Abstract
BACKGROUND AND OBJECTIVES
Inguinal hernia repair is frequently associated with persistent postoperative discomfort and pain and late discharge from the hospital. We evaluated the postoperative analgesic effect of local wound infiltration with tramadol following herniorrhaphy among adult patients.
PATIENTS AND METHODS
Forty-three adult male patients were randomly assigned to two groups; group T (n=23) received tramadol 1 mg/kg in 10 mL 0.9% normal saline and group B (n=20) received 10 mL of 0.25% bupivacaine, both as a local wound infiltration prior to skin closure. Postoperatively, pain severity, time to first analgesic requirement, analgesic consumption, and incidence of side effects were recorded.
RESULTS
During the first postoperative day, there was a significant difference between the two groups in the recorded visual analog scale rating higher in group B (P<.05) and the time to first analgesic requirement (6.6±0.99 hours in group B vs 3.7±0.74 hours in group T; P<.05). There was no difference in the incidence of side effects among the two groups. Postoperative consumption of fentanyl and diclofenac was higher in group B than group T (65% vs 18% and 80% vs 21.7%, respectively, P≤.005).
CONCLUSIONS
Locally infiltrated tramadol prior to herniorrhaphy wound closure provides better pain relief compared to bupivacaine in adult patients.
As the volume and complexity of surgical procedures continue to grow, anesthesiologists are being challenged to provide patients with the “optimal surgical experience”, good operating conditions and rapid recovery time without side effects.1 Inguinal herniorrhaphy is one of the most commonly performed operations. It can be performed under local anaesthetic infiltration, regional or general anaesthesia. Regardless of the anesthetic technique, prevention and treatment of the postoperative pain must be addressed. Theoretically, the same modalities for pain relief should be available for all types of anaesthesia.2 Inguinal hernia repair is frequently associated with persistent postoperative discomfort and distress for the patient and late discharge from the hospital with a loss of the cost-saving benefit.3 Post-herniorrhaphy pain is usually treated by either nonsteriodal anti-inflammatory drugs (NSAIDs) or opioids that are administered either alone or in combination with acetaminophen.4 Opioids provide effective analgesia but side-effects, especially respiratory depression, emesis and sedation, reduce the advantages of these medications in outpatient surgery.5 Some surgeons perform ilioinguinal nerve blockade or deposit local anaesthetics directly into the wound.2,3,6 Tramadol is a centrally acting analgesic. Its analgesic effects are mediated by at least three different mechanisms: it is a weak μ opioid receptor agonist, it inhibits the reuptake of the neurotransmitters hydroxytryptamine (5-HT) and noreadrenaline in the descending inhibitory pain pathways, and facilitates 5-HT release.7,8 Recent studies suggest that tramadol possesses some local anesthetic properties when applied to peripheral nerves.9–11 A dose of 100 mg tramadol added to 40 mL of 1.5% mepivacaine improved the quality of the brachial plexus blockade in patients scheduled for forearm and hand surgery.7 Administration of analgesic agents prior to emergence from general anaesthesia is associated with an acceptable comfort level for the patient in the early postoperative period.12 We conducted a randomized, prospective, double-blinded study to compare tramadol versus bupivicaine wound infiltration in reducing postoperative pain following inguinal hernia repair.
PATIENTS AND METHODS
Following approval from the King Abdalaziz University Hospital ethics committee, 46 male patients aged 18–50 years, with American Society of Anesthesiology classifications of I and II, undergoing an inguinal hernia repair under general anaesthesia, consented to participate in this randomized, double-blinded, clinical trial. Patients with a history of drug dependency, gastritis or duodenal ulceration, difficulty in communication or a history of allergic reaction to the study drugs and patients refusing general anaesthesia were excluded from the study. Patients were allocated randomly into one of two groups: Group T (n=23) received tramadol hydrochloride (Tramadol, Grunenthal, Germany) 1 mg/kg in 10 mL of 0.9% normal saline as a local wound infiltration, starting from the external oblique aponeurosis up to the skin prior to skin closure, while Group B (n=23) received 10 mL of 0.25% isobaric bupivacaine (Marcaine, AstraZeneca, United Kingdom) using the same infiltration technique. Randomization was performed with a computer-generated randomization table using sealed envelopes. Block randomization was used to protect against imbalanced treatment assignment. The anesthesiologist and patient were blinded to the study medicine. A third investigator not involved in the infiltration technique or the assessment of the patient response postoperatively prepared all syringes of the study medications. All patients were pre-medicated with 0.5 mg/kg of oral midazolam. The same anesthetic technique was used for both groups: 3 μg/kg IV fentanyl and 2–3 mg/kg IV propofol as induction agents and 0.15 mg/kg IV cisatracurium as a non-depolarizing muscle relaxant. Maintenance of general anaesthesia was ensured by 1–1.5 vol% isoflurane in 1:1 O2 and N2O. The muscle relaxant effect was reversed at the end of surgery. In the postanesthesia care unit (PACU), pain was assessed using a visual analog scale (VAS) from 0 to 10, with 0 meaning no pain and 10 meaning the worst pain imaginable. Fentanyl IV 25 μg was prescribed for patients with a pain score >4. Patients were discharged from the PACU to the ward after reaching a modified Aldrete score >9.13 In the first postoperative day, assessment of pain was performed using the VAS every 4 hours, unless the patient was sleeping. The onset of pain, time for first analgesic requirement and any side effects were recorded. A diclofenac suppository of 50 mg was prescribed to patients every 8 hour once the VAS ≥4. Nausea and vomiting were recorded using a 3-point ordinal scale (0=none, 1=nausea, and 2=vomiting), 10mg IV metoclopramide was used to treat vomiting. Sedation was evaluated on a scale of 0–4 with 0=fully awake, 1=slight drowsiness, 2=sleepy but easily aroused, 3 = fully asleep but arousable and 4=fully asleep, not arousable. Postoperative assessment was performed by an investigator who was unaware of which treatment each patient had received. All statistical analysis was performed using statistical package SPSS for Windows program, version 10.0, Chicago, Illinois, USA.
Quantitative data (mean scores for the VAS) were skewed and showed a high scatter. On applying Levene’s test for equality of variance, significant results were not found. The non-parameteric Mann-Whitney test was used to compare the two groups. Qualitative variables were compared using the chi-square test. Whenever the expected value in one or more of the cells in a 2×2 table was less than 5, the Fisher exact test was used instead. Statistical significance was considered as a P value <.05. Results are presented as mean, standard deviation (SD) and standard error of the mean unless otherwise specified.
RESULTS
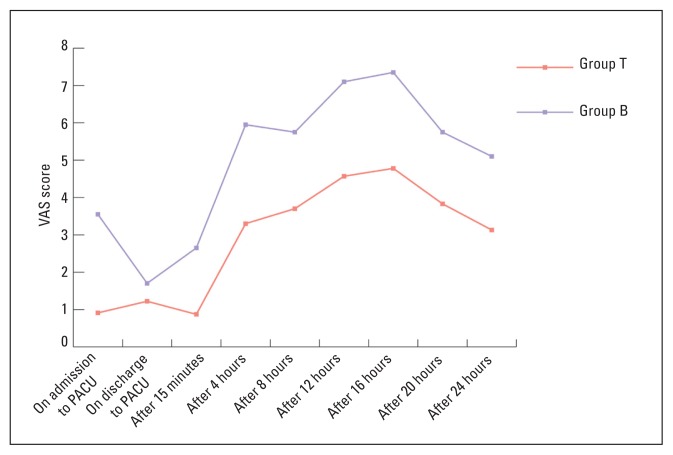
Forty-three patients were included in the study, including 23 in group T and 20 in group B. Three patients initially assigned to group B were excluded due to incomplete data collection. There was no difference between the groups in demographic characteristics or in surgical or anaesthesia times (P>.05, Table 1). In the first postoperative day, VAS was significantly higher among patient in group B than group T (P<.05) (Figure 1). Time for first analgesic requirement was earlier in group B patients than group T patients (3.7±0.745 vs 6.6±0.992 hours, respectively (P<.05). Postoperative nausea and vomiting were absent in both groups, while sedation was unrecordable in almost two-thirds of the patients (67.4%). The percentage of fully awake patients was slightly higher in group B compared to group T (70% vs 65.2%, respectively). However, this difference was not statistically significant (P=.946). Consumption of fentanyl in the PACU was higher among group B patients than group T (65% vs 18%, P=.005) with 35% of group B needing 75 μg of fentanyl and 30% needing 100 μg of fentanyl to relieve pain. Consumption of diclofenac in the first postoperative day was higher in group B (80%) than group T (21.7%) (P<.001, Table 2).
Table 1.
Characteristics of the study groups (n=23 in group T, n=20 in group B).
| Group B (bupivacaine) | Group T (tramadol) | |
|---|---|---|
| Age (years) | 39.7±3.9 | 40.3±3.6 |
| Body mass index (kg/m2) | 27.1±2.6 | 27.5±2.5 |
| Duration of anethesia (min) | 110.80±10.25 | 108.17±11.33 |
| Duration of surgery (min) | 100.55±9.88 | 98.22±10.77 |
Values are mean±standard deviation
Figure 1.
Changes in mean VAS scores during the first 24 hours in the two study groups.
Table 2.
Consumption of fentanyl and diclofenac in postoperative period.
| Study group | Total | P value (Group B vs. Group T) | ||
|---|---|---|---|---|
| Group B | Group T | |||
|
| ||||
| Dose of fentanyl (μg) | .005 | |||
|
| ||||
| No intake | 7 (35%) | 19 (82%) | 26 (60.5%) | |
|
| ||||
| Total dose of 75 μg | 7 (35%) | 3 (13%) | 10 (23.3%) | |
|
| ||||
| Total dose of 100 μg | 6 (30%) | 1 (4.3%) | 7 (16.3%) | |
|
| ||||
| Dose of diclofenac (mg) | .001 | |||
|
| ||||
| No intake | 4 (20%) | 18 (78.3%) | 22 (51.2%) | |
|
| ||||
| Total dose of 100 mg | 16 (80%) | 5 (21.7%) | 21 (48.8%) | |
|
| ||||
| Total number of patients | 20 (100%) | 23 (100%) | 43 (100%) | |
B: Bupivicaine group, T: Tramadol group
DISCUSSION
Although local anesthetic instillation into surgical wounds was found to be effective in many studies,1–3,10–12,14–16 the consequences of inadvertent local anaesthetic injection might be lethal since it can be associated with central nervous system and cardiovascular system toxicity. 5 Clinical studies have shown that tramadol has a local anesthetic effect6,7 with minimal sedation and cardiovascular compromise.8–12,14 Grecek et al observed that subcutaneous tramadol infiltration can provide effective analgesia and anti-inflammatory effects.12 Jou et al suggested that tramadol exerts its sensory blocking action by a mechanism similar to that of local anaesthetics in the form of blocking the voltage-dependent Na channel14 while Mert and colleagues proposed that the calcium concentration in the test solution enhanced the conduction blocking activity of tramadol and reduced it in the presence of lidocaine.15 Guven et al concluded that tramadol applied to rat sciatic nerve might block the Na channel in a manner similar to lidocaine and block potassium channels more than lidocaine.16 They found that the depolarization time of the compound action potential (CAP) was extended equally by applying both lidocaine and tramadol, while tramadol extended half the width of the CAP more than lidocaine due to K channel blocking activity.16 In our study, we found that local wound infiltration with tramadol prior to wound closure in herniorrhaphy provided significant postoperative analgesia when compared to bupivacaine (onset of postoperative pain 6.6±0.9 hours vs 3.7±0.7 hours, respectively).
Altunkaya et al observed that subcutaneous 2 mg/kg tramadol had a local anesthetic action similar to 1 mg/kg lidocaine and they correlated that to its antinociceptive effect, which might be extended into the postoperative period.10 They noticed that the duration of analgesia provided by subcutaneous tramadol with adrenaline was significantly longer than that of lidocaine plus adrenaline (tramadol 4.9±0.3, lidocaine 4.4±0.7 hours). Demiraran and colleagues demonstrated the same postoperative pain relief among post-herniotomy pediatric patients. They used 2mg/kg tramadol in comparison to 0.2 mL/kg of 0.25% bupivacaine and reached the same conclusion.11 In our study, we achieved the same analgesic time in the tramadol group (group T 6.6±0.9 hours) using a smaller dose of tramadol (1 mg/kg) among adult post-herniorrhaphy patients. The achievement of a similar analgesic time despite the use of a smaller tramadol dose might be related to the synergistic effect of the intra-operative fentanyl or the difference in the patient ages in the two studies. The elimination pharmacokinetics of tramadol are appropriately described by a two-compartment model, with a reported elimination half life of 5.1±0.8 hours.17 While parenteral tramadol administered at the time of wound closure relieved postoperative pain for the limited time of 60–90 minutes, 18,19 locally infiltrated tramadol achieved a longer analgesic time than the reported elimination half-life of parenteral tramadol, which might be related to its local effect rather than to systemic absorption.
As in the study by Demiraran et al, there was no significant difference between the study groups in nausea, vomiting and sedation, which might be related to the dose, the route of administration, the timing of infiltration or the small sample size. To our knowledge, no study has evaluated the pharmacokinetics of locally infiltrated tramadol and further study are needed. In conclusion, locally infiltrated tramadol provides an improved postoperative analgesia in comparison to bupivacaine with no significant side effects in this set of patients.
REFERENCES
- 1.Reid MF, Harris R, Phillips PD, et al. Daycase herniotomy in children, A comparison of ilio-inguinal nerve block and wound infilteration for post operative analgesia. Anaesthesia. 1987;42:658–661. doi: 10.1111/j.1365-2044.1987.tb03095.x. [DOI] [PubMed] [Google Scholar]
- 2.Tverskoy M, Cozacov L, Ayache M, et al. Postoperative pain after inguinal herniorraphy with different types of anesthesia. Anesth Analg. 1990;70:29–35. doi: 10.1213/00000539-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hashemi K, Middleton MD. Subcutaneous bupivicaine for postoperative analgesia after herniorraphy. Ann R Coll Surg Engl. 1983;65:38–39. [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf CJ, Chong MS. Preemptive analgesia: treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 5.Covino BG, Wildsmith JAW. Clinical pharmacology of local anesthetic agents. In: Cousins MJ, Bridenbaugh PO, editors. Neuronal Blockade. 3rd ed. Philadelphia: Lippincott-Raven; 1998. p. 107. [Google Scholar]
- 6.Acalovschi I, Cristea T, Margarit S, et al. Tramadol added to lidocaine for intravenous regional anesthesia. Anesth Analg. 2001;92:209–214. doi: 10.1097/00000539-200101000-00040. [DOI] [PubMed] [Google Scholar]
- 7.Robaux S, Blunt C, Veil E, et al. Tramadol added to 1.5% mepivicaine for axillary brachial plexus block improves postoperative analgesia dose-dependently. Anesth Analg. 2004;98:1172–1177. doi: 10.1213/01.ANE.0000108966.84797.72. [DOI] [PubMed] [Google Scholar]
- 8.Tarradell R, Pol O, Farre M, et al. Respiratory and analgesic effects of meperidine and tramadol in patient undergoing orthopedic surgery. Methods Find Exp Clin Pharmacol. 1996;18:211–218. [PubMed] [Google Scholar]
- 9.Houmes R, Voets MA, Verkaaik A, et al. Efficacy and safety of tramadol versus morphine for moderate and severe postoperative pain regarding respiratory depression. Anesth Analg. 1992;74:510–514. doi: 10.1213/00000539-199204000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Altunkaya H, Ozer Y, Kargi E, et al. The postoperative analgesic effect of tramadol when used as subcutaneous local anesthetic. Anesth Analg. 2004;99:1461–1464. doi: 10.1213/01.ANE.0000135640.21229.A0. [DOI] [PubMed] [Google Scholar]
- 11.Demiraran Y, Ilce Z, Kocaman B, et al. Does tramadol wound infiltration offer an advantage over bupivacaine for postoperative analgesia in children following herniotomy? Pediatr Anaesth. 2006;16(100):1047–50. doi: 10.1111/j.1460-9592.2006.01910.x. [DOI] [PubMed] [Google Scholar]
- 12.Gercek A, Eti Z, Gogus FY, et al. The analgesic and anti-inflammatory effect of subcutaneous bupivicaine, morphine and tramadol in rats. Agri. 2004;16:53–58. [PubMed] [Google Scholar]
- 13.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–933. [PubMed] [Google Scholar]
- 14.Jou IM, Chu KS, Chen HH, et al. The effect of intrathecal tramadol on spinal somatosensory-evoked potentials and motor evoked responses in rats. Anesth Analg. 2003;96:783–8. doi: 10.1213/01.ANE.0000049683.58980.30. [DOI] [PubMed] [Google Scholar]
- 15.Mert T, Gunes Y, Guven M, et al. Comparison of nerve conduction blocks by an opioid and a local anesthetic. Eur J Pharmacol. 2002;439:77–81. doi: 10.1016/s0014-2999(02)01368-7. [DOI] [PubMed] [Google Scholar]
- 16.Guven M, Mert T, Gunay I. Effects of tramadol on nerve action potentials in rats: comparisons with benzocaine and lidocaine. Br J Anaesth. 2005;94(4):520–3. doi: 10.1080/00207450590520948. [DOI] [PubMed] [Google Scholar]
- 17.Shipton EA. Tramadol: present and future. Anesth Intensive Care. 2000;28:363–74. doi: 10.1177/0310057X0002800403. [DOI] [PubMed] [Google Scholar]
- 18.Naguib M, Attia M, Samarkandi A. Wound closure tramadol administration has a short-lived analgesic effect. Can J Anesth. 2000;47(8):815–17. doi: 10.1007/BF03019487. [DOI] [PubMed] [Google Scholar]
- 19.Coetzee JF, Van Loggerenberg H. Tramadol or morphine administered during operation: a study of immediate postoperative effects after abdominal hysterectomy. Br J Anaesth. 1998;81:737–41. doi: 10.1093/bja/81.5.737. [DOI] [PubMed] [Google Scholar]