Abstract
BACKGROUND AND OBJECTIVES
Few studies have attempted to delineate the clinical profile of myasthenia gravis (MG) among people of Arab ancestry. Therefore, we sought to clarify the clinical profile, the outcome of treatment and the role of thymectomy in non-thymomatous MG in Saudi Arabia.
PATIENTS AND METHODS
We retrospectively studied 104 patients followed over a mean period of 7.2 years (range, 1 to 22 years) at the King Khaled University Hospital, Riyadh, Saudi Arabia. Disease outcomes were compared among thymectomized and non-thymectomized patients according to the post-intervention status criteria of the Myasthenia Gravis Foundation of America (MGFA).
RESULTS
Age of onset was 22.5±9.3 years (mean±SD) in females and 28.2±15.9 years in males, with peaks in the second and third decades among females and the third and fourth decades among males. At diagnosis, a majority of patients had moderate generalized weakness, equivalent to MGFA class III severity. After medical treatment with or without thymectomy, 9.6% of all patients had achieved complete stable remission, 3.8% had pharmacological remission, 27.9% had minimal manifestations, 23.1% were improved, 20.2% were unchanged and 15.4% were worse. Only thymectomized patients without a thymoma achieved remission, a significant benefit over those who had no thymectomy (P=.02).
CONCLUSION
MG presents at a younger age among Saudi Arabs compared to other racial groups. Thymectomy conferred significant benefits towards achievement of remission.
Myasthenia gravis (MG) is an acquired disease of the neuromuscular junction characterized by muscular weakness and fatigability. The prevalence of MG is 50 to 125 persons per million in Western countries, with the incidence peaking after 60 years of age in males and before 40 years of age in females. 1,2 Long-term treatment of MG includes the use of cholinesterase inhibitors, steroids, immunosuppressive agents and thymectomy, while short-term use of plasma exchange or IV immunoglobulin is reserved for patients with crises or severe weakness.3 An evidence-based review failed to show the benefits of thymectomy in non-thymomatous MG, despite its widespread use in this subgroup of patients.4
Racial factors influence the natural history of MG to a variable extent in different societies. For instance, Chinese and black South Africans are more likely to present with ocular MG compared to whites5 and white South Africans.6 Yet, few studies have attempted to delineate the clinical profile of MG amongst peoples of Arab ancestry. One study from Saudi Arabia discussed the benefits of thymectomy in 48 patients followed for 20 months,7 while a study from Oman described outcomes in 50 patients followed for three years.8 In both studies, data were not subjected to statistical analysis, there were no control groups, and follow-up periods were relatively short for a disease characterized by fluctuating severity. To clarify the clinical profile of MG, the outcome of treatment and the role of thymectomy in non-thymomatous MG, we conducted a retrospective study of all cases of MG seen at our hospital from 1984 to 2006.
PATIENTS AND METHODS
Records of all patients diagnosed with MG at the King Khaled University Hospital, Riyadh, from 1984 to 2006 were reviewed. The diagnosis of MG was based on one or more of the following criteria: the presence of fluctuating muscle weakness with early fatigability, improvement in signs and symptoms after IV edrophonium, a decremental response on repetitive nerve stimulation (RNS) and the presence of serum antibodies to the acetylcholine receptor. Patients were included in the study if they had follow-up records of one year or more. Records reviewed included biographic data, symptoms and signs at presentation and follow-up and results of investigations that included chest CT scans, thymus histology and muscle biopsy in selected cases. Histological findings of the thymus were classified as hyperplastic, thymomatous, atrophied or normal.
Disease severity at presentation was classified according to the Myasthenia Gravis Foundation of America (MGFA) clinical classification:9 grade 0, no symptoms; grade I, ocular muscle weakness only; grade II, mild generalized weakness; grade III, moderate generalized weakness; grade IV, severe generalized weakness; grade V, intubation required. Treatments usually began with pyridostigmine, which was titrated to a maximum dose of 240 mg daily if this was not limited by side effects. When symptoms did not improve, prednisolone was started at a dose of 0.8mg/kg and subsequently tapered to a maintenance dose of 5–15 mg per day. When higher maintenance doses were required or steroids were not tolerated, such as in those with diabetes mellitus, azathioprine (50–150 mg daily) was used. Thymectomy was offered to all patients irrespective of disease severity or chest CT findings. Assessment of outcome was based on the MGFA post-intervention status criteria (PIS) at last outpatient or emergency visit.9 Patients were classified as having achieved complete stable remission (CSR), pharmacological remission (PR), minimal manifestations (MM), had unchanged severity (U), had worse severity (W) or had died (D) from MG. We further grouped CSR, PR and MM as satisfactory outcomes and all other PIS classes as poor outcomes. The three primary endpoints were the achievement of CSR, any remission (either CSR or PR) and a satisfactory outcome.
Data were analyzed with SPSS for Windows software version 11.5. The unpaired t test was used for continuous variables while the chi square and Fisher exact tests were used to compare proportions, with P values <.05 considered significant.
RESULTS
All patients
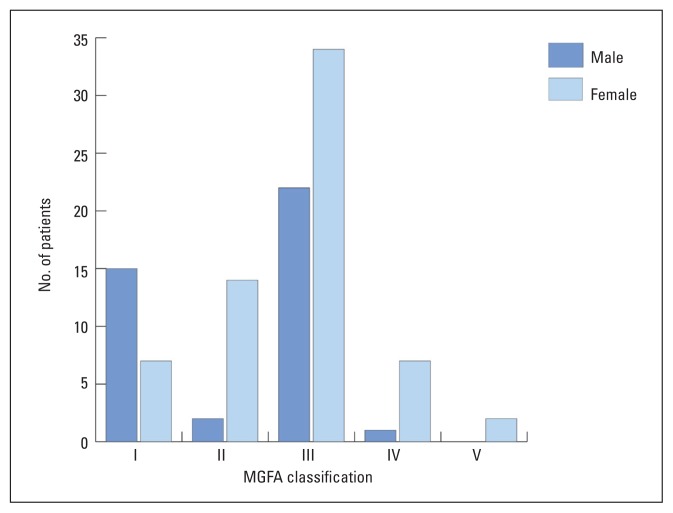
One hundred and four patients were studied, consisting of 40 males and 64 females (M:F ratio=1:1.6). Age at diagnosis of MG ranged from 3 to 73 years, with a mean (±SD) of 22.5±9.3 years in females and 28.2±15.9 years in males, a difference that was statistically significant (P=.024). Peaks of onset were in the second and third decades in females and the third and fourth decades in males. Only one male and no female had disease onset beyond the sixth decade, while four males and three females were diagnosed within the first decade. Duration of follow-up ranged from 1 to 22 years (7.6±4.6 years), with 29% of patients having 10 years follow-up or more. At diagnosis (Figure 1), 22 patients (21.2%) had ocular symptoms only (MGFA I), 15.4% had mild generalized weakness (MGFA II), 53.8% had moderate generalized weakness (MGFA III), 7.7% had severe generalized weakness (MGFA IV) and 1.9% were in crises (MGFA V). During diagnostic work-up, 71 (68.3%) patients showed a positive decremental response on RNS, while serum acetylcholine receptor antibodies were present in 71 of 97 (73.2%) patients for whom results were available. The prevalence of acetylcholine receptor antibodies in patients with pure ocular MG at presentation (MGFA class I severity) was 68.2%, although at last assessment, only 27.3% (6 of 22) of these patients had pure ocular MG, the remainder having progressed to generalized MG, or having attained a remission. In contrast, the prevalence of acetylcholine receptor antibodies was 74.7% in those with generalized MG, or MGFA classes II-V at presentation.
Figure 1.
Myasthenia Gravis Foundation of America clinical classification of 104 myasthenia gravis patients at diagnosis.
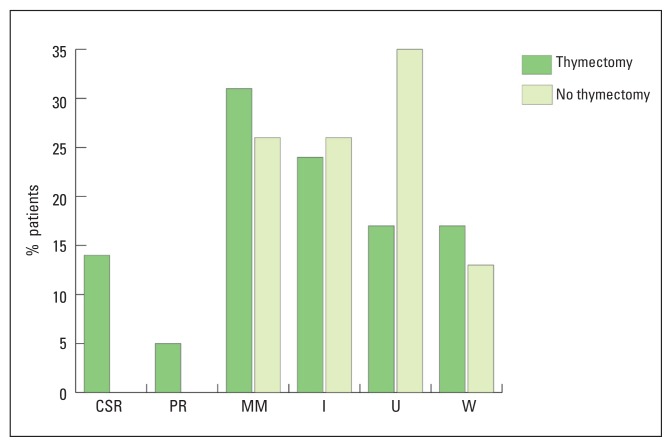
The prevalence of acetylcholine receptor antibodies in those with disease onset younger than age 14 years (10/15, 67%) was similar to that of adult-onset MG (61/82, 74%). Four of the five seronegative patients with childhood-onset MG had muscle biopsies, which excluded mitochondrial disease and muscular dystrophies. Twenty-six patients (25%) had other autoimmune diseases, with thyroid disease being most common (Table 1). At last assessment, 10 patients (9.6%) were in CSR, four patients (3.8%) in PR, 29 patients (27.9%) had MM, 24 patients (23.1%) had significant improvement, 21 patients (20.2%) had unchanged severity and 16 patients (15.4%) were worse (Figure 2). No patient died of MG during the period of study.
Table 1.
Associated autoimmune disease in 104 patients with myasthenia gravis.
| Associated disease | Male | Female | Total |
|---|---|---|---|
| Hyperthyroidism | 2 | 5 | 7 |
| Hypothyroidism | 2 | 3 | 5 |
| Type 1 diabetes mellitus | 1 | 4 | 5 |
| Bronchial asthma | - | 4 | 4 |
| Pernicious anemia | - | 2 | 2 |
| Rheumatoid arthritis | - | 1 | 1 |
| Alopecia totalis | 1 | - | 1 |
| Crohn’s disease | - | 1 | 1 |
| Hypogodotrohic hypogonadism | 1 | - | 1 |
Figure 2.
Postintervention status in non-thymectomized and thymectomized patients without thymoma. CSR: complete stable remission, PR: pharmacological remission, MM: minimal manifestations, I: improved, U: unchanged, W: worse.
Thymectomized versus non-thymectomized patients
Thymectomy was performed in 81 patients (78%), at a median interval of one year from MG diagnosis (range, 2 months to 6 years). Seventy-two of these patients had maximal thymectomy by the trans-sternal approach, while nine patients had laparoscopic surgery. Histology results were not available for two patients. Of the remaining 79 patients, hyperplasia was seen in 54 (68.4%), an atrophied or normal thymus in 17 (21.5%) and thymoma in 8 patients (10.1%). Remission was achieved only by patients who had thymectomy, at a mean interval of 26 months (range, 3 to 72 months) from thymectomy and 46 months (range, 9 to 84 months) from MG diagnosis. Duration of remission at last assessment ranged from 1 year to 18 years (mean, 5 years).
To assess the benefits of thymectomy in non-thymomatous MG, 8 patients with thymoma were excluded from the analysis. The rate of CSR in thymectomized patients was not significantly different from non-thymectomized patients (13.7 % vs. 0.0%; P=.111), but the achievement of any remission (either CSR or PR) was significantly higher in patients who had thymectomy compared to those who had no thymectomy (19.18% vs. 0.0%; P=.02).
Patients seronegative for acetylcholine receptor antibodies tended to have better remission rates compared to seropositive patients (19.23% v. 11.2%), but this difference was not significant (P=.325). No difference was observed in age of onset between those who did and those who did not achieve CSR, any remission or a satisfactory outcome. A higher rate of satisfactory outcome was observed among thymectomized patients (46.6% vs. 26.1%; P=.095), but this difference was not significant. The differences in requirements for steroids or azathioprine were also not significant (Table 2).
Table 2.
Clinical characteristics of 96 patients with non-thymomatous myasthenia gravis.
| Clinical variable | Thymectomy (n=73) | No thymectomy (n=23) | P value |
|---|---|---|---|
| Age (mean±SD) (years) | 21.1±10.2 | 31.2±14.3 | <.001 |
| Females | 49 (67.1%) | 13 (56.5%) | .454 |
| Endpoints | |||
| Complete stable remission | 10 (13.7%) | 0 (0.0%) | .111 |
| Any remission | 14 (19.2%) | 0 (0.0%) | .020 |
| Satisfactory outcome | 34 (46.6%) | 6 (26.1%) | .095 |
| Used prednisolone | 61 (74.0%) | 16 (69.6%) | .789 |
| Used azathioprine | 30 (41.1%) | 5 (21.7%) | .136 |
DISCUSSION
Our results show that MG in Arabs occurs at an age younger than in other racial groups,9,10 findings that are consistent with previous studies.7,8 We did not observe the second peak of onset beyond age 50 years described in European males or in patients with thymoma,10 as the mean age of our thymoma patients was 38.2 years. The younger age of onset observed in our patients may raise the possibility of other childhood diseases mimicking autoimmune MG. However, since most of our patients with disease onset younger than age 14 years had high titers of acetylcholine receptor antibodies, and four of five seronegative patients had muscle biopsies, disorders such as muscular dystrophies, mitochondrial disease and congenital myasthenic syndromes were effectively excluded.
The prevalence of acetylcholine receptor antibodies in MG varies greatly between studies, depending on the patient population. Rates of 94% in adult-onset generalized MG and 29% in pure ocular MG have been reported among whites,10 while in a Chinese cohort composed of both adults and children, the rates were 85% in generalized MG and 59% in purely ocular MG.11 In exclusively pediatric MG populations, reported prevalence rates have ranged from 33% to 81%.12,13 Thus, seroprevalence rates among the different subgroups in the present study were all within the ranges reported elsewhere.
Over half of our patients presented with either moderate or severe disease, but after medical treatment with or without thymectomy, 41% had a satisfactory outcome, 23% had significant improvement and 13% had achieved some form of remission. More significant is the benefit of thymectomy observed among our patients. All 14 patients who achieved either CSR or PR had had thymectomy, compared to non-thymectomized patients who achieved neither, a difference that was significant. If the decision to perform thymectomy was influenced by disease severity in our patients, this could potentially bias our results, but thymectomy at our center was offered to all patients so that non-thymectomized patients were those that refused surgery. A very large Italian study14 reported a remission rate of 11%, which was comparable to our own findings, but other studies have reported better remission rates, depending on criteria used to define remission. For instance, an Italian study reported a six-month CSR rate of 9.5%,15 while remission rates were 34% among thymectomized patients versus 17% among non-thymectomized patients in a Japanese study,16 even though some of the patients in the latter study continued to take anticholinesterases. However, the MGFA post-intervention status criteria used in our study defines CSR as the absence of symptoms and signs of MG for at least one year in a patient who received no therapy during that period.8
Contrary to findings in many studies,17–19 but consistent with the results of one study,15 we did not find a younger age at onset having favorable effects on remission among our patients with non-thymomatous MG. We did observe, however, that patients who had thymectomy in our study were a decade younger than those who did not have thymectomy, suggesting that either younger patients were more likely to give consent to surgery, or surgery was more likely when someone other than the patient gave consent, as in the case of children.
Our study had some limitations, mainly due to its retrospective design. For instance, some investigation results could not be traced, although this could be due to the long follow-up as much as the retrospective design. We observed also that some patients with minimal or no symptoms continued to take anticholinesterases, perhaps due to lack of clear criteria for stopping drug treatment, a situation that would not arise in a prospective study. Although this could negatively influence outcomes, we do not believe that the effect was significant, considering the very small number of such patients among our cohort.
In conclusion, our results show that MG presents at a much younger age among Arabs compared to other racial groups, but the clinical profile and response to therapy is comparable to results obtained elsewhere. While we observed a striking benefit of thymectomy among patients with non-thymomatous MG, over 35% of all patients had unfavorable outcomes at last assessment. Clearly, early diagnosis and timely referral to specialist centers for optimal therapy and thymectomy may result in a better prognosis. The high prevalence of thyroid disease among MG patients should alert physicians to the possibility of co-existing myopathy, especially among patients who do not respond to optimal therapy.
REFERENCES
- 1.Mantegazza R, Baggi F, Antozzi C, et al. Myasthenia gravis (MG): Epidemiological data and prognostic factors. Ann N Y Acad Sci. 2003;998:413–423. doi: 10.1196/annals.1254.054. [DOI] [PubMed] [Google Scholar]
- 2.Grob D, Brunner NG, Namba T. The natural course of myasthenia gravis and effects of therapeutic measures. Ann N Y Acad Sci. 1981;377:652–669. doi: 10.1111/j.1749-6632.1981.tb33764.x. [DOI] [PubMed] [Google Scholar]
- 3.Drachman DB. Myasthenia gravis. N Eng J Med. 1994;330(25):1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 4.Gronseth GS, Barohn R. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review). Report from the Quality Standards Subcommittee of the American Academy of Neurology. Neurol. 2000;55:7–15. doi: 10.1212/wnl.55.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Chui H-C, Vincent J, Newsom-Davis, et al. Myasthenia gravis: population differences in disease expression and acetylcholine receptor antibody titers between Chinese and Caucasians. Neurol. 1987;37:1854–1857. doi: 10.1212/wnl.37.12.1854. [DOI] [PubMed] [Google Scholar]
- 6.Heckmann JM, Owen EP, Little F. Myasthenia gravis in South Africans: Racial differences in clinical manifestations. Neuromusc Dis. 2007;17:929–934. doi: 10.1016/j.nmd.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Ashour MH, Jain SK, Kattan KM, et al. Maximal thymectomy for myasthenia gravis. Eur J Cardiothor Surg. 1995;9:461–464. doi: 10.1016/s1010-7940(05)80083-1. [DOI] [PubMed] [Google Scholar]
- 8.Jacob PC, Tharakan JT, Chand PR, Koul RL, Chacko AP. Clinical profile of myasthenia gravis in the Sultanate of Oman. Saudi Med J. 2003;27(7):774–775. [PubMed] [Google Scholar]
- 9.Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: Recommendations for clinical research standards. Ann Thorac Surg. 2000;70:327–334. doi: 10.1016/s0003-4975(00)01595-2. [DOI] [PubMed] [Google Scholar]
- 10.Beekman R, Kuks JBM, Oosterhuis HJGH. Myasthenia gravis: diagnosis and follow-up of 100 consecutive patients. J Neurol. 1997;244:112–118. doi: 10.1007/s004150050059. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Yang M, Xu J, Zhang M, Lang B, Wang W, et al. Clinical and serological study of myasthenia gravis in Hubei Province, China. J Neurol Neurosurg Psychiatry. 2007;78:386–390. doi: 10.1136/jnnp.2006.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews PI, Massey JM, Sanders DB. Acetylcholine receptor antibodies in juvenile myasthenia gravis. Neurol. 1993;43:977–982. doi: 10.1212/wnl.43.5.977. [DOI] [PubMed] [Google Scholar]
- 13.Lindner A, Schalke B, Toyka KV. Outcome in juvenile-onset myasthenia gravis: a retrospective study with long-term follow-up of 79 patients. J Neurol. 1997;244:515–520. doi: 10.1007/s004150050135. [DOI] [PubMed] [Google Scholar]
- 14.Mantegazza R, Beghi E, Pareyson P, et al. A multicenter follow-up study of 1152 patients with myasthenia gravis in Italy. J Neurol. 1990;237:339–344. doi: 10.1007/BF00315656. [DOI] [PubMed] [Google Scholar]
- 15.Cosi V, Romani A, Lombardi M, et al. Prognosis of myasthenia gravis: a retrospective study of 380 patients. J Neurol. 1997;244:548–555. doi: 10.1007/s004150050142. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi N, Kuwabara S, Nemoto Y, et al. Treatment and outcome of myasthenia gravis: retrospective multi-center analysis of 470 Japanese patients, 1999 – 2000. J Neurol Sci. 2004;224:43–47. doi: 10.1016/j.jns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Beghi E, Antozzi C, Batocchi AP, et al. Prognosis of myasthenia gravis: a multicenter follow-up of 844 patients. J Neurol Sci. 1991;106:213–220. doi: 10.1016/0022-510x(91)90260-e. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson DH, Ansher M, Horan S, Rutherford RB, Ringel SP. The relationship of age to outcome in myasthenia gravis. Neurol. 1990;40:786–790. doi: 10.1212/wnl.40.5.786. [DOI] [PubMed] [Google Scholar]
- 19.Durelli L, Maggi G, Casadio C, Ferri R, Rendine S, Bergamini R. Actuarial analysis of the occurrence of remissions following thymectomy for myasthenia gravis in 400 patients. J Neurol Neurosurg Psychiatry. 1991;54:406–411. doi: 10.1136/jnnp.54.5.406. [DOI] [PMC free article] [PubMed] [Google Scholar]