Abstract
Acremonium species are saprophytic molds widely distributed in nature, existing in soil and decaying vegetation. Penetrating wounds, intravascular catheters and immunosuppression are risk factors for invasive infections of Acremonium. The fungus can also cause cutaneous infections and mycetoma in the immunocompetent; such infections occur in extremities open to trauma. In this paper, a female patient with skin infection due to Acremonium strictum in both legs is described.
Acremonium species are saprophytic molds widely distributed in nature, existing in soil and decaying vegetation. These species are among common laboratory contaminants, but in the last 10 years there has been an increase in cases of Acremonium infection.1 Human diseases due to Acremonium are cutaneous and subcutaneous infections such as superficial skin infections and mycetomas, keratitis and endophthalmitis following use of colonized contact lenses as well as following trauma, and particularly systemic infections involving multiple organs in the immunocompromised patients.2,3 In this paper, a female patient with skin infection due to Acremonium strictum in both legs is presented.
CASE
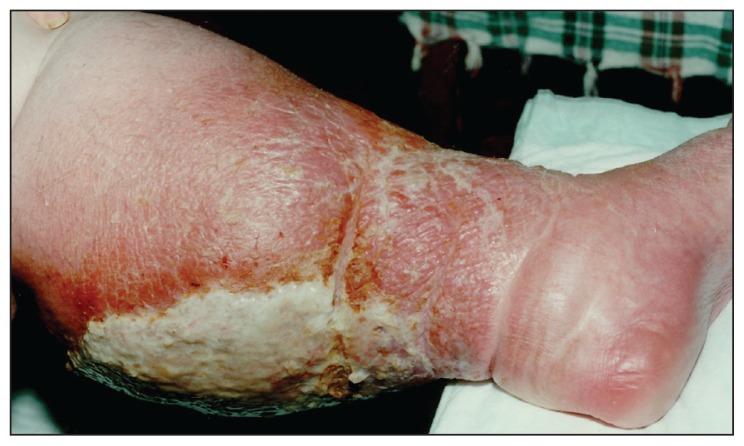
The patient was a 45-year-old female confined to wheelchair because of obesity and gonarthrosis. She had been obese for 20 years (130 kg) and had gonarthrosis for 5 years. She had pustular lesions first on the back of her legs, starting first in the right leg and later appearing also in the left leg (Figure 1a). She did not describe any obvious trauma on the legs before infection. With time the pustules had developed into exudating ulcerations. Later she had been hospitalized in a dermatology clinic where she had received a long duration of antibiotic treatment against Staphylococcus aureus and Pseudomonas aeruginosa isolated from her ulcerations. After limited success with antibacterials, her cultures had been repeated and a “mold” had been isolated. Fluconazole 150 mg/day had been added to her therapy. With the added drug the ulcerations had incompletely subsided, but exudation stopped. Later she left the clinic upon her will.
Figure 1.
Pustular lesions on the back of leg, before treatment.
Six months after leaving the dermatology clinic, she was referred to our clinic for exacerbation of her ulcerations. Swabs and tissue biopsies were taken from exudating ulcerations on both legs for culture and histopathological examination. Later, the patient was hospitalized.
The physical examination of the patient showed that she could not walk because of obesity and gonarthrosis and she was confined to a wheelchair. On the back of both legs there was a wide erythema and ulcerations with ample purulent exudate, extending to front of both legs as well as to her edematious ankles. Clinical laboratory tests revealed an erythrocyte sedimentation rate of 46 mm/h; blood sugar and other test results were within the normal ranges.
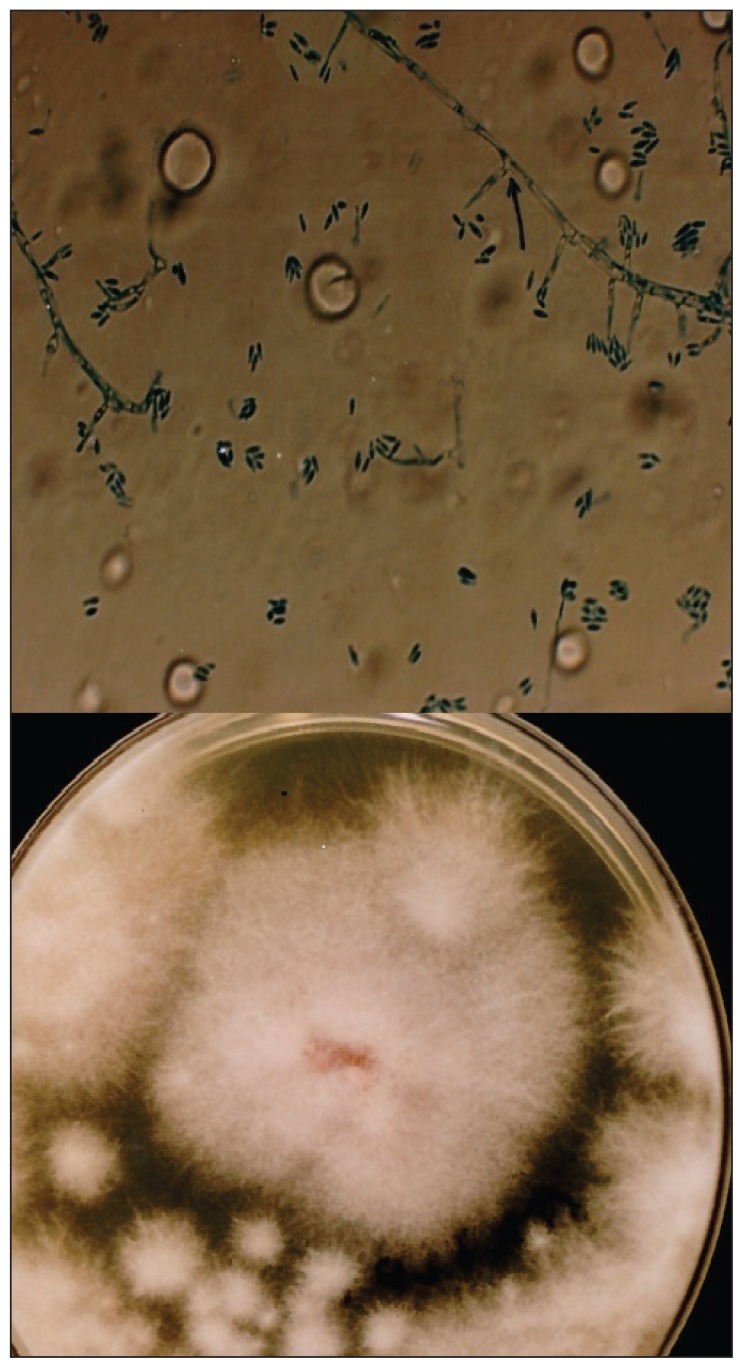
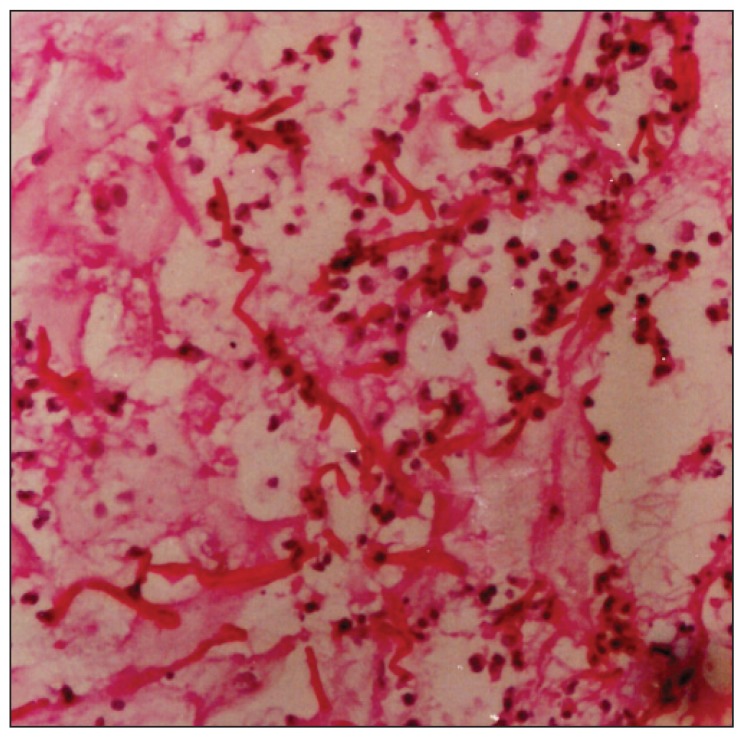
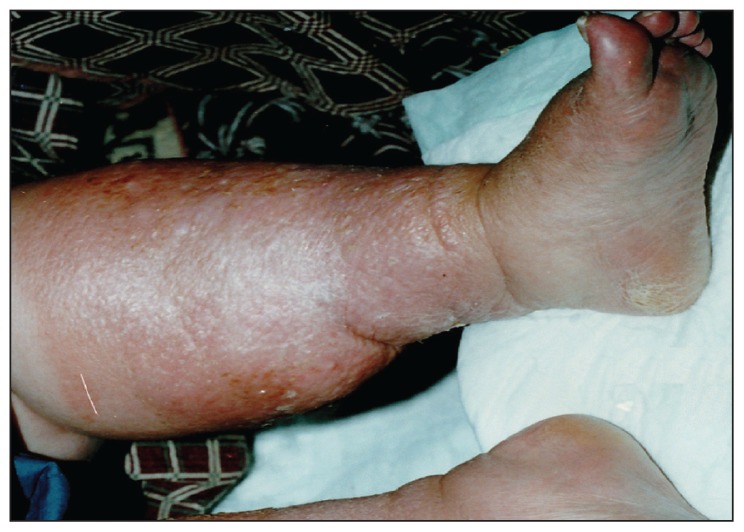
The microscopic examination of KOH preparations made from swabs and tissue biopsy specimens revealed hyalen, septate hyphae. On the fifth day of culture incubation of both specimens (exudate and biopsy material) a mold was grown from each specimen and multiple passages were performed on Sabouraud glucose agar plates at 260°C. White tufted colonies with a pale, salmon pink-coloured base developed. Lactophenol cotton blue preparations from the colonies revealed abundant, small, cylindrical conidia that were produced from the phialidic tips of long, slender, lateral hyphae (Figure 1). These findings identified the fungus as Acremonium strictum (Figure 2a, 2b). Molecular analysis was not performed. The histopathologic examination of biopsy specimens also showed many hyphal segments in the dermis (Figure 3). Conventional amphotericin B was started, but stopped on the sixth day of therapy due to chills, fever, nausea and vomiting, 200 mg/dL serum urea and 1.93 mg/dL serum creatinine. The patient refused therapy with liposomal amphotericin B. By her own will she left discharged the hospital with a prescription for oral itraconazole 400 mg/day and returned for regular weekly checks. At the follow-up visit at the end of 2 months of itraconazole use, there was a marked regression in her lesions with no exudation, but at her visit at 3 months, there was again an exacerbation of exudating ulcerations on both legs. Cultures from lesions were repeated, again yielding A strictum. Therefore, the patient was again hospitalized and treated with amphotericin B lipid complex (3 mg/kg/day) for one month. During this therapy her serum urea and creatinine values increased to 315 mg/dL and 4.65 mg/dL, respectively. Ultrasonographic examination showed focal partial atrophy in the kidneys. The patient underwent hemodialysis five times. Amphotericin B lipid complex was replaced by oral itraconazole 400 mg/ day and she left upon her will. In the following return visits her renal functions returned to normal and her hepatic function was normal. In the fourth month of itraconazole therapy the lesions on both legs completely disappeared. After this period itraconazole was given in gradually decreasing doses four months more, reducing the dose 100 mg/day from the previous month (Figure 4). There was no recurrence of the lesions 3 years after the completion of itraconazole therapy.
Figure 2.
A) KOH preparation of Acremonium strictum; 90 degree branched conidiophores from main hyphae were pointed out with black arrow. B) Sabouraud dextrose agar 10-day colonisation from inoculation.
Figure 3.
Hispathologic examination.
Figure 4.
Lesions healed at end of the fourth month, after therapy with itraconazole.
DISCUSSION
Acremonium species, common within the environment, can be opportunistic human pathogens.4 Penetrating wounds, intravascular catheters and immunosuppression are risk factors for invasive infections of Acremonium. The several cases reported indicate that A strictum is rare, but an important pathogen in Turkey.2,3,5 Infections included facial infection, peritonitis and neonatal septicemia. The fungus can also cause cutaneous infections and mycetoma in the immunocompetent; such infections occur in extremities open to trauma.1 Since Acremonium commonly occurs in the environment, a histopathological diagnosis is necessary to confirm that the fungus is not a contaminant. The patient was not immunocompromised. She did not describe any obvious trauma on the legs preceding the infection. The ulcerating papules developed first on one leg and then on the other. With constant rubbing of the legs to the adjacent metal parts of the wheelchair, the patient might have eventually come in contact with Acremonium with a long latency period between skin trauma and development of lesions.
REFERENCES
- 1.Schinabeck MK, Ghannoum MA. Human hyalohyphomycoses. A review of human infections due Acremonium spp., Paecilomyces spp., Penicillium spp., and Scopulariopsis spp. J Chemother. 2003;15(Suppl 2):5–15. doi: 10.1179/joc.2003.15.Supplement-2.5. [DOI] [PubMed] [Google Scholar]
- 2.Yalaz M, Hilmioglu S, Metin DY, Akisu M, Nart D, Cetin H, et al. Fatal disseminated Acremonium stictum infection in a preterm newborn: a very rare cause of neonatal septicaemia. J Med Microbiol. 2003;52:835–7. doi: 10.1099/jmm.0.05140-0. [DOI] [PubMed] [Google Scholar]
- 3.Sener AG, Yucesoy M, Senturkun S, Afsar I, Yurtsever SG, Turk M. A case of Acremonium strictum peritonitis. Med Mycol. 2008;46(5):495–7. doi: 10.1080/13693780701851729. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Hazarika NK, Barua P, Shivaprakash MR, Chakrabarti A. Acremonium strictum: Report of a Rare Emerging Agent of Cutaneous Hyalohyphomycosis with Review of Literatures. Mycopathologia. 2013 Oct 12; doi: 10.1007/s11046-013-9709-1. [DOI] [PubMed] [Google Scholar]
- 5.Erbagci Z, Tuncel AA, Erkilic S, Zer Y. Successful treatment of antifungal- and cryotherapy-resistant subcutaneous hyalohyphomycosis in an immunocompetent case with topical 5% imiquimod cream. Mycopathologia. 2005;159:521–6. doi: 10.1007/s11046-005-5260-z. [DOI] [PubMed] [Google Scholar]