 73 (51.4) | - |
| Tonsillar swelling or exudate, n (%) | 48 (33.8) | - |
Table 2.
Distribution of the McIsaac scores and the corresponding positive results of RADT and throat culture for GAS.
| McIsaac score | Total number=142 n (%) |
RADT positive n=54 n (%) |
Throat culture positive n=60 n (%) |
|---|---|---|---|
|
| |||
| 1 | 8 (5.6) | 0 (0) | 0 (0) |
| 2 | 22 (15.5) | 2 (9.1) | 3 (13.6) |
| 3 | 43 (30.3) | 8 (18.6) | 10 (23.3) |
| ≥4 | 69 (48.6) | 44 (63.8) | 47 (68.1) |
n: number, RADT: rapid antigen detection test.
All scores were ≥1 because all included patients were from 4–16 years old.
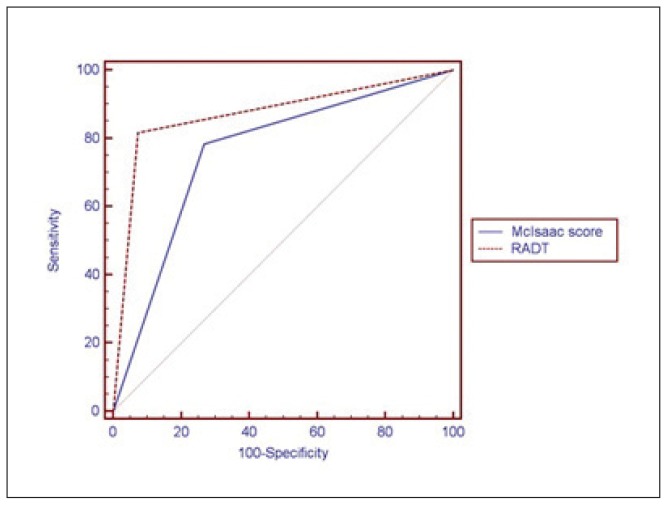
In the current study, when comparing the performance of the McIsaac score and RADT versus routine throat culture, we found a highly significant association between both McIsaac score (P<.001) and RADT (P<.001) and the result of the throat culture (Table 3). An ROC curve was constructed (Figure 1) to show the ability of the McIsaac score and RADT to diagnose patients with GAS pharyngitis. A McIsaac score of 34 had a sensitivity of 78.3% and a specificity of 73.2%, with a positive predictive value (PPV) of 68.1% and negative predictive value (NPV) of 82.2%. The sensitivity and specificity of the RADT for a positive throat culture for GAS was 81.7% and 93.9% respectively, with a PPV of 89.1% and NPV of 87.4%. Of 300 healthy children controls, GAS was isolated from 16% (48/300); 4.3% (13/300) showed growth of β-hemolytic streptococci other than GAS and 79.7% (239/255) yielded no growth of pathogenic bacteria. Among the GAS isolates, 16.7% (30/180) were from girls and 15% (18/120) were from boys. The colonization rate was statistically insignificant between gender (P=.7) and age subgroups (P=.1) (Table 4).
Table 3.
Performance of McIsaac score and RADT versus throat culture.
| Throat culture | Agreement (95% CI) (%) | |||||
|---|---|---|---|---|---|---|
| Positive n=60 | Negative n=82 | Sensitivity | Specificity | Overall | P | |
|
| ||||||
| McIsaac score | ||||||
| Positive, n=69 | 47 | 22 | 78.3 (65.8–87.9) | 73.2 (62.2–82.4) | 75.8 (67.9–82.5) | .0001 |
| Negative, n=73 | 13 | 60 | ||||
|
| ||||||
| RADT score | ||||||
| Positive, n=54 | 49 | 5 | 81.7 (69.6–90.5) | 93.9 (86.3–98.0) | 87.8 (81.2–92.7) | .0001 |
| Negative, n=88 | 11 | 77 | ||||
Figure 1.
Receiver operating characteristic (ROC) curve for the performance of McIsaac score and rapid antigen detection test (RADT) in defining GAS pharyngitis
Table 4.
Association between age and gender and carriage rate among healthy children.
| Variables | Results of throat culture among healthy children | P | |
|---|---|---|---|
| Positive for GAS n=48 | Negative for GAS n=252 | ||
|
| |||
| Age (years), n (%) | |||
| 4–7 (n= 60) | 5 (10.4) | 55 (21.8) | .11 |
| 7–10 (n=82) | 14 (29.2) | 68 (27) | |
| 10–13 (n=106) | 23 (47.9) | 46 (18.3) | |
| 13–16 (n=52) | 6 (12.5) | 83 (32.9) | |
| Gender, n (%) | |||
| Males (n=120) | 18 (37.5) | 30 (62.5) | .7 |
| Females (n=180) | 102 (40.5) | 150 (59.5) | |
Values are numbers (percentage).
Antibiotic susceptibility tests revealed that 100% of GAS isolates recovered from the case group were sensitive to penicillin and vancomycin. The penicillin concentration at which 90% of strains were inhibited (MIC90) was 0.12 μg/mL (Table 4). However, the following resistance pattern was observed for other antibiotics: 70% (42/60) for erythromycin, azithromycin, and clarithromycin, 13.3% (9/60) for clindamycin, 66.7% (40/60) for tetracycline, and 23.3% (14/60) for ceftriaxone. As regards the resistance phenotypes of erythromycin-resistant GAS isolates, 28 (66.7%) had the constitutive (cMLSB) phenotype, 7 (16.7%) had the M phenotype, and 7 (16.7%) had the iMLSB phenotype. The MIC90 of erythromycin for the erythromycin-resistant isolates (cMLS) was 256 μg/mL (Table 5).
Table 5.
The MIC results (μg/mL) of penicillin and erythromycin among the GAS isolates in the case group [No. of isolates with MIC (μg/mL)].
| Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| MIC | 0.015 | 0.03 | 0.06 | 0.12a | 0.25b | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | |
| Penicillin | 1 | 3 | 3 | 53 | / | / | / | / | / | / | / | / | / | / | / | 60 |
| Erythromycin | / | / | / | 18 | / | 7 | / | / | 6 | 7 | / | 3 | 3 | / | 16 | 60 |
MIC=minimal inhibitory concentration,
Penicillin MIC ≤ 0.12 is considered sensitive to penicillin;
Erythromycin MIC ≤0.25 is considered sensitive to erythromycin.
In this study, 28.9% (41/142) of children in the case group suffered from recurrent attacks of tonsillitis and received multiple treatment courses with macrolides. Moreover, we found that 92.8% of macrolide-resistant strains (in the case group) were isolated from children with a history of recurrent attacks of tonsillitis and multiple treatment courses with those antibiotics (P<.001) (Table 6). Regarding the susceptibility pattern of GAS isolates recovered from the control group, they were 100% sensitive to penicillin, clindamycin, ceftriaxone and vancomycin. However, 37.5% (18 out of 48) were resistant to tetracycline and 12.5% (6 out of 48) were resistant to macrolides. The erythromycin resistant isolates were of the M phenotype.
Table 6.
Comparison between susceptibility to macrolides and history of recurrent attacks of tonsillitis and recurrent antibiotic intake among case patients.
| History of recurrent attacks of tonsillitis and recurrent antibiotic intake | Susceptibility to macrolides | P | |
|---|---|---|---|
| Sensitive, n=18 | Resistant, n=42 | ||
|
| |||
| Negative, n (%) | 16 (88.9%) | 3 (7.1%) | a<.001 |
| Positive, n (%) | 2 (11.1%) | 39 (92.8%) | |
n= number, Fisher exact test,
statistically significant.
DISCUSSION
In the present study, the occurrence of GAS among Egyptian children with tonsillitis and pharyngitis was 42.2%. Similar high rates (40% and 41.5%) of GAS pharyngitis have also been reported in other studies from Arab countries.11,12 Previous studies on the prevalence of GAS in Egyptian children with sore throat reported that 24%13 and 31.6%14 had a positive throat culture. The high rate of GAS infection in our study may be due to the exclusion of children younger than 4 years old.
To the best of our knowledge, there have been no previous studies from Egypt on GAS carriage rates among children. This study revealed a high pharyngeal carriage rate (16%) for GAS among healthy asymptomatic Egyptian children. Carriage rate varies among countries.11,15–18 The high carriage rate in our study may be due to dissemination of GAS from one infectious member to other members in overcrowded areas with poor hygiene and lack of awareness. We found no significant statistical association between GAS colonisation rate with gender and age subgroups, as reported in other studies.3,19
Reducing unnecessary antibiotic consumption is needed to reduce the risk of emergence of antibiotic resistant bacterial strains, which represents a considerable public health problem, particularly in developing counties.20 North American guidelines for diagnosis of GAS currently combine clinical judgement, throat culture and/or RADTs.5 In this study, the McIsaac score with a sum of 4 or more had a sensitivity of 78.3% and a specificity of 73.2% for a confirmed GAS pharyngitis. The McIsaac score can be used to identify patients who may need immediate antibiotic therapy, further tests or follow up. Our study demonstrated a sensitivity of 81.7% and specificity of 93.9% for RADT, which may allow rapid and reliable diagnosis of GAS.5 However, a negative RADT result should be confirmed by throat culture, especially in countries with a high incidence of post-streptococcal cardiac sequelae.4,5
To date, S pyogenes has remained universally susceptible to penicillin. Therefore, penicillin remains the first-line drug of choice for pharyngeal infections, as well as for complicated or invasive infections.10 In the current study, none of the GAS isolated from children with acute pharyngitis were resistant to penicillin or vancomycin, which is consistent with data from several countries who reported 100% sensitivity to penicillin and vancomycin.10,21–23 In contrast, Telmesani et al11 reported that only 48% of GAS isolates were sensitive to penicillin among children 1–12 years diagnosed with pharyngitis in Saudi Arabia.
The high MIC to penicillin (0.12 μg/mL) in up to 88% of GAS isolates among the case grouup in our study is an alarming sign. This is in accordance with rising MICs of penicillin (0.25–0.75 μg/mL) in 5% of isolates in Mexico and 20.6% in India.24,25 On the other hand, the highest rates of resistance were against macrolides (70%), tetracycline (66.7%), and ceftriaxone (23.3%). Prevalence of macrolide-resistant GAS varies among different countries and from year to year, ranging from 2.6% to 98%.10,21–23,26–28 These high levels of resistance to macrolides and tetracycline in this study may be due to the frequent use of such antibiotics in our country.20
The distribution of frequency of macrolide-lincosamide-streptogramin B (MLSB) resistance phenotypes of GAS are quite different according to geographical region. In this study, more than 50 percent of the erythromycin-resistant GAS isolates exhibited the cMLSB phenotype (constitutive resistance to macrolide-lincosamide-streptogramin B). Similarly, the prevalent MLSB resistance phenotype of GAS was cMLSB in Central and Eastern European countries (60.5%).29 However, the rates of the M phenotype were 79.9% and 70.9% in Spain and Taiwan, respectively.28,30
In this study, we report a MIC90 of 256 μg/mL for erythromycin-resistant isolates which represents a high level of resistance of about 70% of GAS isolates among case subjects. Moreover, this study showed that the resistant strains for macrolides were isolated from children with a history of recurrent attacks of tonsillitis or pharyngitis and recurrent treatment courses with these antibiotics. This might be due to the irrational use of antibiotics in the Egyptian community. Antibiotics are commonly dispensed from community pharmacies in Egypt without medical prescription.20
As regards the antibiotic resistance pattern in carriers of GAS in our study, all isolates were susceptible to penicillin with an MIC90 of 0.03 μg/mL, vancomycin, clindamycin, and ceftriaxone. However, 12.5% of isolates were resistant to macrolides with MIC90 of 0.25 μg/mL for erythromycin and 37.5% to tetracycline. Studies from different countries have also shown that GAS isolates were sensitive to penicillin and other antibiotics such as erythromycin, gentamycin, cephalexin and amoxicillin.19,27 The variation in antimicrobial resistance pattern among countries might be due to isolates being collected at different seasons, differences in the site of infection, the age of the patients and the geographical location.22
CONCLUSION
In conclusion, this study shows rising rates of GAS infection among Egyptian children with tonsillitis and pharyngitis and highlights the problem of asymptomatic GAS carriage in Egypt. Penicillin is still the treatment of choice for GAS tonsillitis. However, we found a high penicillin MIC90 (0.12 μg/mL) for GAS isolates, which is an alarming sign. High frequency of resistance to macrolides mainly of the M phenotype was observed, especially among children with a history of recurrent attacks of acute tonsillitis and recurrent antibiotic intake.
RECOMMENDATIONS
Further study with greater numbers of cases and controls would provide a better overview of GAS infection and better assessment of carriers with erythromycin-resistance phenotypes. Molecular genotyping would give better insights for detection of responsible genes in macrolide resistance isolates. The McIsaac score (as a sensitive and reliable clinical score) and RADT are potentially useful adjuncts for diagnosis and could be used to minimize the unnecessary use of antibiotics.
Footnotes
Conflict of interests:
The authors declare that they have no conflict of interests.
Grants and financial support: None.
REFERENCES
- 1.Dunne EM, Marshall JL, Baker CA, et al. Detection of group a streptococcal pharyngitis by quantitative PCR. BMC Infect Dis. 2013;13:312. doi: 10.1186/1471-2334-13-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devi U, Borah PK, Mahanta J. The prevalence and antimicrobial susceptibility patterns of beta-hemolytic streptococci colonizing the throats of schoolchildren in Assam, India. J Infect Dev Ctries. 2011;5:804–808. doi: 10.3855/jidc.1465. [DOI] [PubMed] [Google Scholar]
- 3.Dumre SP, Sapkota K, Adhikari N, et al. Asymptomatic throat carriage rate and antimicrobial resistance pattern of streptococcal pyogenes in Nepalese school children. Kathmandu Univ Med J (KUMJ) 2009;7:392–6. doi: 10.3126/kumj.v7i4.2760. [DOI] [PubMed] [Google Scholar]
- 4.McIsaac WJ, Kellner JD, Aufricht P, Vanjaka A, Low DE. Empirical Validation of Guidelines for the Management of Pharyngitis in Children and Adults. JAMA. 2004;291:1587–1595. doi: 10.1001/jama.291.13.1587. [DOI] [PubMed] [Google Scholar]
- 5.Rimon AW, Fischer Walker CL, Hamza HS, et al. The utility of rapid antigen detection testing for the diagnosis of streptococcal pharyngitis in low-resource settings. Int J Infect Dis. 2010;14:1048–53. doi: 10.1016/j.ijid.2010.02.2269. [DOI] [PubMed] [Google Scholar]
- 6.Gysin C. Indications of pediatric tonsillectomy. ORL J Otorhinolaryngol Relat Spec. 2013;75:193–202. doi: 10.1159/000342329. [DOI] [PubMed] [Google Scholar]
- 7.McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ. 1998;158:75–83. [PMC free article] [PubMed] [Google Scholar]
- 8.Binso AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Inect Dis. 2002;35:113–25. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 9.Clinical laboratory standards institute (CLSI) Methods for antimicrobial susceptibility tests for bacteria that grow aerobically twenty four informational supplement: Approved standards M100- S24. Wayne, PA, USA: 2014. [Google Scholar]
- 10.Michos AG, Bakoula CG, Braoudaki M, Koutouzi FI, Roma ES, Pangalis A. Macrolide resistance in Streptococcus pyogenes: prevalence, resistance determinants, and emm types. Diag Microbiol Inect Dis. 2009;64:295–299. doi: 10.1016/j.diagmicrobio.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Telmesani AM, Ghazi HO. A study of group A streptococcal bacteria isolation from children less than 12 years with acute tonsillitis, pharyngitis and healthy primary school children. J Family Community Med. 2002;9:23–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Ba-Saddik IA, Munibari AA, Alhilali AM, et al. Prevalence of Group A beta-hemolytic Streptococcus isolated from children with acute pharyngotonsillitis in Aden, Yemen. Trop Med Int Health. 2014;19:431–9. doi: 10.1111/tmi.12264. [DOI] [PubMed] [Google Scholar]
- 13.Steinhoff MC, Abd El Khalek MK, Khallaf N, et al. Effectiveness of clinical guidelines for the presumptive treatment of streptococcal pharyngitis in Egyptian children. Lancet. 1997;350:918–21. doi: 10.1016/s0140-6736(97)03317-5. [DOI] [PubMed] [Google Scholar]
- 14.Rimon AW, Hamza HS, Vince A, et al. Evaluation of the WHO clinical decision rule for streptococcal pharyngitis. Arch Dis Child. 2005;90:1066–1070. doi: 10.1136/adc.2004.069120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozturk CE, Yavuz T, Kaya D, Yusel M. The rate of asymptomatic throat carriage of group Astreptococcus in school children and associated ASO titers in Duzce, Turky. Jpn J Infect Dis. 2004;5:271–2. [PubMed] [Google Scholar]
- 16.Martin JM, Green M, Baarbadora KA, Wald ER. Group A Streptococci among school aged children: Clinical characteristics and the carrier state. Pediatrics. 2004;114:1212–9. doi: 10.1542/peds.2004-0133. [DOI] [PubMed] [Google Scholar]
- 17.Nabipour F, Tayarzadeh M. Prevalence of beta hemolytic streptococcus carrier state and its sensitivity to different antibiotics among guidance school children in Kerman, Iran. Amer J Infect Dis. 2005;1:128–31. [Google Scholar]
- 18.Dawson KP, Ameen AS, Nsanze H, Bin-Othman, Mustafa N. The prevalence of group A streptococcal throat carriage in Al Ain, United Arab Emirates. Ann Top Paediatr. 1996;16:123–7. doi: 10.1080/02724936.1996.11747814. [DOI] [PubMed] [Google Scholar]
- 19.Prajapati A, Rai SK, Mukhiya RK, Karki AB. Study on carrier rate of streptococcus pyogenes among the school children and antimicrobial susceptibility pattern of isolates. Nepal Med Coll J. 2012;14:169–171. [PubMed] [Google Scholar]
- 20.Sabry NA, Farid SF, Dawoud DM. Antibiotic dispensing in Egyptian community pharmacies: an observational study. Res Social Adm Pharm. 2014;10:168–84. doi: 10.1016/j.sapharm.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Zavadska D, Berzina D, Drukalska L, Pugacova N, Miklasevics E, Gardovska D. Macrolide resistance in group A beta haemolytic Streptococcus isolated from outpatient children in Latvia. APMIS. 2010;118:366–70. doi: 10.1111/j.1600-0463.2010.02607.x. [DOI] [PubMed] [Google Scholar]
- 22.Dhanda V, Chaudhary P, Toor D, Kumar R, Chakraborti A. Antimicrobial susceptibility pattern of beta-haemolytic group A, C and G streptococci isolated from North India. J of Med Microbiol. 2013;62:386–393. doi: 10.1099/jmm.0.046672-0. [DOI] [PubMed] [Google Scholar]
- 23.Jain A, Shukla VK, Tiwari V, Kumar R. Antibiotic resistance pattern of group-A beta-hemolytic streptococci isolated from North Indian children. Indian J Med Sci. 2008;62:392–396. [PubMed] [Google Scholar]
- 24.Capoor MR, Nair D, Deb M, Batra K, Aggarwal P. Resistance to erythromycin and rising penicillin MIC in streptococcus pyogenes in India. Jpn J Infect Dis. 2006;59:334–336. [PubMed] [Google Scholar]
- 25.Hull Vance S, Tucci M, Benghuzzi H. Evaluation of the antimicrobial efficacy of green tea extract (egcg) against streptococcus pyogenes in vitro- biomed. Biomed Sci Instrum. 2011;47:177–182. [PubMed] [Google Scholar]
- 26.Zhou W, Jiang YM, Wang HJ, Kuang LH, Hu ZQ, Shi H. Erythromycin-resistant genes in group A β-haemolytic Streptococci in Chengdu, Southwestern China. Ind J Med Microbiol. 2014;32:290–293. doi: 10.4103/0255-0857.136568. [DOI] [PubMed] [Google Scholar]
- 27.Rijal KR, Dhakal N, Shah RC, et al. Antibiotic susceptibility of group A streptococcus isolated from throat culture of schoolchildren in Pokhara, Nepal. Nepal Med Coll J. 2009;11:238–240. [PubMed] [Google Scholar]
- 28.Rubio-Lopez V, Valdezate S, Alvarez D, et al. Molecular epidemiology, antimicrobial susceptibility and resistance mechanisms of streptococcus pyogenes isolates resistant to erythromycin and tetracycline in Spain. BMC Microbiol. 2012;12:215–226. doi: 10.1186/1471-2180-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagai KPC, Appeblbaum TA, Davis IM, et al. Susceptibility to erythromycin in 1,011 streptococcus pyogenes isolated from 10 centeral and eastern European countries. Antimicrob Agents Chemother. 2002;46:546–549. doi: 10.1128/AAC.46.2.546-549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang CY, Lai JF, Huang IW, et al. Epidemiology and molecular characterization of macrolide-resistant Steptococcus pyogenes in Taiwn. J Clin Microbiol. 2014;52:508–16. doi: 10.1128/JCM.02383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]