Abstract
During the past century, three major influenza pandemics took place, leading to a devastating number of deaths. Pandemics occur through the emergence of a new strain of influenza virus that can infect humans, to which there is little pre-existing immunity and which spreads easily from human to human. The H5N1 influenza virus has the potential of becoming a pandemic virus, since it can infect humans and is highly pathogenic. All that remains is the final step of acquiring the genetic material to enable efficient human-to-human transmission. Therefore, the World Health Organization (WHO) has declared pandemic alert phase 3, the last phase before there is actual evidence of increased and efficient human- to-human transmission. In addition, every case of transmission of an avian influenza virus to humans is regarded by WHO as a cause for heightened alertness and surveillance. The circulation of highly pathogenic avian influenza viruses in large numbers among the poultry population in a growing number of countries is a major concern. Since the influenza viruses are highly unstable, the co-circulation of highly pathogenic animal viruses with human viruses may create opportunities for different species-specific viruses to exchange genetic material, giving rise to a new influenza virus to which humans would have little, if any, protective immunity. In this article, we highlight the current avian influenza situation from its different aspects with a special focus on the Hajj since we host over 2 million pilgrims a year in the holy cities of Mekkah and Medina.
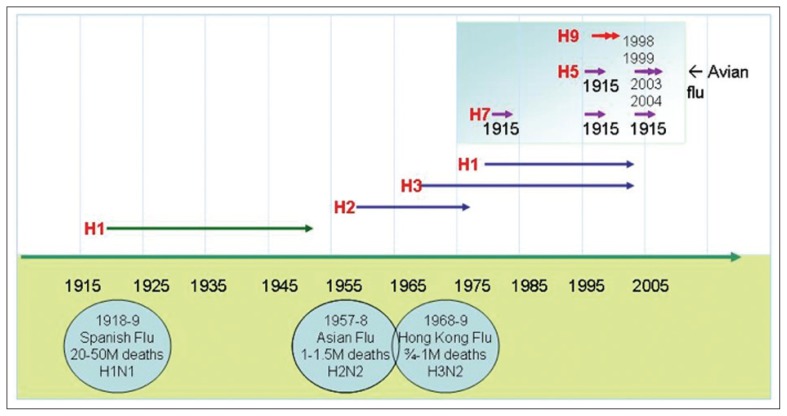
A heightened awareness, or if we may say an escalated fear, of the emergence of a new pandemic influenza virus has been witnessed over the past year, and not without good reason. The memories of previous pandemics are written in the books of history, where not thousands but millions of people around the world passed away from the disease.1 The currently circulating H5N1 avian influenza virus carries the potential to repeat this disaster (Figure 1).2 Wild aquatic birds, notably members of the order Anseriformes (ducks and geese) are the natural reservoir for avian influenza, but other birds may become infected. These and other animals are either asymptomatic carriers or develop disease. In the past, humans have suffered from infection by avian influenza viruses of subtypes H5, H7 and H9 as a deadend host (Table 1).
Figure 1.
Influenza virus epidemic and pandemic time line.
Table 1.
Strains of avian influenza A virus in documented human infections (1959–2006)
| Strain | Symptoms |
|---|---|
| H7N7 | Respiratory (pneumonia – respiratory symptoms in fatal case), Conjunctivitis |
| H5N1 | Respiratory (pneumonia) - respiratory insufficiency |
| H9N2 | Influenza like illness |
| H7N2 | Respiratory symptoms |
| H7N3 | Conjunctivitis |
Today the occurrence of an influenza pandemic is inevitable; the only question is when will it occur. The World Health Organization (WHO) relies on 112 influenza surveillance centers from over 83 countries around the world to decide on the yearly influenza vaccine make-up for each year, allowing for the high likelihood of a match between the antigenic components of the vaccine and the circulating virus.3 However, during the 2003/2004 influenza season the H3N2 circulating virus developed an over 80% drift from the H3N2 virus used to make one of the three major vaccine components.4 Based on this knowledge we have a strong reason to believe that once an avian influenza virus acquires genetic material transforming it into a highly infectious pathogen among humans, a pandemic will take place.
The mortality from the previous century’s three pandemics varied enormously, from 1 million to more than 45 million deaths (Figure 1). Some experts suggest that a future influenza pandemic will cause more deaths than that witnessed in the infamous 1918 Spanish flu pandemic when over 50 million deaths took place.5 The reason for such unavoidable deaths is related to the fact that since 1918 there has been limited development in influenza vaccine manufacturing abilities. Vaccines have never been available early enough and in sufficient quantities to have an impact on morbidity and mortality during a pandemic. Unfortunately, past problems related to the special nature of preparing the vaccine and the inadequacy of the manufacturing capacity to provide adequate doses worldwide continue.6 In addition to the challenges of vaccination, there are limited pharmaceutical options, i.e., few highly efficacious drugs against the influenza virus. Further, sophisticated modes of transportation nowadays allow for the easy movement and spread of influenza virus, facilitating its spread around the globe.
The virus dimension
The viruses that cause influenza are influenza type A viruses, which belong to the family Orthomyxoviridae. If a strain is capable of infecting birds it is called an avian influenza or “bird flu” virus. Two other types of the influenza virus are type B, which leads to sporadic cases among humans, and type C, which does not infect humans. The focus of this article will be on influenza A virus, which may infect humans and birds and most importantly has the capability of developing into a pandemic virus.7 The genome of the virus encodes for two important glycoproteins on the outer surface of the virus: hemagglutinin (H or HA) and neuraminidase (N or NA) proteins. On the basis of the antigenicity of these glycoproteins, influenza A viruses currently cluster into sixteen H (H1–H16) and nine N (N1–N9) subtypes. These clusters are substantiated by phylogenetic analysis of the nucleotide and deduced amino acid sequences of the HA and NA genes.8 Several subtypes of the virus exist as different combinations of the H and N glycoproteins. The influenza virus has a poor ability to proofread its genetic material while replicating, which results in repeated and frequent mistakes in the genetic encoding of the viruses’ outer glycoproteins, H and N. Such minor changes in the H and N protein occur frequently and account for the drift phenomena known in this virus.9 Further, influenza A viruses, including subtypes from different species, can exchange or “reassort” genetic material, resulting in novel subtypes different from both parent viruses. Such major changes in the make up of the virus are known as antigenic shifts, which may lead to emerging pandemic viruses (Table 2).10
Table 2.
Comparison of the antigenic shift and drift phenomena.
| Drift | Shift |
|---|---|
| Minor change, within subtype | Major change, new subtype |
| Point mutations | Exchange of gene segments |
| Occurs in A and B subtypes | Occurs in A subtypes only |
| May cause epidemics | May cause pandemic |
| Example: A/Fujian (H3N2) replaced A/Panama (H3N2) in 2003–2004 | Example: H3N2 replaced H2N2 in 1968. |
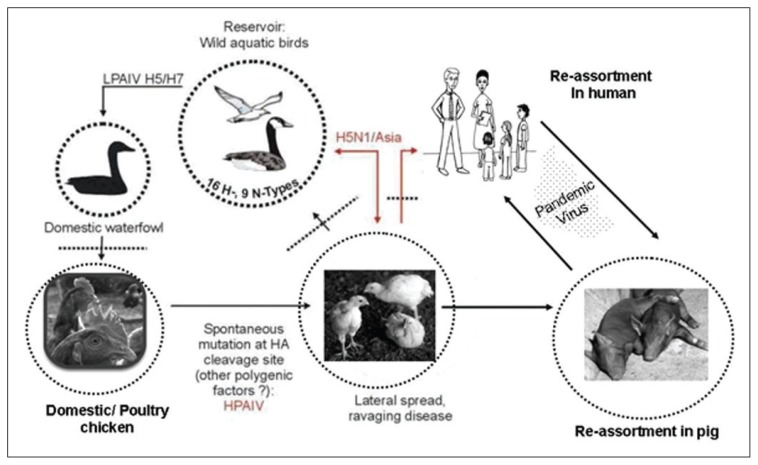
Influenza A viruses that infect birds are divided on the basis of their ability to cause disease into low pathogenic (LPAI) and highly pathogenic avian influenza viruses (HPAI), with the latter having a mortality rate among poultry as high as 100%.11 Since 1959, there have been only 19 reported primary isolates of HPAI from domestic poultry worldwide. Devastating pandemics take place when populations are exposed to a new viral subtype in the absence of immunity and protective vaccines. The infectious capabilities of an emerging new virus are likely to be acquired from the human influenza viruses. Conditions favorable for the emergence of an antigenic shift involve humans living in close proximity to domestic poultry and pigs.12 Because pigs are susceptible to infection with both avian and mammalian viruses, including human strains, they can serve as a “mixing vessel” for the exchange of genetic material between human and avian viruses, resulting in the appearance of novel subtypes (Figure 2). While the influenza pandemic of 1957 (H2N2) and 1968 (H3N2) arose through reassortment of genes between avian viruses and the prevailing human influenza strain, the influenza virus causing the Spanish flu appears to have been derived from an avian source.13,14
Figure 2.
Genetic reassortment of the avian influenza virus.
The animal dimension
Sixteen subtypes of influenza virus are known to infect birds, thus providing an extensive reservoir of influenza viruses potentially circulating among the bird population (Figure 2). Almost all birds are thought to be susceptible to infection with avian influenza virus, though some species are more resistant to infection than others. These birds excrete large quantities of virus in their respiratory secretion and feces (108.7× 50% egg infective dose per gram feces.15 Migratory waterfowl, including wild ducks, are the natural reservoir for avian influenza viruses, and these birds are also the most resistant to infection. Domestic poultry, however, including chickens and turkeys, are particularly susceptible to epidemics of rapidly fatal influenza.11 Some secondary infections or environmental conditions may cause exacerbation of LPAI infections leading to more serious disease. Indeed, LPAI viruses are capable of undergoing a series of mutational events in poultry. After several cycles of infection, the virus adapts to the host and may have the capability to unexpectedly switch to an HPAI strain by insertional mutations leading to more serious disease (Figure 2).
A wide spectrum of symptoms are noticed among birds, ranging from none (asymptomatic) to coughing, sneezing, incoordination, poor egg production, misshapen eggs, swelling around the eyes, comb and wattle, and ischemia and necrosis of the internal organs. Birds with severe disease have been described as being highly contagious and infection is rapidly fatal resulting in severe epidemics among poultry. In cases of HPAI infection, sudden onset of severe illness and rapid death may lead to mortality rates approaching 100%, leading to major economic losses.11
Natural infection with H5N1 has also been described in tigers and other large cats in a zoo in Thailand, the first report of H5N1 in Felidae.16 Infection occurred after the animals were fed with virus-infected chicken carcasses. Cat-to-cat transmission was also reported in the same zoo.
Outbreaks of avian influenza among poultry
It has been shown that viruses of low pathogenicity can, after circulation for short periods in a poultry population, mutate into HPAI viruses, leading to major outbreaks among poultry. A severe epidemic occurred in Italy in 1999/2000, causing 413 outbreaks with 16 million birds affected.17,18 During the 1983–1984 epidemic in the United States, an H5N2 virus initially caused low mortality, but within six months became highly pathogenic, leading to high mortality rates, approaching 90%. Control of the outbreak required the sacrifice of more than 17 million birds at a cost of nearly US $61 million.19 An LPAI H5N1 strain transformed within months to an HPAI strain, leading to the culling of more than 34 million of Vietnam’s 250 million birds in the current epidemic, and efforts to combat the disease are estimated to be US $4 million a day. To date, all outbreaks of HPAI have been caused by influenza A viruses of subtypes H5 and H7. Direct or indirect contact of domestic birds with wild migratory waterfowl has been implicated as a frequent cause of epidemics. Backyard farming and live bird markets also play an important role in the spread of epidemics.
Containment of poultry at the farm level
Apart from being highly contagious, avian influenza viruses are readily transmitted from farm to farm mechanically by contaminated equipment, vehicles, food, cages or clothing. In addition, HPAI viruses may survive for long periods of time in the environment, especially when temperatures are low. Stringent sanitary measures on farms can, however, confer some degree of protection. In the absence of prompt control measures backed by good surveillance, epidemics can last for years.20 For example, an epidemic of H5N2 avian influenza, which began in Mexico in 1992, started with a virus with low pathogenicity and evolved to a highly fatal form, and was not controlled until 1995.
The quarantining of infected farms and destruction of infected or potentially exposed flocks are standard control measures aimed at preventing the spread of the virus to other farms and eventual establishment in a country’s poultry population. Some of the major challenges to containing HPAI outbreaks, especially in far eastern countries, include the backyard farming phenomenon, the economic significance of poultry products, a lack of control experience, a lack of resources, uncertainty over the efficacy of bird influenza vaccines as well as difficulty in vaccinating all birds, and more importantly, the infeasibility of eradicating the reservoir.
The human dimension
Historically, human infections with avian influenza A viruses have been extremely rare. H5N1 has been the exception with more than 216 cases of human infections reported since 1997. The death rate from these cases has exceeded 54%.21 These viruses may also trigger inappropriate innate immune responses in humans, leading to severe respiratory distress and multisystem failure. Most cases of avian influenza infection in humans are thought to have resulted from direct contact with infected poultry or surfaces contaminated with large amounts of the virus excreted in the dropping of infected birds. These droppings dry and become pulverized and are then inhaled. Plucking and preparing diseased birds, children playing with poultry, particularly asymptomatic infected ducks, and consumption of duck’s blood and undercooked poultry have all been implicated as risks for acquiring the disease.22 To date, there is no evidence that properly cooked poultry meat or products are a source for human infections.
No sustained human-to-human transmission of influenza A (H5N1) has been reported. However, reports of several household clusters suggest human-to-human transmission. In one recent report of apparent child-to-mother transmission, very close contact without the use of precautions was documented, 23 but no cases of human-to-human transmission by small particle aerosols have been identified yet. It seems very plausible that H5N1 viruses have the potential to ignite a most ravaging pandemic should they acquire the capacity to be transmitted from person to person either through mutation or reassortment with human influenza viruses. Luckily, evidence for genetic reassortment between human and avian influenza virus genes has not yetbeen found.
There is no definite incubation period for avian influenza in humans. Intervals between cases in household clusters have ranged from 2–5 days and 8–17 days.22 Most patients have been previously healthy young children or adults. Reported symptoms of avian influenza include typical influenza-like illness, such as fever, sore throat, cough and muscle aches, with lower respiratory tract symptoms. Clinically apparent pneumonia has been reported in most cases along with radiological changes.22,24 Besides respiratory symptoms, a large proportion of patients also complain of gastrointestinal symptoms such as diarrhea, vomiting, and abdominal pain, which are more common in children than adults. Unlike human infections with H7 virus, conjunctivitis is not prominent in H5N1-infected patients. The clinical course of the illness in severe cases includes acute respiratory distress syndrome (ARDS) necessitating ventilatory support, multiorgan failure with renal dysfunction, and cardiac compromise. The case fatality rate has been 89% among children less than 15 years of age and most deaths have been attributed to progressive respiratory failure. Striking results on routine laboratory tests, especially in severe cases, were an early onset of lymphopenia, with a pronounced inversion of the CD4+/CD8+ ratio, thrombocytopenia, and increased levels of serum transaminases.24
Rapid and accurate diagnosis of suspected cases of H5N1 infection is of vital importance for the initiation of proper management and, more importantly, for the containment of the infection by implementing strict infection control practices early on. The diagnosis depends on demonstration of the virus by either cell culture, reverse transcriptase polymerase chain reaction (RT-PCR), immunofluorescent testing using monoclonal antibodies to H5N1 or a rising antibody titer using enzyme immunoassay (EIA) methodology. Detection of influenza A/H5 by real-time RT-PCR offers a rapid and highly sensitive method to diagnose H5N1 infection. A fourfold rise in titre from an acute to convalescent specimen is also diagnostic of infection in patients that recover.26 Samples must be transported in clearly labeled containers and the laboratory should be informed of the specimen prior to delivery. Laboratories must apply good laboratory practices and standard precautions. For culture-based isolation, biosafety level-3 (BSL-3) facilities should be used. For RT-PCR, BSL-2 facilities using BSL-3 work practices should be implemented.27
The management of most hospitalized patients with avian influenza requires ventilatory support within 48 hours of admission. Early treatment with oseltamivir, one of two neuraminidase inhibitors licensed for use in influenza, may provide the greatest clinical benefit (Table 3).28 Optimal dosing and duration of treatment with oseltamivir is unclear. Clinical progression leading to death has been described despite early therapy. And high-level antiviral resistance to oseltamivir from the substitution of a single amino acid in N1 neuraminidase (His 274 Tyr) has been detected recently in several patients with influenza A (H5N1) who were treated with the drug. These variants retain full susceptibility to zanamivir and partial susceptibility to the investigational neuraminidase inhibitor permamivir.29 Inhaled zanamivir, the second neuraminidase inhibitor, has not been studied in cases of influenza A (H5N1) in humans, but topical zanamivir was found to be active in animal models of influenza A (H5N1).30 Many of the viruses isolated from humans have been found to be resistant to the adamantanes (amantadine, rimantadine), but resistant strains were less common in isolates from Indonesia (6.3% of isolates), China (8.9% of isolates) and Hong Kong (14.3% of isolates) in a recent study.31 Corticosteroids and interferon alfa have also been used in the treatment of human avian influenza with uncertain effects. Ribavirin should not be used for treatment since there is substantial first-pass hepatic metabolism and therefore no clinical benefit is expected. Intravenous antibiotic therapy should be considered if a secondary bacterial infection is suspected.
Table 3.
Treatment and prophylaxis for human avian influenza (H5N1) with oseltamivir (Tamiflu).28
| Adolescent/Adult | Children (age 1–12 yrs) weight (kg) | |||
|---|---|---|---|---|
| 1–15 | 15–23 | 23–40 | ||
|
| ||||
| Treatment | Mild cases: 75 mg twice daily for 7 days |
30 mg twice daily for 1 week | 45 mg twice daily for 1 week | 60 mg twice daily for 1 week |
|
| ||||
| Severe cases: 150 mg twice daily for 7 days |
||||
|
| ||||
| Prophylaxis (Post-exposure) | 75 mg once daily for 7 days | 30 mg once daily 7–10 days | 45 mg once daily 7–10 days | 60 mg once daily 7–10 days |
The few cases of possible human-to-human cases of transmission of influenza H5N1 involved prolonged close and unprotected contact with infected patients. As in human influenza, droplet and contact transmission are probably the most effective means of transmission of avian influenza virus between humans. Diarrhea in an H5N1-infected patient may contain infectious virus and thus represents a potential non-respiratory route of transmission that needs to be considered in infection control practices. Data on excretion patterns and periods of potential infectivity are lacking for human infections with avian influenza viruses. Based on our current knowledge, strict infection control measures should be implemented during contact with potentially infected birds or with patients with suspected or confirmed infection. Enhanced infection control precautions are warranted in healthcare facilities when dealing with suspected or confirmed cases of avian influenza.32 Standard precautions, including hand hygiene and facial protection, are on top of the list. Full barrier precautions including contact, droplet and airborne should be used when possible knowing that not all facilities will be able to implement full precautions. In addition to enforcing respiratory etiquette, limiting the number of healthcare workers caring for suspected/confirmed cases, enhancing environmental cleaning and disinfection in patient rooms, cohorting patients and limiting visitors all become important infection control measures to contain the disease. Post-exposure prophylaxis for unprotected healthcare workers and close contacts of infected patients is advisable.33
No influenza A (H5) vaccines are commercially available for humans. Vaccine production against HPAI is complicated because of the requirement for high biosafety containment facilities and the difficulty in some cases in obtaining high virus yields in embryonated eggs because of virus pathogenicity. Reverse genetic techniques, generation of recombinant hemagglutinin, DNA vaccination and the use of apathogenic H5 viruses have overcome some of the vaccine production obstacles. An experimental H5N1 vaccine in which important virulence determinants were altered using plasmid-based reverse genetics was recently used in a multicenter, doubleblind safety and immunogenicity study.34 Four hundred fifty-one healthy adults between the ages of 18 and 64 years received two intramuscular doses of the subvirion influenza A (H5N1) vaccine with different concentrations of hemagglutinin antigen or placebo. Although the vaccine was safe, the only group where more than 50% of the subjects reached the immunogenicity threshold of an antibody titer of 1:40 or greater were the subjects who received two doses of 90 microgram each, 28 days apart, a dose 12 times that of the seasonal influenza vaccine. Provision of an adequate supply of this vaccine for the world’s population would pose a great difficulty. Several points not answered by previous studies include the protective titer levels for influenza since people with low titers can show protection against influenza and people with high titers can have symptomatic infection. Another important issue is whether the vaccine offers cross protection against other H5N1 strains of influenza.34 Previous studies of new influenza A (H5N3) and (H2N2) vaccines administered with adjuvants (MF59 and aluminum hydroxide) showed higher immunogenicity that resulted in cross neutralizing antibodies against influenza A (H5N1).35 The Department of Health and Human Services at the National Institutes of Health has funded studies of more than 30 candidate vaccines. Results from these trials should be available in the next 12 months.
The real risk of bird flu in Saudi Arabia and the Hajj dimension
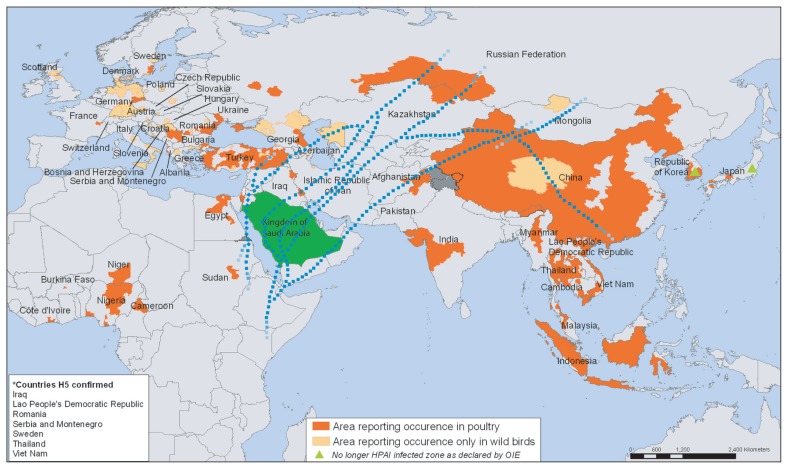
The HPAI H5N1 virus is of greatest concern at present. Of all avian viruses known to infect humans, H5N1 has caused the greatest morbidity and mortality. Moreover, the H5N1 virus has the potential to start an influenza pandemic. The virus has also proved to be particularly difficult to control in poultry populations and the H5N1 subtypes have established endemicity in birds in large parts of Southeast Asia. Wild migratory birds have played an important role in the spread of H5N1 from one country to another and across continents so there is little hope of preventing the spread of the virus worldwide. The Kingdom is on the flight paths of migratory birds (Figure 3), so the question is not whether the virus will arrive but when and are we ready? The immediate threat of outbreaks among poultry and the possible human-to-human transmission of avian influenza are clearly imminent. The Kingdom has the honor of hosting over 2 million pilgrims during the Hajj season as well as thousands of visitors throughout the year for Omrah. This on its own adds a different dimension to the existing challenge. Due to the extreme crowding during these religious events the facilitation of the rapid spread of the virus would seem inevitable.
Figure 3.
Areas of the world reporting confirmed occurrence of H5N1 avian influenza in poultry and wild birds since 2003 and bird migration routes reaching Saudi Arabia (from the WHO, Public Health Mapping and GIS Map Library, adapted with permission)
Experts believe that only countries with a pandemic preparedness plan and pre-existing care capacities will be able to respond quickly to pre-empt the pandemic or minimize its adverse impact. The Ministry of Health, the Ministry of Agriculture, and the National Commission of Wildlife Conservation and Development have taken initial steps for a contingency plan for influenza pandemic preparedness. However, there are still urgent needs to expand and strengthen the capacity of surveillance networking in the Kingdom for both human and animal influenza strains in all regions all year round. The formation of a National Influenza Center recognized by WHO has become a top priority for optimal country preparedness. We need to improve the infrastructure for rapid detection and diagnosis of early cases in the event of a pandemic by developing or collaborating with a central, recognized virology laboratory with experience in avian influenza. We must develop an adequate system to alert, communicate, and manage disasters with the involvement of veterinarians (e.g., for rapid sharing of data on poultry and bird infections), biologists, environmentalists, and physicians. An early warning system is critical because it will allow the government and citizens to take the appropriate action to mitigate the impact of a pandemic. This will require formation of a team of experts from all relevant sectors, but most importantly from the Ministries of Agriculture and Health to conduct regular meetings to review, update, and monitor the preparedness plan so any gaps can be covered as the infection will evolve rapidly.
In short, in view of the limited access to antivirals and almost non-existent pandemic influenza vaccines, a strong surveillance and early warning system is the cornerstone for proper pandemic preparedness. If the threat of an influenza pandemic is not ruffling our feathers, we are not sure what will.
References
- 1.Beveridge WIB. The chronicle of influenza epidemics. Hist Phil Life Sci. 1991:223–235. [PubMed] [Google Scholar]
- 2.BWHO 2004. World is ill-prepared for “inevitable” flue pandemic. Bull World Health Organ. 2004;82:317–318. [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. 2005k. [Accessed May 2006]. WHO Consultation on the composition of influenza vaccine for the Northern Hemisphere 2006–2007. http://www.who.imt/csr/disease/influenza/vaccinesnorth2006_7/cn/index.html/
- 4.Simson L. The threat of pandemic influenza: Are we ready? The National Academic Press; 2004. Pandemic influenza and mortality: Post evidence and projections for the future in: Board on global health. [Google Scholar]
- 5.Johnson NP, Muller J. Updating the accounts: Global mortality of the 1918–1920. “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105–15. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 6.Poland GA. Vaccine against avian influenza. A race against time. N Engl J Med. 2006;354(13):1411–13. doi: 10.1056/NEJMe068047. [DOI] [PubMed] [Google Scholar]
- 7.Dowdle WR. Influenza A virus recycling revisited. Bull World Health Organ. 1999;77:820–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Fouchier RA, Munster V, Wallensten A, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from block headed gulls. J Virol. 2005;79:2814–22. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson NM, Galvani AP, Bush RM. Ecological and immunological determinants of influenza evolution. Nature. 2003;442:428–33. doi: 10.1038/nature01509. [DOI] [PubMed] [Google Scholar]
- 10.Webster RG, Hulse DJ. Microbial adaptation and change: avian influenza. Rev Sci Tech. 2004;23:453–65. doi: 10.20506/rst.23.2.1493. [DOI] [PubMed] [Google Scholar]
- 11.Swayne DE, Suarez DL. Highly pathogenic avian influenza. Rev Sci Tech. 2000a;19:463–8. doi: 10.20506/rst.19.2.1230. [DOI] [PubMed] [Google Scholar]
- 12.Guan T, Poon LL, Cheum CY, et al. H5N1 Influenza: a proteam pandemic threat. Proc Natl Acad Sci USA. 2004;101:8156–61. doi: 10.1073/pnas.0402443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid AH, Fanning TG, Hultin JV, et al. Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proc Natl Acad Sci USA. 1999;96:1651–6. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaoka Y, Krauss S, Webster RG. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J Virol. 1989;63:4603–8. doi: 10.1128/jvi.63.11.4603-4608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster RG, Yakhno MA, Hinshaw VS, et al. Intestinal influenza: replication and characterization of the influenza virus in ducks. Virology. 1978;84:268–78. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thanawongnuwech R, Amonsin A, Tantilertcharoen R, et al. Probable tiger-to-tiger transmission of avian influenza H5N1. Emerg Infect Dis. 2005;11:699–701. doi: 10.3201/eid1105.050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banks J, Speidel ES, Moore E, et al. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of high pathogenic H7N1 avian influenza viruses in Italy. Arch Virol. 2001;456:963–73. doi: 10.1007/s007050170128. [DOI] [PubMed] [Google Scholar]
- 18.Capua I, Mitenellum F, Merangon S, et al. H7N1 avian influenza in Italy (1999–2000) in intensively reared chicken and turkeys. AV Pathol. 2000;29:537–43. doi: 10.1080/03079450020016779. [DOI] [PubMed] [Google Scholar]
- 19.Bean WJ, Kawaoka Y, Wood JM, et al. Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza A viruses: potential role of detecting interfering RNAs in nature. J Virol. 1958;54:151–60. doi: 10.1128/jvi.54.1.151-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Avian influenza A(H5N1)-update 31: Situation (poultry) in Asia: need for a long-term response, comparison with previous outbreaks. 2004. Mar 02, [Accessed May 2006]. http://www.who.int/csr/don/2004_03_02/eu/index/html/ [PubMed]
- 21.WHO. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported by WHO. 2006. [Accessed May 2006]. http://www.int.csr.disease/avian_influenza/country/eu.
- 22.WCWHO. The writing committee of the World Health Organization (WHO) consultation in human influenza A/H5. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;2005;353:1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 23.Ungchusakk A, Vewarakul P, Dowel SF, et al. Probable person-to-person transmission of avian influenza: A (H5N1) N Engl J. 2005;352:333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 24.Chot P, Tayasumond HT, Ungchusakk A, Hanshaoworakal U, et al. Human disease from influenza A (H5N1) Thailand 2004. Emerg Infect Dis. 2005;11:201–9. doi: 10.3201/eid1102.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jong MD, Back VC, Paht Q, et al. Fatal avian influenza A (H5N1) in child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–91. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 26.Yuen KY, Chan PK, Peris M, et al. Clinical feature and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 27.WHO. WHO global influenza preparedness plan: The role of WHO and recommendations for national measures before and during pandemics. 2005. [Accessed May 2006]. http://www.who.int/entity/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_5/en/index.html.
- 28.CDC Fact Sheet. Antiviral Agents for Influenza: Background Information for Clinicians. http://www.cdc.gov/flu/professionals/antiviralback.htm. Last modified March 31, 2006.
- 29.Hayden FG. Antivirals for pandemic influenza. IV peramivir in influenza A/duck/MN/1525/81 (H5N1) infection in mice. Presented at Second International Conference on Community-Acquired Pneumonia; Montreal. Sept 17–19, 2005; www.shea-online.org/Assets/files/W_-_Antivirals_for_Pandemic_Influenza.ppt. [Google Scholar]
- 30.Leneva IA, Goloubeva O, Fenton RJ, et al. Efficacy of Zanamavir against avian influenza A viruses that possess genes encoding H5N1 internal proteins and are pathogenic in mammals. Antimicrob Agents Chemoth. 2001;45:1216–24. doi: 10.1128/AAC.45.4.1216-1224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung C-L, Rayner JM, Smith GJD, et al. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J Infect Dis. 2006;193:1626–1629. doi: 10.1086/504723. [DOI] [PubMed] [Google Scholar]
- 32.WHO. Infection control recommendations for avian influenza in health care facilities. 2006. [Accessed May 2006]. http://www.who.int.csr/
- 33.WHO. WHO interim guidelines on clinical management of humans infected by influenza A (H5N1) 2004. [Accessed May 2006]. http://www.who.int/csr/disease_avia_influenza/guidelines/clinicalmanage/eu/index.html/
- 34.Treanor JJ, Campbell JD, Zangwill KM, et al. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 35.Polan GA. Vaccines against avian influenza. A race against time [Editorial] N Engl J Med. 2006;354(13):1411–13. doi: 10.1056/NEJMe068047. [DOI] [PubMed] [Google Scholar]