Abstract
BACKGROUND AND OBJECTIVES
Describe the epidemiology and characteristics of Middle East respiratory syndrome coronavirus (MERS-CoV), which are essential for control and treatment.
METHODS
We conducted a retrospective review of all cases of MERS-CoV reported in four cities of the Makkah Region from March to June 2014. Exposure factors and comorbid conditions were analyzed using Epi Info.
RESULTS
Analysis of the 261 cases revealed that the incidence peaked in mid-April 2014 and the fatality rate was 42%. Cough, fever, radiological evidence of pneumonia, and shortness of breath were identified as significant risk factors for a diagnosis of MER-CoV infection. Healthcare workers (HCWs) are at a higher risk of acquiring MERS-CoV than non-HCWs. Males in Jeddah are at higher risk due to greater outdoor exposure while females in Taif are at higher risk due to domestic caregiving. Filipino nurses are at highest risk among all HCWs.
CONCLUSION
The findings indicate the need to screen all contacts of HCWs to improve MERS control and form public–private partnerships to investigate the true burden of MERS.
Middle East respiratory syndrome (MERS) is caused by one of many coronaviruses. The MERS virus is found primarily in some animals, such as dromedary camels1 and bats, and is likely transmitted to humans through an intermediate host MERS-CoV is phylogenetically related to bat coronaviruses. MERS-CoV is also found in dromedary camels and is a potential risk factor for human transmission. It acts as an intermediate host and its transmission cycle remains unresolved.2–4
MERS has a variable incubation period and is transmitted through respiratory droplets and contact with contaminated surfaces. Infection with the virus causes mild to acute and fulminant disease in patients with co-morbid conditions and may be fatal if not diagnosed promptly and treated appropriately.5,6 It may also cause upper-respiratory tract infections leading to simple cold, rhinitis, cough, sore throat, fever, or gastrointestinal infections such as nausea, vomiting, or diarrhea.6,7 Occasionally, MERS may cause serious pneumonia, particularly in populations who are immunocompromised; 8 or have cardiopulmonary or renal illnesses; or are considered vulnerable, such as the elderly, young, obese, or pregnant.7 If not diagnosed promptly, MERS may lead to acute respiratory syndrome or death.6
In humans, transmission can occur through direct contact with droplets emitted by coughing or sneezing,9 indirect contact with contaminated objects or surfaces, or close contact by such means as touching or shaking hands.7 History of travel to endemic regions has also been associated with infection in recent studies in the Netherlands.10,11 Currently, no specific vaccine or treatment is available for immunization against coronaviruses. 6 Therefore, to reduce risk and prevent further spread from asymptomatic sources,12 simple measures, such as maintaining good respiratory hygiene; washing hands;13 avoiding touching the eyes, mouth, and nose; and proper waste disposal are recommended.13–16 Along with these measures, vigilance and enhanced surveillance is needed to forecast any clusters or outbreaks.13,17–19
Since initially reported in Saudi Arabia in mid-2012,13 MERS has been reported in other Middle Eastern countries and on other continents.13 The origin of the virus is reflected in the naming of the virus as Middle East respiratory syndrome coronavirus (MERS-CoV).20 In the Makkah region of Saudi Arabia, the majority of confirmed cases have been reported in Jeddah (203 cases), followed by Makkah (39 cases), Taif (13 cases), and Kunfudah (6 cases). To assist in the development of strategies for the reporting, diagnosis, control, and treatment of the virus, we investigated the epidemiology and characteristics of confirmed MERS-CoV infection cases in the Makkah Region of Saudi Arabia from March to June 2014.
METHODS
Healthcare system of the Makkah region
The Kingdom of Saudi Arabia is divided into thirteen regions that each has a governorate and sub-governorates with a capital. Makkah is the capital of region while Jeddah is the largest port city. The Ministry of Health (MoH) is the main public institution responsible for provision of preventive, curative, and rehabilitative healthcare services for the entire population. The MoH is not only responsible for the management, planning, financing, and regulation of the healthcare sector through primary healthcare centers (PHCs), generalized and specialized hospitals, but also for overall supervision of private healthcare facilities. After a report of a suspected case of MERS is generated at a PHC, it is sent to secondary or tertiary care hospitals and the relevant health directorates of the MoH for strategic action.
Study setting and design
This descriptive study was conducted at the Department of Public Health, Directorate General of Health Affairs, Makkah, Kingdom of Saudi Arabia. Secondary data were collected from all confirmed cases that had been reported by public and private hospitals serving four cities of the Makkah Region ( Jeddah, Makkah, Kunfudah, and Taif ) between March and June 2014. The data were compiled in Makkah for retrospective review and analysis.
Data collection and sample selection
A preformed Excel data sheet listing demographic, clinical, laboratory, radiological, outcome, and epidemiological risk factors was e-mailed to all cities for reporting of data on these factors by designated health officials. The data were cross-checked and clarified and missing data were provided through direct communication with the appropriate health directorates. The variables for which data were collected were patient code; age; sex; nationality; occupation; clinical signs; symptoms; co-morbid conditions; white blood cell (WBC) and platelet (PLT) counts; real-time polymerase chain reaction results; radiological evidence of pneumonia; epidemiological risk factors (i.e., status as a healthcare worker, travel to endemic regions, and/or exposure to a laboratory-confirmed case or MERS-associated animal); time and place of symptom onset; and outcome as recovered, transferred, died, and discharged.
Data analysis
The Excel sheets were carefully reviewed by the responsible physicians before the data were cleaned of any missing information and then cross-tabulated. Using the Centers for Disease Control (CDC) Epi Info program, data on demographic, clinical, laboratory, and exposure factors and comorbid conditions were analyzed. The odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated as indicators of the strength of associations.
A P value of <.05 was considered an indication of statistical significance. The case fatality rate was calculated by dividing the number of deaths by the total number of confirmed cases.
Ethical considerations
The study protocol was approved by the Ethics Review Committee of Makkah Region. Unique patient codes were issued to each study participant to maintain anonymity. Confidentiality was maintained throughout the study and no personal identifiers were listed on the Excel data sheet.
MERS case definition
The initial case definition of MERS was the presence of one or more of the symptoms of acute respiratory infection:fever ≥38°C; cough; shortness of breath with radiological evidence of pneumonia; clinical or radiological evidence of pneumonia; and, subsequently, intensive care unit admission. A revised “highly sensitive“ case definition that included broader clinical and laboratory criteria was published on May 13, 2014 and subsequently adopted by the MoH.21
RESULTS
As of September 6, 2014, 261 confirmed cases had been reported in the Makkah Region between March and June 2014, indicating a MERS incidence of 3.49 per 100 000 (95% CI 3.09–3.95). The majority of cases were reported in Jeddah (203, 78%), followed by Makkah (39, 15%), Taif (13, 5%), and Kunfudah (6, 2%). Of these confirmed cases, 147 (56%) were Saudis (incidence 3.39 per 100000, 95% CI 2.86–3.95) and 114 (44%) non-Saudis (incidence 3.70 per 100 000, 95% CI 3.07–4.43; Table 1). In order of decreasing incidence, the non-Saudis were from the Philippines (27), India (12), Yemen (12), Syria (11), Egypt (9) Palestine (9), Bangladesh (7), Indonesia (6), Sudan (5), Pakistan (4), Burma (3), Jordan (2), Turkey (1), Ethiopia (1), Morocco (1), Lebanon (1), Eritrea (1), South Africa (1), and Australia (1).
Table 1.
Nationality of patients with confirmed MERS-CoV infections in the Makkah Region, March–June 2014.
| Saudi | Non-Saudi | |
|---|---|---|
|
| ||
| Makkah City | 20 (51%) | 19 (49%) |
| Kunfudah | 5 (83%) | 1 (17%) |
| Taif | 10 (77%) | 3 (23%) |
| Jeddah | 112 (55%) | 91 (45%) |
| Total | 147 (56%) | 115 (44%) |
Data are n and percentage.
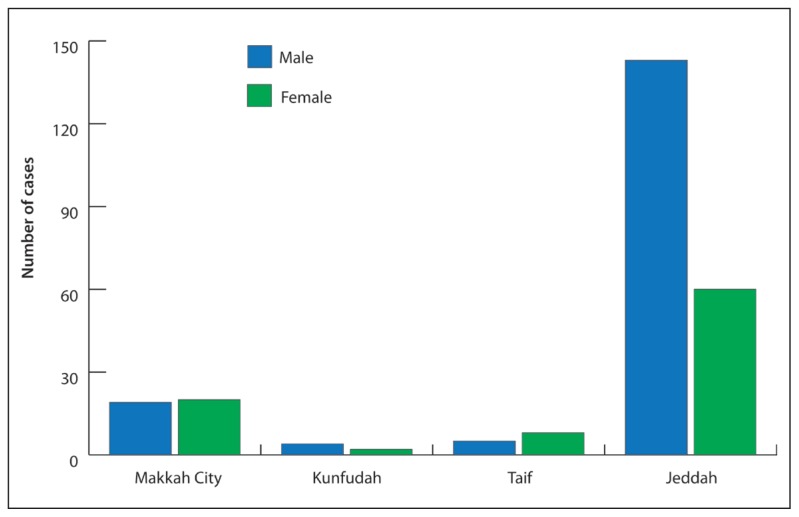
Of confirmed cases, 171 (66%) were males and 90 (34%) females with a ratio of (male: female; 2:1) (Figure 1). In Jeddah, 143 (70%) were males (mean age 49.7 years, range 8–90 years) and 60 (30%) females (mean age 45.0 years, range 13–75 years). In Makkah, 19 (49%) were males (mean age 44.3 years, range 14–84 years) and 20 (51%) females (mean age 39.5 years, range 18–69 years). In Taif, 5 (38%) were males (mean age 43.2 years, range 31–59 years) and 8 (62%) females (mean age 57.2 years, range 30–74 years). In Kunfudah, 4 (67%) were males (mean age 50.5 years, range 36–65 years) and 2 (33%) females (mean age 35.5 years, range 26–45 years).
Figure 1.
Sex distribution of confirmed cases of MERS in the Makkah Region, March–June 2014.
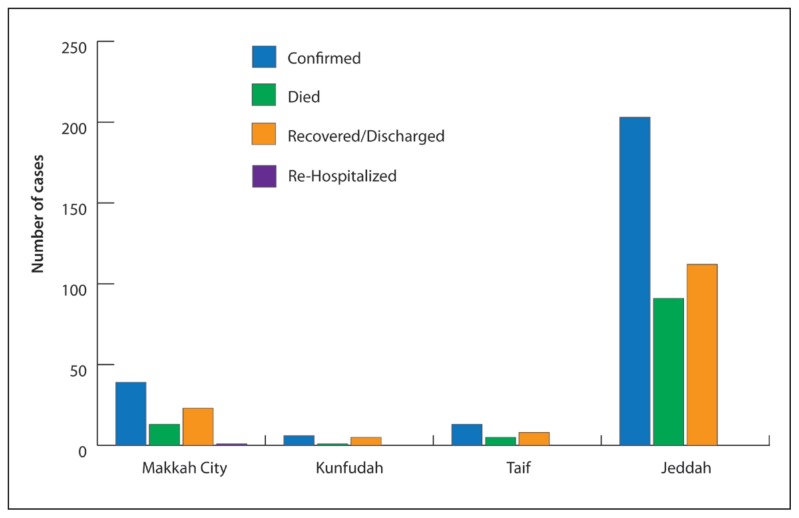
The first index case reported was diagnosed in the last week of March 2014. According to a review of the report, the peak in the number of confirmed cases occurred in mid-April 2014 before the number began to decline in the last week of April 2014, with the last confirmed case reported in June 2014 (Figure 2, 3). In Makkah, Taif, and Kunfudah, all confirmed cases were reported by public healthcare facilities (HCFs). In Jeddah alone, the cases were reported by both public HCFs (64%) and private HCFs (21%). Among all confirmed cases, only 148 (57%) had recovered or been discharged. The majority of fatal cases had been reported in Jeddah (91 cases, 45%), followed by Makkah (13 cases, 33%), Taif (5 cases, 38%), and Kunfudah (1 case, 16%). Only 2 (5%) cases had been re-hospitalized in Makkah city due to complications (Figure 2). The fatality rate among all confirmed cases had been 42% (110 cases). As the data is between a limited period (March to June 2014), seasonality cannot be determined.
Figure 2.
Outcomes of confirmed cases of MERS-CoV infection in the Makkah Region, March–June 2014.
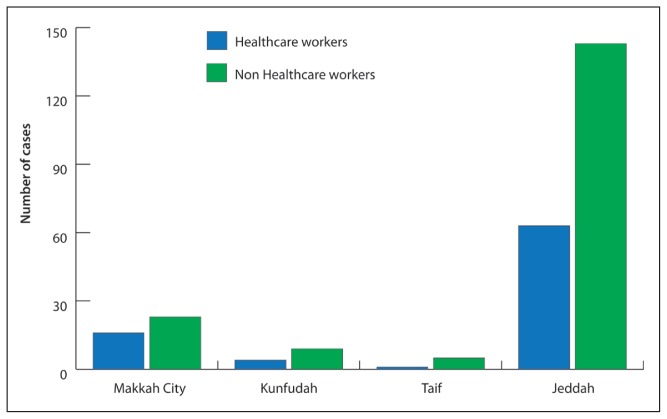
Figure 3.
Distribution of cases of healthcare workers and non healthcare workers in the Makkah Region, March–June 2014.
Among all confirmed cases, 84 (32%) had been healthcare workers (HCWs) and 177 (68%) had been non-health care workers (non-HCWs; Figure 3). The majority of HCW cases had been reported in Jeddah (63 cases, 31%), followed by Makkah (16 cases, 41%), Taif (4 cases, 31%), and Kunfudah (1 case, 17%). The majority of HCW cases were nurses (36 cases, 43%), followed by physicians (22 cases, 26%), technicians (8, 9%), administrative clerks (7 cases, 8%), healthcare-related cleaners (5 cases, 6%), and other healthcare-related workers (6 cases, 7%), (Table 2). Epidemiological risk factors for cases in Makkah were travel to Turkey (1 case, 3%), attendance at a festival or pilgrimage (1 case, 3%), contact with an infected human (16 cases, 41%), contact with an animal (3 cases, 7%), and visit to an HCF 14 days prior to symptom onset (22 cases, 56%).
Table 2.
Distribution of confirmed cases of MERS-CoV infection in healthcare workers (n= 84) and non healthcare workers (n=177) in the Makkah Region, March–June 2014.
| Type of HCW | Cases (n, %) | Type of non-HCWs | Cases (n, %) | |
|---|---|---|---|---|
|
| ||||
| Makkah City | Nurses | 10 (62%) | Unemployed workers | 12 (52%) |
| Physicians | 3 (20%) | Housewives | 6 (26%) | |
| Healthcare-related cleaners | 2 (12%) | Students | 2 (9%) | |
| Administrative clerks | 1 (6%) | Non-healthcare-related cleaners | 2 (9%) | |
| Policemen | 1 (11%) | |||
| Total HCWs | 16 (41%) | Total non-HCWs | 23 (59%) | |
|
| ||||
| Taif | Nurses | 3 | Unemployed workers | 4 |
| X-ray technicians | 1 | Housewives | 4 | |
| Teachers | 1 | |||
| Total HCWs | 4 (31%) | Total non-HCWs | 9 (69%) | |
|
| ||||
| Kunfudah | Nurses | 1 | Soldiers | 2 |
| Teachers | 1 | |||
| Housewives | 1 | |||
| Factory workers and others | 1 | |||
| Total HCWs | 1 (17%) | Total non-HCWs | 5 (83%) | |
| Jeddah | Nurses | 22 (35%) | Retired 32 | |
| Physicians | 19 (30%) | Housewives | 26 | |
| Technicians | 7 (11%) | Employees | 18 | |
| Administrative clerks | 6 (10%) | Unemployed/not workers | 42 | |
| Other healthcare-related workers | 6 (10%) | Students | 5 | |
| Healthcare-related cleaners | 3 (4%) | Drivers | 5 | |
| Policemen/security | 4 | |||
| Housemaids | 3 | |||
| Laborers/farmers | 3 | |||
| Teachers | 1 | |||
| Children | 1 | |||
| Total HCWs | 63 (31%) | Total non-HCWs | 140 (69%) | |
HCW, healthcare workers; non-HCW, non-healthcare workers.
Epidemiological risk factors for cases in Jeddah were visit to an HCF 14 days prior to symptom onset (59 cases, 29%), travel to domestic cities (2 cases, 1%), attendance at a festival or pilgrimage (3 cases, 1%), contact with an infected human (23 cases, 11%), and contact with an animal (3 cases, 1%) were identified. In Taif, visit to an HCF 14 days prior to symptom onset (1 case, 7%), travel to domestic cities (3 cases, 23%), contact with an infected human (1 case, 7%), and contact with an animal (3 cases, 23%) were identified. In Kunfudah, contact with an infected human (6 cases, 100%) and travel to domestic cities (2 cases, 33%) were identified.
Regarding symptomatology, 185 cases (71%) showed signs and symptoms while 42 cases (16%) remained asymptomatic. The most common signs and symptoms were cough (170 cases, 65%), fever >38°C (165 cases, 63%), radiological evidence of pneumonia (146 cases, 60%), shortness of breath (136 cases, 52%), joint pain or swelling (137 cases, 52%), fatigue (108 cases, 41%), sore throat (96 cases, 37%), fever with chills (95 cases, 36%), tachypnea (78 cases, 30%), wheezing or abnormal breathing (68 cases, 26%), chest pain (66 cases, 25%), headaches (59 cases, 23%), altered consciousness (53 cases, 20%), pharyngitis (39 cases, 15%), vomiting (32 cases, or 12%), runny nose (30 cases, 11%), diarrhea (27 cases, 10%), abdominal pain (26 cases, 10%), nausea (20 cases, 7%), hemoptysis (11 cases, 4%), local neurological deficit (10 cases, 4%), and conjunctivitis (6 cases, 2%). Of the 122 cases (47%) with co-morbid conditions, the most common were hypertension (76 cases, 29%), diabetes (72 cases, 28%), heart disease (47 cases, 18%), obesity (41 cases, 16%), smoking (41 cases, 16%), chronic renal disease (39 cases, 15%), immune-compromised status (24 cases, 9%), chronic lung disease (19 cases, 7%), steroid use (19 cases, 7%), asthma (12 cases, 5%), chronic liver disease (8 cases, 3%), cancer (5 cases, 2%), pregnancy (5 cases, 2%), chronic hematologic disease (2 cases, 0.7%), systemic lupus erythematosus (2 cases, 0.7%), eclampsia (1 case, 0.4%), and hypothyroid (1 case, 0.4%). Using the WBC count cut-off values of low (<4×109/L), normal (4–11×109/L), and high (>11×109/L), 50 (19%) had a low, 85 (33%) a normal, and 16 (6%) a high WBC count, while data were unavailable for 110 (42%) (Table 3). Using the PLT count cutoff values of very low (<50×109/L), low (<150×109/L), normal (150–400×109/L), and high (>400×109/L), 9 (3%) had a very low, 40 (15%) had a low, 98 (38%) had a normal, and 7 (%) had a high PLT count, while data were unavailable for 108 (41%).
The mortality among non-HCW cases was higher (OR 12.8, 95% CI 6.02–27.2, P<.001) compared to HCW cases, higher among males (OR 1.8, 95% CI 1.08–3.12, P<.02) compared to females, higher among those aged ≥50 years (OR 6.1, 95% CI 3.56–10.46, P<.001) compared to those aged <50 years, and higher among primary cases (OR 3.34, 95% CI 1.97–5.73, P<.001) compared to secondary cases. The symptoms identified as significant risk factors at the time of diagnosis were shortness of breath (OR 2.57, 95% CI 1.55–4.26, P<.001), chest pain (OR 2.1, 95% CI 1.13–3.91, P<.02), altered level of consciousness (OR 3.51, 95% CI 1.88–6.58, P<.001), tachypnea (OR 1.2, 95% CI 1.1–3.25, P<.02), and radiological evidence of pneumonia (OR 11.15, 95% CI 5.82–21.38, P<.001). Smoking (OR 0.78, 95% CI 0.4–1.55, P=.48), diabetes mellitus (OR 0.82, 95% CI 0.47–1.41, P=.47), and chronic renal disease (OR 1.5, 95% CI 0.75–3.00, P=.25) were not found to be significant risk factors, nor was contact with animals for either primary or secondary cases (OR 3.29, 95% CI 0.8–14.11, P=.13).
DISCUSSION
One major finding of this study is that susceptibility to MERS-CoV infection by sex varies by region. In Jeddah, where the bulk of MERS cases have been reported, males appear more susceptible to infection than females probably because most males must work outside of the home, either in a healthcare setting or in the community. In contrast, females appear more susceptible to infection in Taif despite remaining indoors probably due to close contact with or exposure to confirmed cases. In Makkah and Kunfudah, both sexes appear equally susceptible. Further, review of the data appears to indicate that non-HCWs (177 or 68%) are at higher risk of infection than HCWs. However, review of the difference between the MERS incidence rate for non-HCWs and HCWs revealed that the risk ratio (RR) was statistically significant (RR 0.005, 95% CI 0.003–0.006, P<.001), indicating HCWs are at higher risk of acquiring MERS, consistent with the findings of a Jordanian study.22 In contrast, the difference between the RR for Saudis and non-Saudis was insignificant (RR 0.91, 95% CI 0.71–1.16, P=.71), indicating that they face almost the same risk. Among non-Saudis, nurses from the Philippines (n=26,23%) appear to be at highest risk, as the majority of nurses working in Saudi Arabia are from that country. Among non-HCWs, domestic workers and homemakers comprised a large proportion of cases (137 cases, 52%), likely due to their care for and close contact with confirmed cases.23 Among HCWs, nurses, physicians, and technicians appear at highest risk due to their direct contact with patients. In contrast, hospital administrative clerks and health-related cleaners are indirectly exposed in the workplace.
Among private healthcare facilities, those in Jeddah are most actively engaged in reporting of confirmed cases. Therefore, it is unsurprising that no confirmed cases were reported by private healthcare facilities in Makah, Taif, and Kunfudah. The actual number of suspected cases remains unknown in these cities, and the burden may thus be underestimated. In Makkah, 56% of confirmed cases had hospital-acquired infection from a prior visit to an HCF and 41% had community-acquired infection due to close contact or unknown exposure. In Taif, 23% of cases had some history of domestic travel and 23% had exposure to domestic pets but not to camels or other known animal sources. In Kunfudah, all cases had been exposed to patients with respiratory illness, vomiting, diarrhea, or community-acquired infections. In Jeddah, 29% of cases had prior exposure to an HCF. The King Fahad General Hospital Jeddah, the largest facility with over 600 beds and to which the majority of cases were admitted, reported the highest number (n=82, 31%) of cases in the region.
The overall fatality rate was 42%, which was lower than that reported in previous studies in the region.24 Among all confirmed cases, 16% remained asymptomatic, having been infected via contact with confirmed cases, thus indicating that screening of all HCW contacts is an important step in disease containment. In accordance with other regional studies,6,24 cough (170 or 65%), fever >38°C (165 or 63%), radiological evidence of pneumonia (146 or 60%), and shortness of breath (136 or 52%) were common in confirmed cases, thereby validating their inclusion as symptoms in the revised case definition for MERS.21
Study limitation
The primary limitation was that private healthcare facilities in Makkah, Taif, Kunfudah, and other regional cities did not report whether they had treated any confirmed cases during the study period. As the actual MERS-related morbidity and mortality for these cities could not be determined, the fatality rate (42%) may have been underestimated. As suspected, cases that presented at community or private clinics in these cities may not have been included in the analysis, so the results may not truly reflect the severity and impact of MERS.
CONCLUSION
This study yielded several valuable findings on the epidemiology and characteristics of MERS cases. Regarding nationality, Saudis and non-Saudis appear to have almost the same risk of exposure. Among all nationalities, Filipinos appear to be at highest risk, as the majority of nurses in Saudi Arabia are Filipino. Regarding occupation, healthcare workers are at higher risk of acquiring MERS than non-HCWs. Regarding sex, males in Jeddah appear more susceptible, probably due to their participation in outdoor activities, while females in Taif appear more susceptible, probably due to their exposure to confirmed cases in the home. Regarding symptomatology, the frequent finding of cough (65%), fever >38°C (63%), radiological evidence of pneumonia (60%), and shortness of breath (52%) among symptomatic cases validates the current MERS case definition and the findings of other regional studies. A particularly important finding was that despite strict MoH requirements that public and private healthcare facilities report cases of any notifiable diseases, including MERS,21 and the establishment of hotlines throughout the country, the reporting of all MERS cases remains challenging. As the regular reporting of cases to higher authorities is essential for key decision making,21 this challenge must be overcome.
RECOMMENDATIONS
Knowledge of epidemiological, demographic, clinical, and laboratory characteristics will help identify gaps; set goals for further research; and strengthen strategic decision-making in MERS reporting, control, and treatment. This knowledge will assist in the establishment of health facilities and isolation wards; procurement of personal protective gear, ambulances, ventilators, laboratory equipment, and supplies; hiring of additional healthcare staff; and enhancing capacity through appropriate training. Central to this effort is establishing private–public healthcare partnerships for both effective response and collaborative research. Further regional studies performed collaboratively by experts from universities, the private sector, regional health directorates, the WHO, and the CDC are required to gain knowledge of the true epidemiology and burden of MERS.
REFERENCES
- 1.Hemida MG, Chu DKW, Poon LML, Perera RAPM, Alhammadi MA, Ng HY, et al. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. 2014;20(7):1231–4. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Memish ZA, Zumla AI, Al-Hakeem RF, Al-Rabeeah AA, Stephens GM. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368(26):2487–94. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 3.Cotton M, Lam TT, Watson SJ, Palser AL, Petrova V, Grant P, et al. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis. 2013;19(5):736–42. doi: 10.3201/eid1905.130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perera RA, Wang P, Gomaa MR, El-Shesheny R, Kandeil A, Bagato O, et al. Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18(36) doi: 10.2807/1560-7917.es2013.18.36.20574. pii-20574. [DOI] [PubMed] [Google Scholar]
- 5.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–61. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014 doi: 10.1016/j.ijid.2014.09.003. pii:S1201-9712(14)01622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Regional Office for Europe (EURO), N.I.F.P.f.T. Middle East respiratory syndrome coronavirus (MERS-CoV)–Turkey. 2014. (cited 2014 Oct 29). http://www.who.int/csr/don/24-october-2014-mers/en/
- 8.Guery PB, Poissy J, el Mansouf L, Séjourné C, Ettahar N, Lemaire X, et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381(9885):2265–72. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hijawi B, Abdallat M, Sayaydeh A, Alqasrawi S, Haddadin N, Jaarour N, et al. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr Health J. 2013;19(Suppl 1):S12–8. [PubMed] [Google Scholar]
- 10.Kraaij–Dirkzwager M, Timen A, Dirksen K, Gelinck L, Leyten E, Groeneveld P, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) infections in two returning travellers in the Netherlands, May 2014. Euro Surveill. 2014;19(21) doi: 10.2807/1560-7917.es2014.19.21.20817. [DOI] [PubMed] [Google Scholar]
- 11.Al-Tawfiq JA, Zumla A, Memish ZA. Coronaviruses: severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus in travelers. Curr Opin Infect Dis. 2014;27(5):411–7. doi: 10.1097/QCO.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 12.Omrani AS, Matinb MA, Haddadc Q, Al-Nakhlid D, Memishe ZA, Albarraka AM, et al. A family cluster of Middle East Respiratory Syndrome Coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17(9):e668–72. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Memish ZA, Assiri A, Alhakeem R, Yezli S, Almasri M, Zumla A, et al. Middle East respiratory syndrome corona virus, MERS-CoV. Int J Infect Dis; Conclusions from the 2nd Scientific Advisory Board Meeting of the WHO Collaborating Center for Mass Gathering Medicine; Riyadh. 2014. pp. 51–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gautret P, Vu Hai V, Sani S, Doutchi M, Parola P, Brouqui P, et al. Protective measures against acute respiratory symptoms in French pilgrims participating in the Hajj of 2009. J Travel Med. 2011;18(1):53–5. doi: 10.1111/j.1708-8305.2010.00480.x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Tawfiq JA, Zumla A, Memish ZA. Respiratory tract infections during the annual Hajj: potential risks and mitigation strategies. Curr Opin Pulm Med. 2013;19(3):192–7. doi: 10.1097/MCP.0b013e32835f1ae8. [DOI] [PubMed] [Google Scholar]
- 16.Health Protection Agency (HPA) UK Novel Coronavirus Investigation team. Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. 2013;18(11):20427. doi: 10.2807/ese.18.11.20427-en. [DOI] [PubMed] [Google Scholar]
- 17.Mailles A, Blanckaert K, Chaud P, van der Werf S, Lina B, Caro V, et al. First cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections in France, investigations and implications for the prevention of human-to-human transmission, France, May 2013. Euro Surveill. 2013;18(24) pii:20502. [PubMed] [Google Scholar]
- 18.Bauch CT, Oraby T. Assessing the pandemic potential of MERS-CoV. Lancet. 2013;382(9893):662–4. doi: 10.1016/S0140-6736(13)61504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Tawfiq JA, Zumla A, Gautret P, Gray GC, Hui DS, Al-Rabeeah AA, et al. Surveillance for emerging respiratory viruses. Lancet Infect Dis. 2014;14(10):992–1000. doi: 10.1016/S1473-3099(14)70840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groot RJ, Baker SC, Ziebuhr J. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87(14):7790–2. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madani TA. Case definition and management of patients with MERS coronavirus in Saudi Arabia. Lancet Infect Dis. 2014;14(10):911–3. doi: 10.1016/S1473-3099(14)70918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Abdallat MM, Payne DC, Alqasrawi S, Rha B, Tohme RA, Abedi GR, et al. Hospital-associated outbreak of middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59(9):1225–33. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drosten C, Meyer B, Müller MA, Corman VM, Al-Masri M, Hossain R, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371(9):828–35. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 24.Al-Tawfiq JA1, Assiri A, Memish ZA. Middle East respiratory syndrome novel corona MERS-CoV infection. Epidemiology and outcome update. Saudi Med J. 2013;34(10):991–4. [PubMed] [Google Scholar]