Abstract
BACKGROUND
Hirschsprung disease [HD] is a predominantly childhood disorder of intestinal motility with a multifactorial and polygenic etiology. The objective of this study was to document the clinical and pathological features of HD in Kuwait, which has an estimated consanguinity rate of 54%.
METHODS
We analyzed all rectal and colonic biopsies (n=268) for suspected HD identified from the records in the Pathology Department of Al-Sabah Hospital for the period between 1994 and 2004.
RESULTS
One hundred and two patients (87 males and 15 females) had histologically confirmed HD. Fifty-eight (57%) were neonates (<1 month of age), while 21% were more than 4 months old. The diagnosis was based on open biopsy in 11 cases and rectal biopsies in 91 cases. Nine patients with open biopsies presented as intestinal obstruction, necrotizing enterocolitis, or perforation. The extent of the disease was unknown in 13 patients. There were 67 males and 3 females with short segment HD. Nine had long segment, two ultra-short segment and eight total colonic aganglionosis (TCA). Five TCA cases involved the small intestine. A skip area was observed in two cases. Six patients had other anomalies. A positive family history for HD was established in three patients. Two of these were male siblings from a consanguineous marriage and had Waardenburg syndrome.
CONCLUSION
This study has highlighted an exceptionally strong male predominance of short segment and a relatively high frequency (5.6%) of small intestinal involvement in HD in Kuwait. These data call for a more detailed epidemiological study with special emphasis on genetics.
Hirschsprung disease (HD), also known as congenital megacolon, is a developmental disorder characterized by an absence of enteric ganglion cells for a variable distance starting from the rectum. This has been attributed to defective migration or destruction of enteric nervous system precursor cells of neural crest origin.1 Its clinical manifestations include chronic constipation, delayed passage of meconium or intestinal obstruction. Although most cases are diagnosed during the neonatal period, a small percentage of patients present after the first year of life or in adulthood.
The etiology of HD is multi-factorial and polygenic. The occurrence of discordant forms of the disease in monozygotic twins suggests that adverse intrauterine environmental factors may have etiological significance. 2,3 In this regard, the role of pyrexia in early pregnancy has attracted attention.4 While the etiological significance of environmental factors remains speculative, there is sufficient evidence to implicate genetic factors. Mutations have been demonstrated in at least eight genes in HD patients,5,6,7 which strongly supports a genetic basis for the disease. The complex, rather than simple Mendelian inheritance observed in this disease has been attributed to variations in gene penetrance and expression. Recessive mutations may be propagated by consanguineous marriages.
Based on the extent of intestinal involvement, HD is classified as ultra-short segment (USSHD), short segment (SSHD), long segment (LHSD) or total colonic aganglionosis (TCA). The criteria for the diagnosis of USSHD remain controversial. Some authors reserve this diagnosis for cases with clinical, manometric and radiological features of HD but in which ganglion cells are present in rectal biopsies. Abnormal innervations in the lower rectoanal region and achalasia have been suggested as the possible etiological factors in these cases.1,8,9 Others classify cases with aganglionosis up to 4 cm above the pectinate line as USSHD.10 A peculiar pattern of acetylcholinesterase activity has been noted in some of these cases.11
A definitive diagnosis of HD is based on histological examination of rectal or colonic biopsies. Although constipation is the primary clinical presentation, it must be stressed that only about 12% to 17% of constipated children have HD.12 Radiological investigations and/or rectal manometry are considered mandatory prebiopsy screening procedures to reduce the number of unnecessary rectal biopsies.
Despite a high rate of consanguinity, there are few reports of HD in the Gulf States in the English literature.13,14 The objective of this study was to document the clinical and pathological features of HD in Kuwait, an Arabian Gulf State with an estimated consanguinity rate of 54%.15
Methods
The study was retrospective and covered the period 1994 to 2004 (11 years). During this period, the Department of Pediatric Surgery, Ibn Sina Hospital, managed all pediatric HD cases in Kuwait. Tissue examination was done at the Department of Pathology, Al-Sabah Hospital, Kuwait. All rectal and colonic biopsies for suspected HD done during the study period were identified in the records of the Pathology Department, Al-Sabah Hospital. Pullthrough specimens were also culled and the extent of intestinal involvement recorded. Age, sex, clinical presentation and diagnosis were analyzed.
A minimum of 30 haematoxylin and eosin (H&E) stained serial sections were examined before a diagnosis of HD was made on rectal biopsies taken at a distance of not less than 2 cm above the pectinate line. An entire block of biopsies with squamo- columnar junction or insufficient submucosa were serially sectioned and examined. They were reported as inadequate for assessment if ganglion cells were absent. The ideal biopsy is 0.2 cm in diameter and shows a thickness of submucosa equal to that of mucosa.9 All cases in which aganglionosis were observed in the recto-sigmoid colon, irrespective of distance, were classified as short segment. Ultrashort segment was reserved for cases with clinical and radiological features of HD and the presence of ganglion cells in the rectum.
Results
During the study period, a total of 268 pediatric patients had forceps/suction rectal or open biopsies as part of an investigation for HD. HD was confirmed in 102 (38%). The diagnosis was made from suction or forceps rectal biopsy in 91 cases and open biopsy in 11 cases. Six of the latter presented with intestinal obstruction and three with intestinal perforation.
The age range of HD patients was between 1 day and 13 years. The relative frequency of HD peaked during the neonatal period (57%) and markedly declined afterwards. Only about 21% of HD patients were older than 4 months. A total of 14 patients presented after the age of 1 year. In this group, 8 (54%) were aged between 2 and 3 years. There were 87 males and 15 females. In the group under the age of 1 year, a strong male predominance was observed. The group of patients older than one year comprised 8 males and 6 females. No significant sex difference was seen in this group.
Most patients (82%) presented with constipation, defined as either failure to pass meconium within 48 hours in most neonates or infrequent passage of stool of increased consistency in older children. This was the most common presenting complaint (Table 1), irrespective of age group and was associated with abdominal distension in 4 cases and necrotizing enterocolitis in 1 patient. Patients presenting with intestinal obstruction were all less than 3 months old. The majority (4/6) were neonates and in 2 patients the intestinal obstruction was associated with necrotizing enterocolitis. Three patients presented with intestinal perforation. In 2 neonates, the perforation was caecal. The third patient was 50 days old and had rectal perforation.
Table 1.
Frequency of presenting complaints.
| Clinical Presentation | Number of Cases (%) |
|---|---|
| Constipation | 84 (82.4)) |
| Abdominal distension | 9 (8.8) |
| Intestinal obstruction | 6 (5.9) |
| Intestinal perforation | 3 (2.9) |
| Total | 102 (100) |
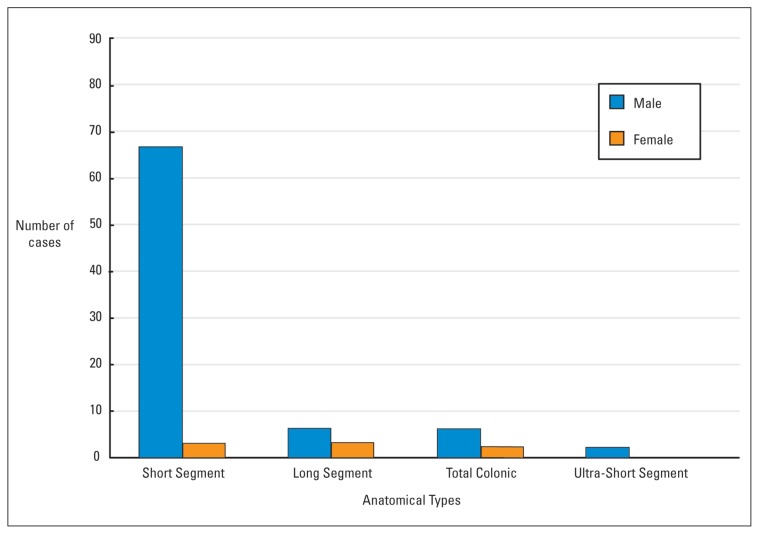
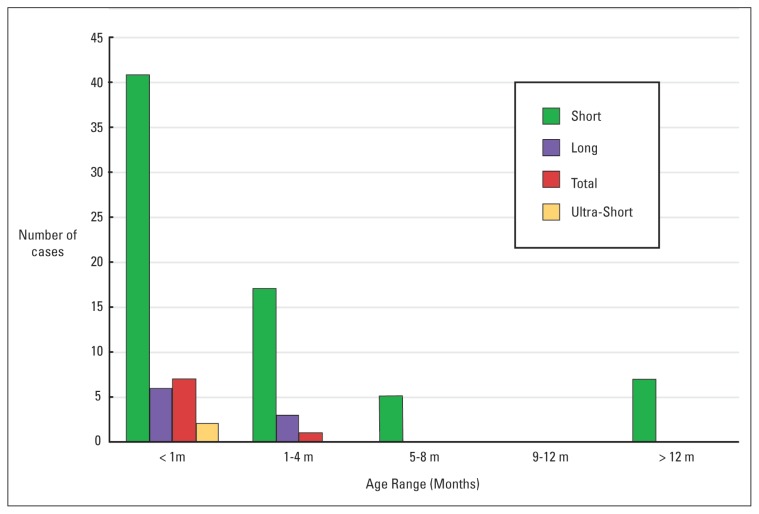
Information on the extent of the disease was available for 89 patients. Of the remainder, one died of necrotizing enterocolitis and sepsis shortly after the rectal biopsy. The rest were lost to follow up or had gone abroad for treatment. Seventy patients (79%) had short segment disease in which aganglionosis was confined to the rectosigmoid region. This pattern showed a striking male predisposition (67 males, 3 females) (Figure 1) and was the only form of disease seen in patients older than one year (Figure 2). Eight patients (6 males, 2 females) had total colonic aganglionosis. In two of these, a skip lesion was observed at the mid-transverse colon and proximal caecum, respectively. In 5 patients (4 males and 1 female) aganglionosis extended up to the ileum or jejunum. There were 9 cases of long segment and 2 cases of ultra-short segment HD.
Figure 1.
Sex distribution of anatomical types of Hirschsprung disease in 102 cases.
Figure 2.
Age distribution of anatomical types of Hirschsprung disease in 102 cases.
Other congenital anomalies were found in 6 patients (5.8%). Two patients had Waardenburg syndrome. Both were males from a consanguineous marriage and had 3 normal female siblings. The aganglionosis extended up to the ileum in one and the jejunum in the other. The second sibling also had malrotation of the appendix. There were 2 patients with Down’s syndrome and 1 with Rubenstein-Taybi syndrome and 1 with Meckel’s diverticulum. All four had either short segment (3 cases) or ultrashort segment HD.
Discussion
The results of this study indicate that there is a strong male predominance (5.8:1) in HD in Kuwait. This is consistent with reports from other parts of the world, which give sex ratios that vary from about 2.9 to 4.5:1.14,16 However, the male:female ratio of about 22.3:1 observed in the SSHD in this series is exceptionally high and inconsistent with most reports.17,18 This warrants further investigation. Similarly, the majority of cases were diagnosed during the neonatal period. While this confirms the general tendency of early diagnosis worldwide, the percentage of neonatal cases in Kuwait (57%) is significantly lower than what has been observed in Australia (90%).19 Besides, a relatively high percentage (13%) of HD patients in Kuwait were more than 1 year old. Late presentation of HD patients has been reported in many parts of the world and has been attributed to cultural differences, neglect of initial symptoms and prolonged home treatment with laxatives.20 Increased awareness of the disease by parents will probably decrease the frequency of late presentation.
The relative frequency of TCA was high in this series (8.9%). Although this is higher than what has been reported in most studies, it is consistent with the observation by Rescorla et al.16 Skip area, also known as segmental or zonal aganglionosis,21,22,23 is a condition in which an area with ganglion cells is present within an otherwise aganglionic colon. The existence of skip areas in a subset of patients with HD is a rare phenomenon that poses practical challenges to both pathologists and surgeons and has been attributed to an extramural phase of neuroblast migration, which is unique to the colon.22 Ignorance of this variant may result in inadequate management with a disastrous effect on the patient. This was observed in 2 patients in this series. One died of enterocolitis and sepsis.
In addition, there was a high relative frequency of involvement of the small intestine by HD (5.61%) in this series. Male predominance was more pronounced in the group of patients with extension of the disease to the small intestine (4:1) than in those in which the disease was confined to the colon (3:1). Both observations appear peculiar to Kuwait.24 The frequency of other congenital anomalies was low (5.8%) in this series. Although consanguinity was established in 2 patients, it was not specifically analyzed for the other patients.
The results of this study underscore the need for more parental education and further epidemiological study of HD in Kuwait with special emphasis on genetics and consanguinity.
References
- 1.Reyes-Mugica M. Hirschsprung Disease. Path Case Rev. 2000;5:51–59. [Google Scholar]
- 2.Siplovich L, Carmi R, Bar-Ziv J, Karplus M, Mares AJ. Discordant Hirschsprung’s disease in monozygotic twins. J Pediatr Surg. 1983;18:639–640. doi: 10.1016/s0022-3468(83)80383-2. [DOI] [PubMed] [Google Scholar]
- 3.Moore TC, Landers DB, Lachman RS, Ament ME. Hirschsprung’s disease discordant in monozygotic twins: a study of possible environmental factors in the production of colonic aganglionosis. J Pediatr Surg. 1979;14:158–161. doi: 10.1016/0022-3468(79)90009-5. [DOI] [PubMed] [Google Scholar]
- 4.Smith MS, Edwards MJ, Upfold JB. The effects of hyperthermia on the fetus. Dev Med Child Neurol. 1986;28:806–809. doi: 10.1111/j.1469-8749.1986.tb03936.x. [DOI] [PubMed] [Google Scholar]
- 5.Kapur RP. Hirschsprung disease and other enteric dysganglionoses. Crit Rev Clin Lab Sci. 1999;36:225–273. doi: 10.1080/10408369991239204. [DOI] [PubMed] [Google Scholar]
- 6.Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin Pediatr. 2000;12:610–617. doi: 10.1097/00008480-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet. 2001;38:729–739. doi: 10.1136/jmg.38.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neilson IR, Yazbeck S. Ultrashort Hirschsprung’s disease: myth or reality. J Pediatr Surg. 1995;11:1135–1138. doi: 10.1016/0022-3468(90)90748-x. [DOI] [PubMed] [Google Scholar]
- 9.Moore EI, Baxendene JJ. Diagnosis and molecular pathology of Hirschsprung’s disease. In: Lowe David G, Underwood James CE., editors. Recent Advances in Histopathology. Vol. 19. Edinburgh: Churchill Livingstone; 2001. pp. 51–63. [Google Scholar]
- 10.Ballard ET. Ultrashort segment Hirschsprung’s disease: a case report. Pediatr Pathol Lab Med. 1996;2:319–325. [PubMed] [Google Scholar]
- 11.Meier-Ruge WA, Bruder E, Holschneider AM, Lochbuhler H, Piket G, Posselt HG, Tewes G. Diagnosis and therapy of ultrashort Hirschsprung’s disease. Eur J Pediatr Surg. 2004;6:392–397. doi: 10.1055/s-2004-830354. [DOI] [PubMed] [Google Scholar]
- 12.Lewis NA, Levitt MA, Zallen GS, Zafar MS, Iacono KL, Rossman JE, et al. Diagnosing Hirschsprung’s disease: Increasing the odds of a positive rectal biopsy result. J Pediatr Surg. 2003;38:412–416. doi: 10.1053/jpsu.2003.50070. [DOI] [PubMed] [Google Scholar]
- 13.Khan AR, Vujanic GM, Huddart S. The Constipated child: how likely is Hirschsprung’s disease? Pediatr Surg Int. 2003;6:439–442. doi: 10.1007/s00383-002-0934-9. [DOI] [PubMed] [Google Scholar]
- 14.Rajab A, Freeman NV, Patton MA. Hirschsprung’s disease in Oman. J Pediatr Surg. 1997;32:724–727. doi: 10.1016/s0022-3468(97)90015-4. [DOI] [PubMed] [Google Scholar]
- 15.Hijazi Z, Haider MZ. Influence of consanguinity and IgE receptor phenotypes on clinical manifestations of asthma in Kuwaiti children. J Trop Pediatr. 2001;47:13–16. doi: 10.1093/tropej/47.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Rescorla FJ, Morrison AM, Engles D, West KW, Grosfeld JL. Hirschsprung’s disease. Evaluation of mortality and long term function in 260 cases. ArchSurg. 1992;127:934–941. doi: 10.1001/archsurg.1992.01420080068011. [DOI] [PubMed] [Google Scholar]
- 17.Klein MD, Philippart AI. Hirschsprung’s disease: three decades experience at a single institution. J Pediatr Surg. 1993;10:1291–1293. doi: 10.1016/s0022-3468(05)80315-x. [DOI] [PubMed] [Google Scholar]
- 18.Russel MB, Russel CA, Niebur E. An Epidemiological study of Hirschsprung’s disease and additional anomalies. Acta Pediatr. 1994;1:68–71. doi: 10.1111/j.1651-2227.1994.tb12955.x. [DOI] [PubMed] [Google Scholar]
- 19.Singh SJ, Croaker GD, Manglick P, Wong CL, Athanasakos H, Elliot E, Cass D. Hirschsprung’s disease: The Australian Pediatric Surveillance Unit’s experience. Pediatr Surg Int. 2003;19:247–250. doi: 10.1007/s00383-002-0842-z. [DOI] [PubMed] [Google Scholar]
- 20.Archibong AE. Pattern of aganglionic megacolon in Calabar, Nigeria. S Afr Med J. 2002;92:642–644. [PubMed] [Google Scholar]
- 21.Martin LW, Buchino JJ, LeCoultre C, Ballard ET, Neblett WW. Hirschsprung’s disease with skip area (segmental aganglionosis) J Pediatr Surg. 1979;6:686–687. doi: 10.1016/s0022-3468(79)80245-6. [DOI] [PubMed] [Google Scholar]
- 22.de Chadarevian JP, Slim M, Akel S. Double zonal aganglionosis in long segment Hirschsprung’s disease with a “skip area” in transverse colon. J Pediatr Surg. 1982;2:195–197. doi: 10.1016/s0022-3468(82)80213-3. [DOI] [PubMed] [Google Scholar]
- 23.Kapur RP, deSa DJ, Luquette M, Jaffe R. Hypothesis: pathogenesis of skip areas in long-segment Hirschsprung’s disease. Pediatr Pathol Lab Med. 1995;1:23–37. doi: 10.3109/15513819509026937. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K, Goto S. Total colonic aganglionosis with or without small bowel involvement:an analysis of 137 patients. JPediatr Surg. 1986;21:319–322. doi: 10.1016/s0022-3468(86)80193-2. [DOI] [PubMed] [Google Scholar]