Abstract
Tilletiopsis minor, a blastoconidia-forming yeast, was isolated from a 4-year-old boy suffering from severe pneumonia. Chest x-rays revealed the progression of widespread and multiple nodular lesions, nonsymmetrical interstitial and airspace infiltrates, and consolidations. Creamy yellow, irregular, wrinkled yeastlike organisms were isolated from the pleural fluid specimens when cultured on Sabouraud dextrose agar for 5 days and incubated at 30°C. Microscopically, the organisms showed broad, irregular filaments with blastoconidia but no budding cells. Manual bench tests and automated phenotypic analyses failed to recognize the organism. This unique and rare organism (AB7-11; DSM 29469) was identified using the sequence analysis of the internal transcribed spacer region of the nuclear ribosomal DNA. It showed a precise alignment with the type strains of T minor. Subsequent to this diagnosis, and the earlier nonresponse to vancomycin and meropenem, the patient was put on liposomal amphotericin. However, the condition continued to deteriorate, and then, intravenous voriconazole was added to control the infection. Finally, the patient’s condition improved, and he was discharged in good condition after 1 month of stay in the hospital.
Tilletiopsis is a fungus that is categorized with a group of dimorphic yeastlike fungi in the class Exobasidiomycetes, which are characterized by the formation of blastoconidia but no budding cells. The genus contains few species renounced for generating potential biological control agents against a number of powdery mildew diseases.1,2 The genus Tilletiopsis was first described by Derx in 1930 when examining infected plant leaves; he suggested that the infection was due to Tilletia species, a smut fungus belonging to the family Tilletiaceae.1
Apart from the original description of the species Tilletiopsis minor by Ramani et al,3 no other report is available to incriminate this species as a causal agent of human or animal diseases. Ramani et al3 isolated T minor from cystic lesions in an immunocompromised patient. These authors observed yeastlike growth with small satellite colonies at the periphery of the culture plate. When grown on potato dextrose agar at 30°C, T minor showed delicate, hyaline hyphae bearing curved conidia, which turned out into dark hyphae with scattered, curved conidia and globose chlamydospores with age.3 Tilletiopsis species, in general, were found to resemble Entyloma species in morphological terms, but blastospores of Entyloma do not bud in a yeastlike manner.1 The biochemical tests for differentiation of Tilletiopsis species have been studied by Ramani et al.3 T minor was found positive for arbutin, lactose, and phenylalanine but negative for mannitol and ammonium sulfate. T minor was found sensitive to amphotericin B (MIC, 0.06 mg/mL), fluconazole (MIC, 8 mg/mL), and itraconazole (MIC, 0.5 mg/mL).3 Tilletiopsis species, for example T washingtonensis, were found to have a unique ability to assimilate a number of compounds including ethanol, butanol, acetate, propionate, butyrate, and ethyl acetate.4
A genetic study of Tilletiopsis species concluded that group I introns can be transferred horizontally among species, and subsequently, when inherited, they can be diverged vertically.5 Four yeastlike fungi with blastoconidia, which were isolated from the plants in Thailand, were allocated to the genus Tilletiopsis based on morphological and chemotaxonomical characteristics. The 18S rDNA sequence analysis and DNA–DNA reassociation analyses indicated that the strains merited new species ranks, namely Tilletiopsis derxii, Tilletiopsis oryzicola, and Tilletiopsis penniseti.6
This report aimed to describe a serious pulmonary illness in a child caused by a rare yeast fungus.
CASE
A previously healthy 4-year-old male patient from Abha region, Saudi Arabia, presented to Asser Central Hospital (a tertiary hospital in the southwest of Saudi Arabia) with fever lasting 4 weeks. The fever was associated with cough and shortness of breath in the last 2 weeks prior to admission to the hospital. The patient’s history was unremarkable with no evidence of immunodeficiency, neither primary immunodeficiency disorders nor immunosuppressive medications. The child received the scheduled vaccination. His maternal grandfather died of pulmonary tuberculosis 1 year back.
On clinical examination, the patient was found febrile (39°C); respiratory rate, 35/min; pulse rate, 25/min; blood pressure, 107/68 mm Hg; and oxygen saturation 93% in room air. The mucous membranes were neither pale nor jaundiced; however, palpable, small bilateral auxiliary lymph nodes were observed. He had a bacillus Calmette Guerin scar on the left upper arm. The chest examination revealed markedly diminished breath sound on the right side and a bilateral crepitation. He was conscious with an unsteady gait. Other systemic examinations were ordinary. Chest x-ray showed bilateral pulmonary infiltrates with a right-sided pleural effusion. A bilateral lower lobe partial opacity was prominent on the right side (Figure 1). No pulmonary nodules or cavitation was noted. The mediastinal lymph node was normal.
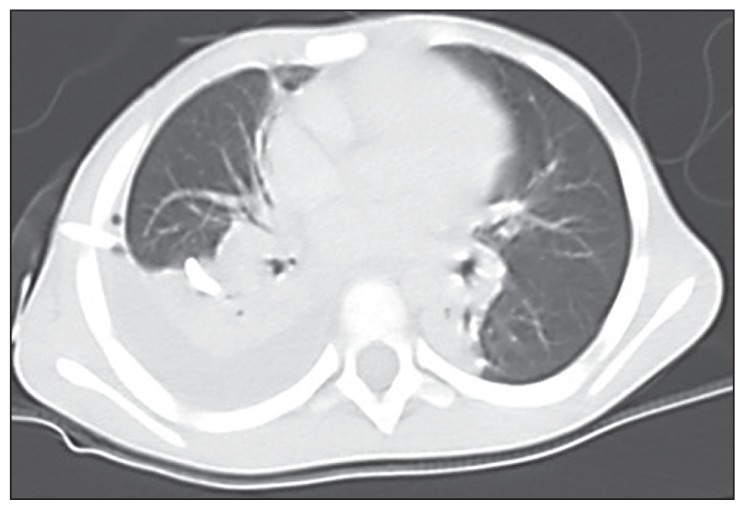
Figure 1.
CT-scan of the chest of the 4-year-old male patient presented to Aseer Central Hospital with severe pneumonia showing moderate right pleural effusion, bilateral lower lobe partial opacity prominent in the right side but no pulmonary nodules or cavitation was noted.
The blood hematological and chemical tests of the patient revealed the following results: white blood cells (WBC), 24 000/mm3; hemoglobin, 13.6 gm/dL; platelet, 219 000/mm3. Na, 132 mmol/L; K, 4.2 mmol/L; urea, 18 mg/dL; glucose, 91 mg/dL; albumin, 3.4; gamma-glutamyl transferase, 41 IU/L; alanine aminotransferase, 34 IU/L; and erythrocyte sedimentation rate, 42 mm Hg. The pleural fluid analysis showed the following results: WBC, 200/mm3, neutrophil, 75%; lymphocytes, 25%; protein, 5.3 gm/L; glucose, 43 mg/dL; and lactate dehydrogenase, 594 IU/L.
The cytological examination showed the presence of acute and chronic inflammatory cells with few mesothelial cells, but no evidence of granulomatous inflammation. No malignant cells were seen. Serology for human immunodeficiency virus was negative, and the investigations revealed no underlying defect of humoral or cell-mediated immunity.
The patient was initially started on broad-spectrum antibiotics that included intravenous vancomycin and meropenem. No response to these medications was reported, and the patient’s condition worsened with progression of wide spread lesions. The computed tomography scan of the chest revealed a moderate right pleural effusion that was noted particularly with pleural drainage.
On day 18 of hospital admission, the pleural fluid specimens were submitted to microbiology laboratory and processed following standard methods. A creamy yellow, irregular, wrinkled yeastlike organism was isolated from the pleural fluid specimens when cultured on Sabouraud dextrose agar (SDA; Difco, Becton, Dickinson and Company, Sparks, Maryland) for 5 days and incubated at 30ºC (Figure 2). The suspected fungal growth was subcultured on a fresh SDA plate to improve the growth and appearance of distinguished fungal elements. The organism was labeled AB7-11 (DSM 29469) and identified on the basis of colony morphology appeared on SDA and on the basis of microscopic features following recommended guiding principles.
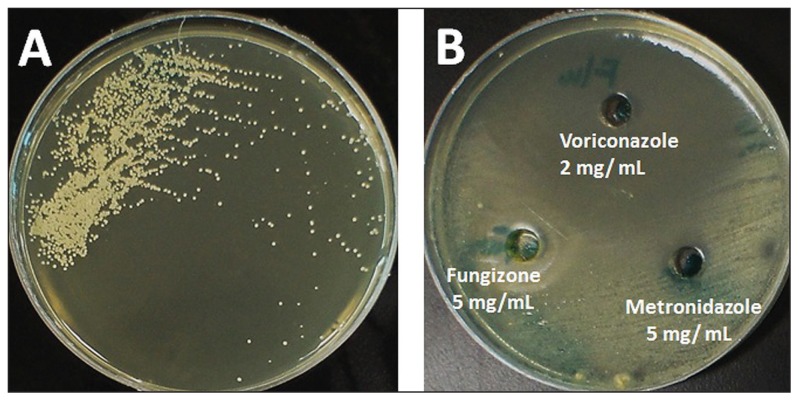
Figure 2.
Growth of a 5-day old culture of Tilletiopsis minor grown on Sabouraud’s dextrose agar at 30°C showing creamy yellow, irregular, wrinkled yeast-like colonies (A). Plate (B) shows the reaction of the organism to selected anti-fungal agents.
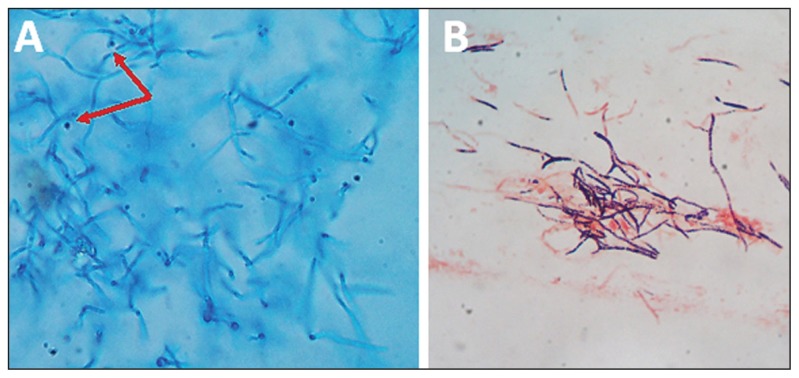
Microscopically, the organisms showed broad irregular filaments with blastoconidia but no budding cells. Manual bench tests and the automated phenotypic analyses failed to recognize the organism. Gram-stained smears made from the culture revealed medium-sized irregular hyphae with the evidence of blastoconidia, but no budding yeast cells were visible (Figure 3).
Figure 3.
Microscopic appearance of a 5-day old culture of Tilletiopsis minor grown on Sabouraud’s dextrose agar at 30°C showing: A) irregular hyaline filaments with ballistoconidia (arrows) in a lacto-phenol cotton blue wet mount); B) a Gram-stained smear showing variable gram positive and gram negative filaments.
The strain was found to have phenotypic properties (morphological, biochemical, and physiological) predictable of yeasts. Subsequently, a report of yeast fungal infection was submitted.
In vitro susceptibility assay was performed using the well method on SDA. The following drugs were tested: amphotericin B 100 mg/mL (Sigma, Missouri, USA), which revealed 15-mm inhibition zones; co-trimoxazole 25 μg (Liofil Chem, Italy), 16-mm inhibition zones; fluconazole 2 mg/mL (Diflucan I.V. Roerig/Pfizer Inc., France ), 70-mm inhibition zones; fungizone 5 mg/mL (E. R Squibb & Sons Ltd, England), 21-mm inhibition zones; itraconazole 10 mg/mL Sporanox I.V, Janssen Biotech N. V, Belgium), 70-mm inhibition zones; metronidazole 5 mg/mL (PSI Pharmaceutical Co., Jeddah, Saudi Arabia), 8-mm inhibition zones; nystatin 100 mg/mL (Sigma, Missouri, USA ), 37-mm inhibition zones; and voriconazole 2 mg/mL (Vfend, Amgen technology, Ireland), 70-mm inhibition zones (Figure 2).
Given this diagnosis, and the earlier nonresponse to vancomycin and meropenem, the patient was put on liposomal amphotericin. However, the condition continued to deteriorate, and then, intravenous voriconazole was added to control the infection. The patient’s condition improved, and he was discharged in good condition after 1 month of stay in the hospital (Figure 4). Then, he was continued on itraconazole (150 mg daily) and followed up in the outpatient department.
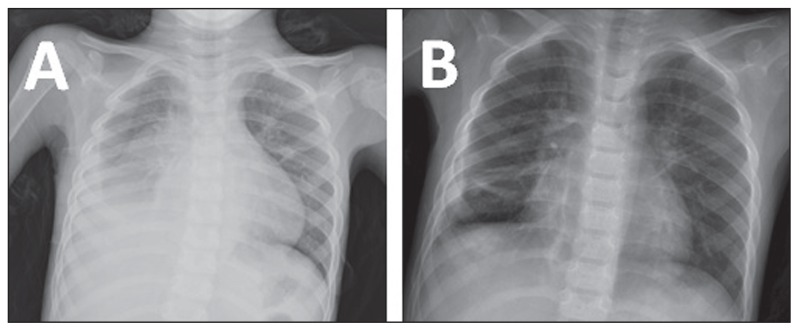
Figure 4.
Chest X-rays of the 4-year-old male patient presented to Aseer Central Hospital, with severe pneumonia showing obliteration of the right costophrenic angle (A) and clear right costophrenic angle and normal lung volume after treatment (B).
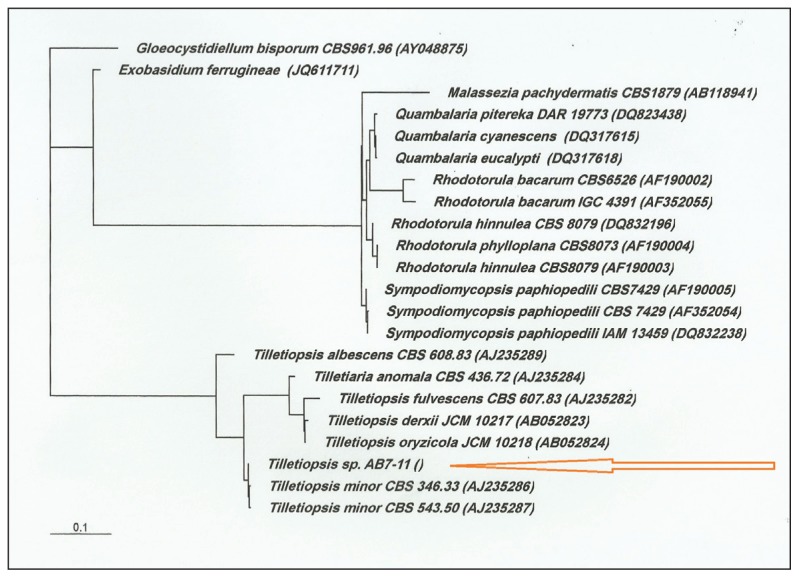
Definitive identification of this unique and rare organism was made using the sequence analysis of the internal transcribed spacer (ITS) region of the nuclear ribosomal DNA.7 A neighbor-joining phylogenetic tree based on the domains 1 and 2 (D1/D2) of the large subunit rDNA showed the joining of the strain AB7-11 to T minor (Figure 5; arrow) and relatively remote connection to other closely related yeast species. Accordingly, the strain AB7-11 (DSM 29469) was identified as T minor.
Figure 5.
Neighbor-joining phylogenetic tree based on the D1/D2 domain of the LSU rDNA showing the association of strain AB7-11 to Tilletiopsis minor (arrow) and to closely related yeast species. Bar, 0.1 substitution per nucleotide position.
DISCUSSION
In the recent years, significant developments in diagnostic technologies have been made for detecting and identifying yeasts in clinical specimens. One of these technologies is the application of gene sequence analyses, especially in the D1/D2 of large subunit rRNA and the ITS. The increasing application of molecular and phylogenetic analyses has resulted in important changes in yeast systematics.7 These have led to redefining many old genera and defining new ones as per the present case (Figure 5).
Identification of yeasts based on phenotypic characterization is strenuous and time-consuming, and in many situations, it is not conclusive. In the present report, the routine microscopic and culture-based diagnostic tests as well as the preset automated procedures were unsuccessful in distinguishing the isolated organism. 8 Infections caused by T minor were described to occur rapidly and become invasive in up to 50% of cases of extensive soil contamination.3 The case presented in this report was unfortunate to have such a fatal illness but blessed to have received precise therapy on time. Initial assumption of a bacterial infection might have worsened the condition of the child. The isolated fungus showed a relatively slow growth; no abundant growth on original or subsequent subcultures on SDA was observed (Figure 2). The unique structures of this fungus, notably the blastoconidia, hyphae, and absence of budding cells (Figure 3), have guided us to consider molecular analyses to reach to a definitive diagnosis.
The isolation of the yeast T minor from a respiratory specimen in this report is considered a unique finding. This is because T minor is not a common pathogen worldwide, and the species belonging to this genus are mainly plant saprophytes.1,2 The diagnosis made in this report is an example of how important it is to submit appropriate specimens and consider rare causal agents such as yeasts of this type. This circumstance was supported by the fact that the child did not respond to antibacterial agents (vancomycin and meropenem). In this case, T minor was isolated from an immunocompetent child. This is contrary to the first reported human case of infection by T minor, which was a subcutaneous cyst and affected an elderly immunocompromised male patient.3 Since the frequency of the yeast in this report was similar to that reported by Ramani et al,3 repeated cultures were performed to ensure that the isolate was an authentic causative agent.
The in vivo application showed that amphotericin B did not improve the condition promptly; nonetheless, the intravenous application of voriconazole was successful. These developments were consistent with the in vitro antifungal assays, which showed that voriconazole was an outstanding agent as it showed a wider inhibition zone (Figure 2B).
In conclusion, it is, advised to not only consider fungal infection in routine respiratory illnesses bit also perform in vitro antimicrobial testing, since unique yeasts or molds may respond differently to empirical antifungal agents.9
Acknowledgments
The authors thank the administration of Aseer Central Hospital particularly the Head of Laboratories and the College of Medicine, King Khalid University for facilitating the completion of this project.
Footnotes
Conflict of interest
None-declared.
REFERENCES
- 1.Nyland G. The genus Tilletiopsis. Mycologia. 1950;42:487–496. [Google Scholar]
- 2.Kurtzman CP, Fell JW, Boekhout T. The Yeasts: A Taxonomic Study. Elsevier Science; 2011. [Google Scholar]
- 3.Ramani R, Kahn BT, Chaturvedi V. Tilletiopsis minor: a new etiologic agent of human subcutaneous mycosis in an immunocompromised host. Journal of clinical microbiology. 1997;35:2992–2995. doi: 10.1128/jcm.35.11.2992-2995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vishniac HS, Anderson JA, Filonow AB. Assimilation of volatiles from ripe apples by Sporidiobolus salmonicolor and Tilletiopsis washingtonensis. Antonie van Leeuwenhoek. 1997;72:201–207. doi: 10.1023/a:1000363510580. [DOI] [PubMed] [Google Scholar]
- 5.Takashima M, Nakase T. A phylogenetic analysis of three group I introns found in the nuclear small subunit ribosomal RNA gene of the ballistoconidiogenous anamorphic yeast-like fungus Tilletiopsis flava. Genes & genetic systems. 1997;72:205–214. doi: 10.1266/ggs.72.205. [DOI] [PubMed] [Google Scholar]
- 6.Takashima M, Nakase T. Tilletiopsis derxii, Tilletiopsis oryzicola and Tilletiopsis penniseti, three new species of the ustilagionomycetous anamorphic genus Tilletiopsis isolated from leaves in Thailand. Antonie van Leeuwenhoek. 2001;80:43–56. doi: 10.1023/a:1012218108640. [DOI] [PubMed] [Google Scholar]
- 7.Kurtzman CP. Use of gene sequence analyses and genome comparisons for yeast systematics. International journal of systematic and evolutionary microbiology. 2014;64:325–332. doi: 10.1099/ijs.0.054197-0. [DOI] [PubMed] [Google Scholar]
- 8.Chao QT, Lee TF, Teng SH, Peng LY, Chen PH, Teng LJ, Hsueh PR. Comparison of the Accuracy of Two Conventional Phenotypic Methods and Two MALDI-TOF MS Systems with That of DNA Sequencing Analysis for Correctly Identifying Clinically Encountered Yeasts. PloS one. 2014;9:e109376. doi: 10.1371/journal.pone.0109376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcazar-Fuoli L, Mellado E. Current status of antifungal resistance and its impact on clinical practice. British journal of haematology. 2014;166:471–484. doi: 10.1111/bjh.12896. [DOI] [PubMed] [Google Scholar]