Abstract
BACKGROUND
The illegal use of melamine in powdered baby formula resulted in a widespread outbreak of melamine-associated pediatric urolithiasis and kidney damage in China in 2008. We conducted this study because more needs to be known about the long-term effects of melamine-associated urolithiasis and kidney damage.
OBJECTIVES
To determine the prognosis and long-term implications of chronic kidney damage in children with urolithiasis resulting from melamine consumption.
DESIGN
Prospective cohort study.
SETTING
Children’s Hospital of Fudan University.
PATIENTS AND METHODS
Children six years of age or older with a history of having consumed melamine-contaminated milk powder were voluntarily screened. We measured urinary microprotein profiles [microalbumin (ALBU), immunoglobulin G (IgG), and N-acetyl-β-D-glucosidase (NAG)] and creatinine (CR) results at 6 and 18 months in children with melamine-associated urolithiasis. This study was conducted from September 17 to October 15, 2008.
MAIN OUTCOME MEASURES
Changes in urinary microprotein profiles.
RESULTS
Of 8335 children screened, 102 children (1.22%) were diagnosed with melamine-associated urolithiasis. Follow-up rates at 6 and 18 months were 91.4% (96/105) and 89.2% (91/102), respectively. Eighteen months later, 90.3% patients had spontaneously passed a stone. The incidence rates of proteinuria and microscopic hematuria at 6 months were significantly higher than at 18 months (P=.029 and P=.017, respectively). The proportion of patients with abnormal ALBU/CR, IgG/CR and NAG/CR at 6 months (27.6%, 17.1% and 21.1%, respectively) was significantly higher than at 18 months (6.4%, 5.1% and 12.8%, respectively). The high concentration of melamine consumed was the primary factor correlated with the high microprotein levels. Approximately 90% melamine-associated urolithiasis cases can be resolved within 18 months by non-surgical therapy.
CONCLUSION
The long-term presence of stones associated with a previous exposure to melanine can cause chronic kidney glomerular and tubular injuries. Passing these stones as soon as possible can reduce kidney injury and accelerate recovery.
LIMITATIONS
We could not control for possible selection bias due to more visits to our hospital or visits to our hospital after diagnosis at other hospitals, which might have increased the rate of diagnosis.
Melamine is widely used as an industrial chemical and has been used in China as an adulterant to increase apparent protein levels in infant formula and other products. An outbreak of melamine-associated pediatric urolithiasis occurred in China in September 2008.1 Some of these children also experienced kidney damage.2 Previous studies primarily focused on acute kidney injury caused by the melamine-associated stones, which were mainly localized to the renal interstitium.3 Recent studies have shown that risk factors for acute kidney injury in infants with melamine-associated urolithiasis are high melamine concentrations in infant formula, age, symptoms of upper respiratory tract infection, diarrhea, dehydration and fever.4 Epidemiologic investigations have also shown that urolithiasis is an independent factor in the development of chronic kidney disease (CKD).5 Urinary calculi in animals without acute renal injury can also lead to chronic kidney disease.6 A similar incident occurred in the United States in 2007 when melamine was used as an adulterant in pet food.7 Some reports have shown that kidney stones in childhood can herald the future deterioration of renal function and the development of hypertension. 8,9 However, the long-term and chronic effects of melamine-associated urolithiasis on the kidney are unknown. Our research mainly focused on chronic kidney injury caused by melamine-associated urolithiasis. The preliminary 6-month follow-up study showed that melamine-associated urolithiasis caused kidney tubular and glomerular damage.10 Furthermore, 30% of the children had not yet discharged their stones. The prognosis and long-term implications of chronic kidney damage in these patients has been unknown. A systematic review of 26 studies also proposed that these patients need for further investigation.11 This 18-month follow-up study aimed to investigate the long-term prognosis and renal effects in children with melamine-associated urolithiasis.
PATIENTS AND METHODS
From September 17 to October 15, 2008, children (age ≤6 years) with a history of consuming melamine-contaminated milk powder were voluntarily screened at Children’s Hospital of Fudan University. The study was approved by the Regional Research Ethics Board, Children’s Hospital of Fudan University, China. The patients’ guardians or parents provided informed consent for data acquisition.
The diagnostic criteria for melamine-associated urolithiasis were the presence of a definite history of consuming melamine-contaminated milk powder before screening; an absence of urinary system disorders caused by other primary or secondary disease; and an abnormal urinary system by multi-sectional ultrasonography (clump of sharp hyper-echoic foci with or without acoustic shadow in the pelvis, calices of the kidney or ureter, punctiform sharp hyper-echoic foci with “comet-tail” artifacts or acoustic shadow on gray-scale ultrasonography). 12,13 Ultrasonography was performed using a probe unit of 3.5 to 5 MHz frequency (SEQUOIA 512, Siemens, Germany) in the supine, lateral decubitus, and dorsal positions for each child. Ultrasonographic findings were confirmed by two ultrasonographic physicians. The smallest detectable calculus diameter was 1.0 mm.
After definitive diagnosis, we recorded sex, age, mode of exposure, melamine concentration consumed, duration of exposure, symptoms and findings on physical examination, and results of urinalysis and urinary system ultrasonography for patients diagnosed with melamine-associated urolithiasis. Appropriately therapy was administered immediately upon diagnosis, including encouraging liquid intake, alkalinization of the urine in all patients and hospitalization for patients with severe disease.
Twenty-two types of milk powder were tainted with melamine. Milk powder produced by the Sanlu Group (https://www.wikiwand.com/en/Sanlu_Group) before August 2008 had the highest melamine concentration, ranging from 162 to 2563 mg/kg. The melamine concentrations of the other brands of milk powder ranged from 0.09 to 150 mg/kg in our screening.14 We considered Sanlu, Sanlu combined with the other brand(s), and the other brand(s) as high-, middle-, and low-melamine concentration milk powders, respectively.
Children with melamine-associated urolithiasis were followed up at 6 and 18 months after diagnosis. For patients who did not live in Shanghai or were unable to come back to our hospital for subsequent visits, the follow-up was carried out by telephone to record their symptoms. The results of urinalysis and renal ultrasonography were recorded by local physicians. During the two follow-up visits in our hospital, renal and urinary tract ultrasonography were repeated. Creatinine (CR) levels were measured as part of urinary microprotein profiles, including microalbumin (ALBU)/CR (reference range, 0 to 26.5 mg/g), immunoglobulin G (IgG)/CR (reference range, 0 to 14 mg/g), and N-acetyl-β-D-glucosidase (NAG)/CR (reference range, 0.3 to 1.2 U/mmol (based on internal data of Wako Corporation]).15,16 The levels of urinary melamine and cyanuric acid were measured by high performance liquid chromatography-triple quadrupole mass spectrometry (HPLC-MS/MS) at the 18-month follow-up visit.15
Statistical analysis
Quantitative data are expressed as the mean and standard deviation (SD). A t test or one-way analysis of variance (ANOVA) was used as appropriate to compare quantitative data. For patients with two tests of their urinary microprotein profile (n=55), a comparison of the reduction in protein from 6 months to 18 months was analyzed by analysis of covariance (ANCOVA), and the first measured value was used as a covariate. The distribution of each group was expressed as frequencies and percentages. The chi-squared test or Fisher exact test were used as appropriate to analyze categorical data. A logistic regression model was used to analyze factors influencing urinary calculi status (passed or persistent, n=91), and a linear regression model was used to analyze factors influencing the ALBU/CR, IgG/CR and NAG/CR levels (n=78) at 18 months, including sex, age, duration of exposure, size of calculi at initial presentation, and status of calculi during follow-up. A two-tailed P value less than 0.05 was considered statistically significant. Statistical analyses were performed using Statistical Package for Social Science (SPSS) version 11.5 and Statistical Analysis Software (SAS) version 9.0.
RESULTS
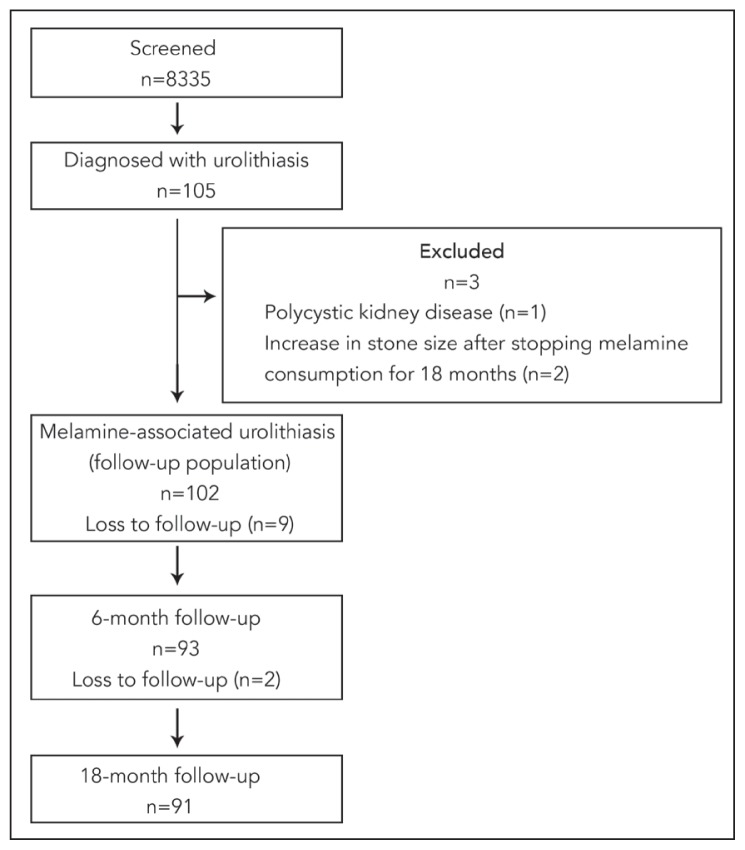
Of the 8335 screened, 105 (1.3%) were diagnosed with melamine-associated urolithiasis. Three of these children were subsequently excluded from the study (Figure 1). The causes of loss to follow-up were incorrect addresses and telephone numbers, living in remote areas, and/or unwillingness to return due to working a busy job. Seven patients had surgical removal of calculi within 6–18 months after diagnosis. The initial diameter of the calculi ranged from 1.1 mm to 19.3 mm. A single calculus was found in 65 cases (63.7%), and multiple calculi were found in 37 cases (36.3%).
Figure 1.
Enrollment and outcomes in the assessment of melamine-associated pediatric urolithiasis.
Of the patients, 77 (75.5%) cases were asymptomatic, and 25 (24.5%) cases showed symptoms such as vomiting, convulsive crying, fever, malaise, and urinary tract infection. Only 1 case (1.0%) presented with acute renal failure and recovered quickly after surgical treatment to remove calculi (ureter retrograde catheterization).
Six months later, 96 children with urolithiasis were followed up (follow-up rate: 91.4%, 62 boys and 34 girls); 18 months after screening, 91 children with urolithiasis were followed up (follow-up rate: 89.2%, 55 boys and 36 girls). Three cases were later found to have unrelated kidney disease and were excluded to avoid bias. The follow-up enrollment and outcome are shown in Figure 1.
Ultrasonography
Sixty-five cases (69.9%) passed stones within 6 months, and 82 cases (90.3%) passed stones within 18 months. Of the 12 patients with stones larger than 10 mm, 4 passed their stones spontaneously, 7 underwent surgical removal of their calculi (5 cases: minimally invasive percutaneous nephrolithotomy; 2 cases: placement of ureteral stent), and 1 was lost to follow-up. Nine patients still had calculi after 18 months, but the sizes of the calculi had all decreased. Logistic regression analysis showed that patients with small calculi were more likely to pass the calculi within 18 months (P<.001) (Table 1).
Table 1.
Logistic regression analysis of patient factors influencing the passage of calculi at 18 months after initial presentation (n=91).
| Characteristics | Persistent stones, n (%) | Passed stone, n (%) | Odds ratio (95% CI) | P |
|---|---|---|---|---|
|
| ||||
| Sex | ||||
| Male | 3 (5.5) | 52 (94.5) | 5.401 (0.971–31.811) | .062 |
| Female | 6 (16.7) | 30 (83.3) | 1.0 | |
| Age (months) | ||||
| 0 to ≤12 | 1 (2.9) | 33 (97.1) | 22.206 (0.874–546.488) | .060 |
| >12 to ≤36 | 2 (6.9) | 27 (93.1) | 3.054 (0.423–21.879) | .269 |
| >36 to ≤72 | 6 (21.4) | 22 (78.6) | 1.0 | |
| Duration of exposure (months) | ||||
| ≤6 | 1 (9.1) | 30 (90.9) | 0.519 (0.044–6.115) | .602 |
| 6–12 | 3 (5.9) | 32 (94.1) | 2.959 (0.332–26.407) | .331 |
| >12 | 5 (16.7) | 20 (83.3) | 1.0 | |
| Concentration of melamine consumed | ||||
| High | 7 (14.3) | 42 (85.7) | 0.154 (0.014–1.643) | .121 |
| Middle | 1 (11.1) | 11 (91.7) | 0.562 (0.016–20.315) | .753 |
| Low | 1 (11.1) | 29 (96.7) | 1.0 | |
| Diameter of calculi (mm) | ||||
| ≤5 | 6 (6.6) | 60 (90.9) | 5.838 (4.259–8.000) | <.001 |
| >5 to ≤10 | 3 (3.3) | 11 (78.6) | 1.0 | |
| >10 | 0 (0)* | 11 (100.0) | ||
Laboratory analysis of urine
Urinalysis was performed in 82 and 78 children at 6 and 18 months, respectively. The incidence of proteinuria and microscopic hematuria was 6 cases (7.3%) and 14 cases (17.1%) at 6 months and 0 cases (0) and 4 cases (5.1%) at 18 months, respectively. The differences in the incidence rates of proteinuria and microscopic hematuria between the 6-month and 18-month follow-up visits were significant (P=.029 and P=.017, respectively). Seventy-four patients were tested for the concentration of melamine and cyanuric acid in their urine at 18 months. No melamine or cyanuric acid residue was detected.
Urinary microprotein profiles were separately measured in 76 patients at 6 months and in 78 patients at 18 months. There were significant differences in the proportions of patients with abnormal ALBU/CR and IgG/CR between the two follow-up visits (P=.001 and P=.018, respectively); The result also showed that the ALBU/CR, IgG/CR and NAG/CR levels in patients at 18 months were significantly lower compared with the levels at the 6 months follow-up (P<.001, P=.038 and P=.026, respectively) (Table 2). Linear regression showed that at 18 months, high levels of ALBU/CR were primarily associated with younger age (−1.778; 95% confidence interval [CI], −3.338 to −0.218; P=.026) and high melamine concentration consumed (−1.528; 95% CI, −2.779 to −0.277; P=.018); high IgG/CR was primarily associated with a high melamine concentration consumed (−1.435; 95% CI, −2.468 to −0.402; P=.007) and size of calculi (1.658; 95% CI, 0.231 to 3.140; P=.024); and NAG/CR might have been related to the high melamine concentration consumed (−0.130; 95% CI, −0.273 to 0.012; P=.072) (Table 3).
Table 2.
Comparison of urinary microprotein profiles at 6 and 18 months of follow-up.
| Urinary microprotein profiles | First follow-up (6 mo), n=76 | Second follow-up (18 mo), n=77 | P | |
|---|---|---|---|---|
|
| ||||
| ALBU/CR | Abnormal number, n (%) | 21 (27.6) | 5 (6.4) | .001 |
| Value (mg/g) | 26.06 (17.78) | 13.64 (3.65) | <.001 | |
| IgG/CR | Abnormal number, n (%) | 13 (17.1) | 4 (5.1) | .018 |
| Value (mg/g) | 15.9 (10.53) | 9.63 (2.30) | .038 | |
| NAG/CR | Abnormal number, n (%) | 16 (21.1) | 10 (12.8) | .173 |
| Value (u/mmol) | 0.92 (0.34) | 0.66 (0.32) | .026 | |
Values are mean (standard deviation).
Table 3.
Linear regression analysis of factors influencing urinary microprotein profiles at 18 months after initial presentation.*
| Factors | B | 95% CI | P | R2 | |
|---|---|---|---|---|---|
|
| |||||
| ALBU/CR | Concentration of melamine consumed | −1.528 | −2.779–−0.277 | .018 | 0.213 |
| Age | −1.778 | −3.338–−0.218 | .026 | ||
| IgG/CR | Concentration of melamine consumed | −1.435 | −2.468–−0.402 | .007 | 0.144 |
| Size of stones | 1.685 | 0.231–3.140 | .024 | ||
| NAG/CR | Concentration of melamine consumed | −0.130 | −0.273–0.012 | .072* | 0.100 |
The table only shows the statistically significant independent factors for the three dependent variables. Other independent variables included sex, age, duration of exposure, size of calculi at initial presentation, and status of calculi during follow-up.
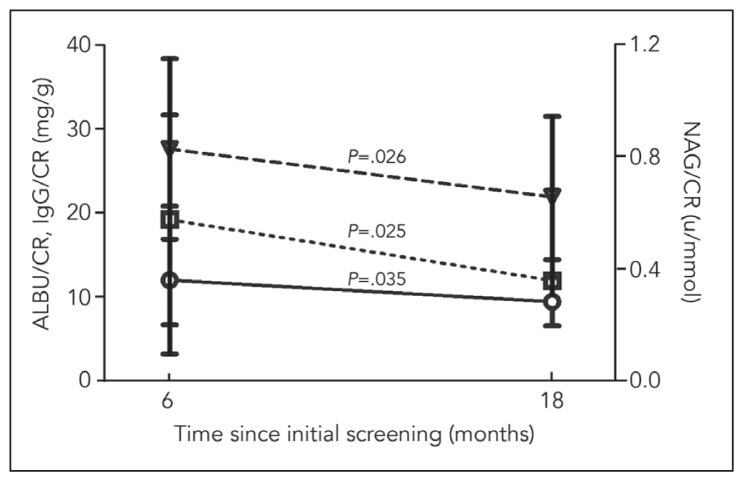
Fifty-five patients had urinary microprotein profiles at both 6 and 18 months. IgG/CR and NA/CR after 18 months was significantly decreased compared with that after 6 months (Figure 2). We analyzed the extent of the reduction in urinary microprotein from 6 to 18 months between children with persistent stones and children who passed their calculi. The reduction in NAG/CR in children who passed calculi was significantly higher than in children with persistent calculi at 6 or 18 months (P=.019 and P=.041, respectively). The reduction of ALBU/CR in children who passed calculi was significantly higher than in children with persistent calculi at 18 months (P<.001), which was not observed in our previous study. We also analyzed the urinary microprotein profile between children who passed calculi within 6 months and children who passed calculi after 6 to 18 months. The reduction in NAG/CR and IgG/CR in children who passed calculi within 6 months was higher than in children who passed calculi after 6 but before 18 months, indicating that earlier passage might aid in recovery from renal injury (Table 4).
Figure 2.
Changes in the mean urinary microprotein profiles between 6 and 18 months.
Table 4.
Comparison of the reductiona in urinary microprotein from 6 to 18 months between patients with and without persistent stones by ANCOVA.b
| Status of calculi | ALBU/CR | IgG/CR | NAG/CR | ||||
|---|---|---|---|---|---|---|---|
| Reduced extent (mg/g) | P | Reduced extent (mg/g) | P | Reduced extent (u/mmol) | P | ||
|
| |||||||
| First follow-up (6 months) | Passed stones (n=40) | 10.45 (21.23) | .387 | 0.25 (0.66) | .086 | 0.39 (0.52) | .019 |
| Persistent stones (n=15) | 14.25 (22.07) | 0.06 (0.52) | 0.05 (0.57) | ||||
| Second follow-up (18 months) | Passed stones (n=48) | 7.68 (13.00) | <.001 | 0.24 (0.66) | .225 | 0.36 (0.55) | .041 |
| Persistent stones (n=7) | 4.44 (4.98) | −0.03 (0.23) | −0.13 (0.41) | ||||
| Within 6 months (n=39) | Passed stones | 4.53 (5.47) | .506 | 0.25 (0.67) | .276 | 0.38 (0.55) | .120 |
| After 6 but before 18 months (n=6) | 4.95 (9.96) | −0.05 (0.35) | 0.012 (0.35) | ||||
The mean of the second follow-up minus the mean of the first follow-up.
ANCOVA was used to eliminate the impact of the first follow-up measures on the extent of reduction.
DISCUSSION
The combination of melamine and cyanuric acid can produce renal toxicity due to significant chemical accumulation in the kidney accompanied by low excretion.16 The long-term prognosis and chronic effects on the kidney of melamine-associated urolithiasis are the main concerns in our long-term follow-up study. After 18 months, the vast majority of the patients had passed their calculi. Therefore, for most patients with melamine-associated urolithiasis, conservative treatment was effective and had a lower risk of adverse effects. For patients with large urinary calculi (≥10 mm), our findings showed that most of the calculi were not passed even after 18 months. For these patients, surgical intervention should be considered as early as possible.
It has been reported that the urinary melamine levels of children with melamine-associated calculi were significantly increased in the acute stage.17 However, it was still unknown whether the body retains melamine in the long term. In our study, we found that the urine melamine and cyanuric acid levels of all of the patients were normal. Moreover, no patient formed new calculi or had a recurrence during follow-up. It is important for these children to stop the intake of melamine.
After 18 months, the incidence rates of proteinuria and microscopic hematuria and the levels of ALBU/CR, IgG/CR and NAG/CR were all significantly reduced. However, 20.8% of these children still had abnormal urinary microprotein profiles; the main abnormal factors were the concentration of melamine consumed, the size of the calculi and the age of the patient. The consumption of higher concentrations of melamine can lead to a slower recovery from renal injury.
To assess the effects of melamine-associated stones on recovery from renal injury, we analyzed the extent of changes in the urinary microprotein profiles between 6 and 18 months. We found that the presence of long-term persistent calculi can result in chronic glomerular injury, which was not observed in our previous 6-month follow-up study.10 The children who passed their calculi recovered from renal tubular injury more quickly than children who had not passed their calculi. Moreover, the reduction in IgG/CR and NAG/CR was greater for patients who passed their calculi within 6 months compared with patients who passed their calculi after 6 months. These results indicate that passage of the melamine-associated calculus was an important factor for recovery from renal tubular injury.
Our study has certain limitations. Our hospital is thought to attract more patients than other hospitals and as a result, selection bias might have increased the rate of diagnosis. There may have been more visits to our hospital than other hospitals or patients may have come to our hospital after diagnosis at other hospitals. We also could not completely exclude confounding bias caused by non-melamine-related renal stones.
Over 18 months of follow-up, we discovered that most patients with melamine-associated calculi can pass their calculi spontaneously. Long-term persistent calculi can result in chronic glomerular injury that recovers slowly. Earlier passage of calculi is associated with a more rapid recovery from renal injury.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (NSFC 81260117) and Natural Science Foundation of Ningxia Province (NZ12184). The authors thank their colleagues in the W.F. Maternal & Child Health Hospital and Ningxia Medical University and the Children’s Hospital of Fudan University for their assistance with this work.
Footnotes
Compliance with Ethical Standards
The authors declare that they have no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.Guan N, Fan Q, Ding J, Zhao Y, Lu J, Ai Y, et al. Melamine-contaminated powdered formula and urolithiasis in young children. N Engl J Med. 2009;360(11):1067–1074. doi: 10.1056/NEJMoa0809550. [DOI] [PubMed] [Google Scholar]
- 2.Lam CW, Lan L, Che X, Tam S, Wong SS, Chen Y, et al. Diagnosis and spectrum of melamine-related renal disease: plausible mechanism of stone formation in humans. Clin Chim Acta. 2009;402(1–2):150–155. doi: 10.1016/j.cca.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Cianciolo RE, Bischoff K, Ebel JG, Van Winkle TJ, Goldstein RE, Serfilippi LM. Clinicopathologic, histologic, and toxicologic findings in 70 cats inadvertently exposed to pet food contaminated with melamine and cyanuric acid. J Am Vet Med Assoc. 2008;233(5):729–737. doi: 10.2460/javma.233.5.729. [DOI] [PubMed] [Google Scholar]
- 4.He Q, Yue Z, Tang X, Chang H, Wang W, Shi W, et al. Risk factors for acute kidney injury (AKI) in infants with melamine-associated urolithiasis and follow-up: a multi-center retrospective analysis. Ren Fail. 2014;36(9):1366–1370. doi: 10.3109/0886022X.2014.945215. [DOI] [PubMed] [Google Scholar]
- 5.Bhalla V, Grimm PC, Chertow GM, Pao AC. Melamine nephrotoxicity: an emerging epidemic in an era of globalization. Kidney Int. 2011;7(8):232–239. doi: 10.1038/ki.2009.16. [DOI] [PubMed] [Google Scholar]
- 6.Dobson RL, Motlagh S, Quijano M, Cambron RT, Baker TR, Pullen AM, et al. Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicol Sci. 2008;106(1):251–262. doi: 10.1093/toxsci/kfn160. [DOI] [PubMed] [Google Scholar]
- 7.Brown CA, Jeong KS, Poppenga RH, Puschner B, Miller DM, Ellis AE, et al. Outbreaks of renal failure associated with melamine and cyanuric acid in dogs and cats in 2004 and 2007. J Vet Diagn Invest. 2007;19(5):525–531. doi: 10.1177/104063870701900510. [DOI] [PubMed] [Google Scholar]
- 8.Saarela T, Lanning P, Koivisto M. Prematurity-associated nephrocalcinosis and kidney function in early childhood. Pediatr Nephrol. 1999;13(9):886–890. doi: 10.1007/s004670050721. [DOI] [PubMed] [Google Scholar]
- 9.Kaste SC, Thomas NA, Rai SN, Cheon K, McCammon E, Chesney R, et al. Asymptomatic kidney stones in long-term survivors of childhood acute lymphoblastic leukemia. Leukemia. 2009;23(1):104–108. doi: 10.1038/leu.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Xu H, Kuang XY, Huang WY, Zhao NQ, Rao J, et al. Follow-up results of children with melamine induced urolithiasis: a prospective observational cohort study. World J Pediatr. 2011;7(3):232–239. doi: 10.1007/s12519-011-0293-5. [DOI] [PubMed] [Google Scholar]
- 11.Wang P-X, Li H-T, Zhang L, Liu J-M. The clinical profile and prognosis of Chinese children with melamine-induced kidney disease: a systematic review and meta-analysis. Biomed Res Int. 2013;2013:868202. doi: 10.1155/2013/868202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho SS, Chu WC, Wong KT, Li CK, Wong W, Ng PC, et al. Ultrasonographic evaluation of melamine-exposed children in Hong Kong. N Engl J Med. 2009;360(11):1156–1157. doi: 10.1056/NEJMc0809955. [DOI] [PubMed] [Google Scholar]
- 13.Jia LQ, Shen Y, Wang XM, He LJ, Xin Y, Hu YX. Ultrasonographic diagnosis of urinary calculus caused by melamine in children. Chin Med J (Engl) 2009;122(3):252–256. [PubMed] [Google Scholar]
- 14.General Administration of Quality Supervision, Inspection, and Quarantine of the People’s Republic of China. 2008. [accessed September 28, 2008]. http://www.aqsiq.gov.cn/ztlm/nf/qwfb/200809/t20080916_89960.htm.
- 15.Tran BN, Okoniewski R, Storm R, Jansing R, Aldous KM. Use of methanol for the efficient extraction and analysis of melamine and cyanuric acid residues in dairy products and pet foods. J Agric Food Chem. 2010;58(1):101–107. doi: 10.1021/jf903040z. [DOI] [PubMed] [Google Scholar]
- 16.Kim GH, Kang MJ, Noh K, Oh do G, Kang W, Jeong HG, et al. Nephrotoxic potential and toxicokinetics of melamine combined with cyanuric Acid in rats. J Toxicol Environ Health A. 2014;77(22–24):1346–1358. doi: 10.1080/15287394.2014.951592. [DOI] [PubMed] [Google Scholar]
- 17.Cheng WC, Chen SK, Lin TJ, Wang IJ, Kao YM, Shih DY. Determination of urine melamine by validated isotopic ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23(12):1776–1782. doi: 10.1002/rcm.4071. [DOI] [PubMed] [Google Scholar]