Abstract
BACKGROUND AND OBJECTIVES
Health-related quality of life (HRQOL) is an important health outcome, representing one of the most important goals of all health interventions. The objectives of this study were to determine HRQOL and the factors affecting it in type 2 diabetic patients.
PATIENTS AND METHODS
This cross-sectional study was conducted in five primary health care (PHC) centers in the Al-Khobar area. From a random sample of 225 type 2 diabetic patients, 216 patients were included in the study along with 216 age-, sex- and nationality-matched controls. Nine patients refused to participate. Type 2 diabetic patients and controls were interviewed with the translated Arabic SF-12 questionnaire.
RESULTS
The mean ages were 50.0±10.0 years for cases and of 49.3±10.3 years for controls (P=.526). Type 2 diabetic patients had lower socioeconomic status and educational level than controls. Obesity was significantly higher in diabetics than controls. HRQOL in type 2 diabetic patients was significantly lower than controls. The mean physical component score was 41.3±8.9 for cases vs. 47.5±9.5 for controls (P<.001), and the mean mental component score 47.8±9.1 in cases vs. 51.5±9.4 in controls (P<.001). HRQOL was significantly lower in females than males (P<.001). HRQOL was impaired in uncontrolled patients (fasting plasma glucose [FPG]>130 mg/dL) in comparison with controlled patients (FPG≤130 mg/dL) (P<.05).
CONCLUSIONS
HRQOL was lower in type 2 diabetic patients than controls and was affected by many factors. Females had lower HRQOL than males, possibly because of a higher incidence of obesity. Uncontrolled diabetic patients had a lower HRQOL than controlled diabetics. Improving HRQOL in diabetic patients is important.
Type 2 diabetes mellitus is a chronic disease that has a high prevalence in Saudi Arabia, and the prevalence continues to increase in the Saudi population, making it a major public health problem.1 In recent years, quality of life has been increasingly recognized as an important outcome of medical treatment and has become a core issue in diabetes management. Health-related quality of life (HRQOL) is an important health outcome in its own right, representing an extremely important goal of all health interventions. The role of primary healthcare centers (PHC) in achieving the goal of improving awareness among diabetic patients and their families cannot be underestimated. Several studies have shown that diabetes can negatively affect the physical and mental well-being of patients through development of short and long-term complications, physical symptoms and lifestyle changes, and feelings of helplessness and emotional distress.2–6 Many factors had been shown to affect HRQOL in diabetic patients. These include demographic factors, body mass index (BMI), blood glucose, glycated hemoglobin (HbA1c), duration of disease, obesity, dyslipidemia, hypertension, and pre-existing heart disease.7 HRQOL in diabetic patients has several manifestations. These include an inability to do physical work (physical function) because of diabetes-related complications and feeling fatigued and depressed because of high blood glucose level (mental function). Therefore, there is a need to explore HRQOL in diabetic patients.
The short form health survey (SF-12) is a valid generic measure of health outcome to examine HRQOL.8 Its Arabic translation and administration in type 2 diabetic patients will improve field research in this area. The aim of this study was to assess HRQOL and the different factors that affect it among type 2 diabetic patients.
PATIENTS AND METHODS
This was a case-control study conducted in PHC centers in Al-Khobar City, Eastern Province of Saudi Arabia. The study population from which cases were selected consisted of 1144 Saudi male and female type 2 diabetic patients registered in the 11 PHC centers. According to AlHazmi et al,9 the prevalence of diabetes in the Dammam area was 6.34% in males and 7.04% in females, so we chose a prevalence of diabetes of 8% in calculating the sample size for the study (n=[(z)2 (p) (p-1)]/(d)2 where n: sample size, z: reliability coefficient [z=1.96 for 95% confidence interval], p: expected population proportion having diabetes=8%=0.08, d: desired interval width =4.0%, type I error α=0.05 and type II error β=0.2, with a confidence interval of 0.95). The sample size according to the equation was 177. The investigators expected a response rate of 80%, therefore the estimated sample size was 225 patients.10
A two-stage random sampling technique was used. In the first stage, 5 of the 11 PHC centers were selected using a simple random sampling technique. In the second stage, a stratified systematic random sampling was used to select diabetic patients using their records in each PHC Center. The selection was stratified by sex. Controls were selected by matching from non-diabetic patients registered in the five PHC Centers. Matching was done for age (within ±5 years), sex and nationality.
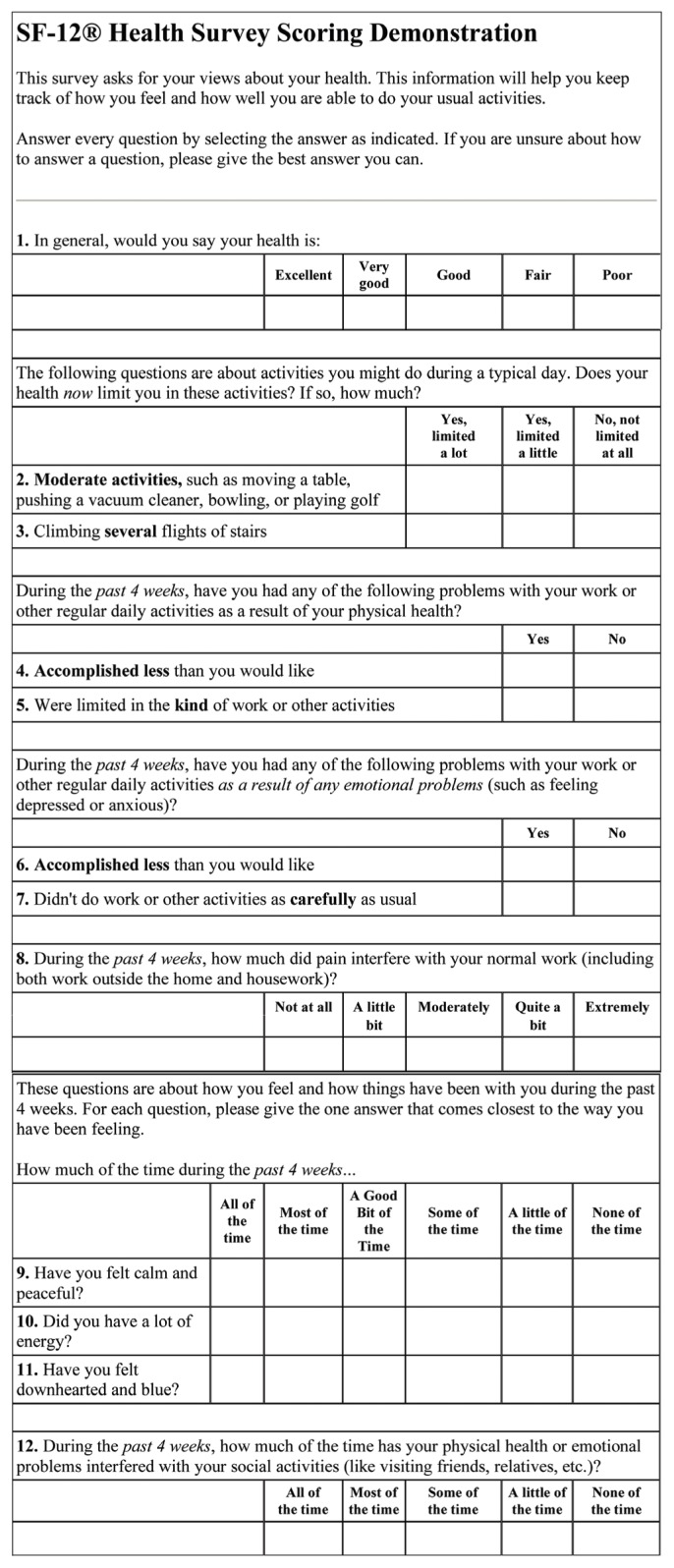
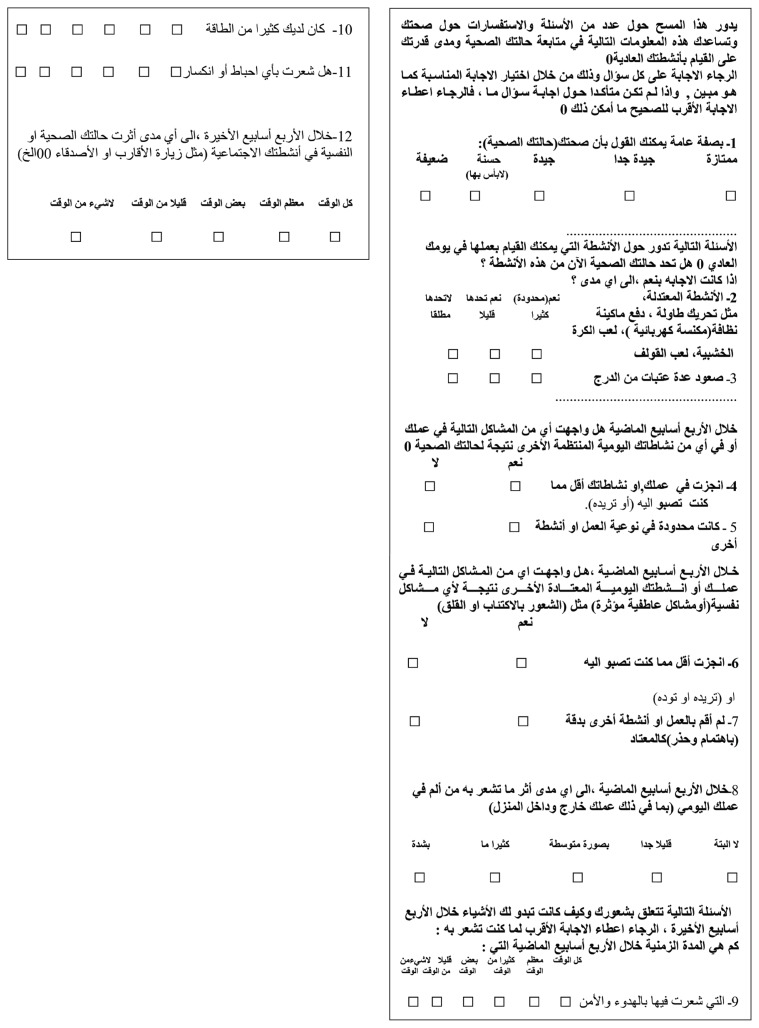
Data was collected using an interviewer-administered questionnaire containing two parts. The first part covered sociodemographic and clinical data about diabetes. The second part was the Arabic version of the SF-12 questionnaire. The SF-12 is a valid alternative to the SF-36 for use in large surveys of general and specific populations. 10–12 The SF-12 contains 12 items (see Appendix 1). All SF-12 items came from the SF-36. It includes eight dimensions: physical functioning, role limitations due to physical health problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems and mental health. Translation of the original SF-12 questionnaire from English to Arabic (Appendix 2) was done after obtaining permission from the author of the questionnaire (www.qualitymetric.com).8 It was translated by the academic translator in King Fahd University Hospital Medical Education Center. For content validity, three consultants from the Department of Family and Community Medicine reviewed the Arabic version. They translated it to English again and their translation was compared with the original English version of the questionnaire. A pilot study was conducted in one PHC center among 20 patients (10 males and 10 females) with diabetes. As a result, some wording was added for better understanding of the questions. In testing the reliability of the translated questionnaire using the test-retest method, the Chronbach alpha was equal to 0.84, which is considered very good reliability.
The SF-12 questionnaire was administered to diabetic patients and controls by six Saudi female nurses who received two weeks of training. The reliability of the interviewers was ensured through daily continuous training and supervision of the interviewers by the first author. A sub-sample of 43 case patients (20%) were reinterviewed by the first author, within 2 to 4 weeks from the initial interview to test the reliability of the questionnaire. The scoring system used for scoring of the SF-12 questionnaire was based on the scoring of SF-12 from Ware and colleagues.8 A weighted number was given to each physical and mental item of the SF-12 questionnaire. Using specific calculations, the mean physical component score (PCS) and mental component score (MCS) were then derived. These means were used as measures of physical HRQOL and mental HRQOL and were subsequently used for comparing HRQOL between diabetic patients and controls.
Diabetes was diagnosed as a fasting plasma glucose (FPG) of ≤126 mg/dL, or a 2-hour postprandial glucose of ≤200 mg/dL or if the individual had symptoms of diabetes and a random plasma glucose ≤200 mg/dL (confirmed by repeat testing).14 The term controlled diabetes was defined as an FPG from 90 to 130 mg/dL, with more than 130 mg/dL considered uncontrolled.14 In this study a glycated hemoglobin level of ≤9.0% was considered as evidence of control and >9% as uncontrolled, which was based on the study of HRQOL among diabetic patients done by Hill-Briggs et al.15 Body mass index (BMI) was classified as: 20–24.9 kg/m2 normal, 25–29.9 kg/m2 overweight, 30–34.9 kg/m2 mild obesity, ≤35 kg/m2 morbid obesity.16 Exercise was classified as 1) regular: exercise for a duration of 30 minutes or more, three times or more per week; 2) irregular: exercise less than 30 minutes; 3) no exercise.17
Data were coded and entered into a personal computer using Statistical Package for Social Sciences (SPSS) version 10.0.18 HRQOL was scored for all related variables. The t-test was used to differentiate between mean scores of HRQOL for both cases and controls. The chi-square test was used to measure associations among qualitative variables. Multiple linear regression was used for comparing the different factors associated with PCS and MCS. Statistical significance was set at <0.05.
RESULTS
Two hundred twenty-five type 2 diabetic patients were included in this study; 9 patients refused to participate. The response rate was 216 out of 225 (96%). Controls were 216 in number, for a total of 432 participants interviewed. The sociodemographic characteristics of the diabetic patients and controls are shown in Table 1. There was no significant statistical difference in age between cases and controls. Almost half of the diabetic patients were illiterate (49.5%) compared to 28.2% of the controls. The difference between cases and controls in education was statistically significant. More than one-third of both cases and controls were housewives, (Table 1). There were more subjects working in the military among controls than cases. There was a statistically significant difference between cases and controls in occupation (P=.014). Diabetes complications were reported by 72.6% of the cases. There was a high prevalence of coronary artery disease (13.9%), hypertension (22.2%), retinopathies (47.2%) in cases. Obesity was more prevalent in cases (29.2%) than controls (18%) (P=.015). Cases with uncontrolled diabetes had a mean BMI of 30.4±5.9 kg/m2 vs 27.0±3.6 kg/m2 for controlled cases. About 51% of uncontrolled patients had a BMI≤30, which was significantly higher than controlled patients (26.2%) (P=.013).
Table 1.
Sociodemographic characteristics of cases and controls.
| Variables | Cases n=216 |
Controls n=216 |
Total | P value (χ2-test) | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
|
| |||||||
| Age group | NS | ||||||
|
| |||||||
| 20–29 years | 4 | 1.85 | 4 | 1.85 | 8 | 1.85 | |
|
| |||||||
| 30–39 years | 27 | 12.0 | 27 | 12.0 | 54 | 12.0 | |
|
| |||||||
| 40–49 years | 79 | 36.5 | 79 | 36.5 | 158 | 36.5 | |
|
| |||||||
| 50–59 years | 52 | 24.1 | 52 | 24.1 | 104 | 24.1 | |
|
| |||||||
| ≤60 years | 54 | 24.9 | 54 | 24.9 | 108 | 24.9 | |
|
| |||||||
| Age (years) (mean±SD) | 50.0±10.0 | 10.02 | 49.3±10.3 | 10.27 | |||
|
| |||||||
| Sex | NS | ||||||
|
| |||||||
| Males | 106 | 49.1 | 106 | 49.1 | 212 | 49.1 | |
|
| |||||||
| Females | 110 | 50.9 | 110 | 50.9 | 220 | 50.9 | |
|
| |||||||
| Education | <.001 | ||||||
|
| |||||||
| Illiterate | 107 | 49.5 | 61 | 28.2 | 168 | 38.9 | |
|
| |||||||
| Read and write/primary | 72 | 33.3 | 83 | 38.4 | 155 | 35.9 | |
|
| |||||||
| Intermediate | 19 | 8.8 | 33 | 15.3 | 52 | 12.0 | |
|
| |||||||
| Secondary | 13 | 6.0 | 30 | 13.9 | 43 | 10.0 | |
| College and above | 5 | 2.3 | 9 | 4.2 | 14 | 3.2 | |
|
| |||||||
| Occupation | .014 | ||||||
|
| |||||||
| Housewife | 100 | 46.3 | 89 | 41.2 | 189 | 43.8 | |
|
| |||||||
| Government (employee and teacher) | 30 | 13.9 | 45 | 20.8 | 75 | 17.4 | |
|
| |||||||
| Student | 2 | 0.9 | 1 | 0.5 | 3 | 0.7 | |
|
| |||||||
| Private | 30 | 13.9 | 28 | 13.0 | 58 | 13.5 | |
|
| |||||||
| Military | 16 | 7.4 | 34 | 15.7 | 50 | 11.4 | |
|
| |||||||
| Unemployed | 10 | 4.6 | 4 | 1.9 | 14 | 3.2 | |
|
| |||||||
| Retired | 28 | 13.0 | 15 | 6.9 | 43 | 10.0 | |
|
| |||||||
| Income/month | NS | ||||||
|
| |||||||
| Low <1999 SR | 49 | 22.7 | 42 | 19.4 | 91 | 21.1 | |
|
| |||||||
| Middle 2000–4999 SR | 95 | 44.0 | 104 | 48.1 | 199 | 46.1 | |
|
| |||||||
| High ≤5000 SR | 72 | 33.3 | 70 | 32.4 | 142 | 32.9 | |
NS = Not significant
The mean PCS for type 2 diabetic patients was 41.3±8.9, which was statistically significantly lower than that of the controls (47.5±9.5; P<.001) (Table 2). The mean MCS for type 2 diabetic patients was 47.8±9.1, which was significantly lower than that of the controls (51.5±9.4; P<.001). Older subjects scored less than younger subjects but the difference was not statistically significant. Females scored significantly less than males. Patients who exercised for 30 minutes or more scored significantly more than those who exercised for less than 30 minutes. Patients who reported emergency visits scored less than those who did not visit the emergency department for diabetes symptoms, but the difference was not statistically significant. Patients who reported hospital admission scored significantly less than those who did not have admission to the hospital. The HRQOL score decreased as the number of complications increased (P=.004). Patients with high income scored more than those with lower income, but the difference was not statistically significant (P=0.051).
Table 2.
Summary of the differences in the means for PCS and MCS in the three different variables.
| Variable | PCS (mean±SD) | P value | MCS (mean±SD) | P value |
|---|---|---|---|---|
| Type of patient | ||||
| Cases | 41.3±8.9 | <.001 | 47.8±9.1 | <.001 |
| Controls | 47.5 ±9.5 | 51.5±9.4 | ||
| Gender | ||||
| Male | 47.8±9.3 | <.001 | 52.5±8.3 | <.001 |
| Female | 41.2±9.1 | 46.9±9.6 | ||
| Control status | ||||
| Controlled | 44.3±9.5 | .022 | 51.9±8.5 | .002 |
| Uncontrolled | 40.7±8.8 | 47.1±8.9 |
PCS: Physical component score, MCS: Mental component score.
Fourteen independent variables were regressed with PCS as a dependent variable and 14 independent variables were regressed with MCS as a dependent variable by the backward method (Tables 3, 4). The coefficient of determination (R2) was 0.242 for the PCS variables in Table 3, meaning that 24.2% of the variability in PCS was determined by the independent variables. The coefficient of determination (R2) was 0.151 for the MCS variables in Table 4, meaning that 15.1% of the variability in MCS was determined by the independent variables. Obese patients scored a lower HRQOL than non-obese patients.
Table 3.
Multiple linear regression model to predict HRQOL from physical component scores for diabetes patients.
| Variables* | Regression coefficient (B) | SE B | P value |
|---|---|---|---|
| Age | −0.109 | 0.063 | .086** |
| Female gender | −4.561 | 1.328 | .001 |
| Exercise time | 3.626 | 1.527 | .019 |
| Emergency visit | −2.686 | 1.571 | .090** |
| Hospital admission | −3.298 | 1.661 | .049 |
| Number of complications | −1.990 | 0.669 | .004 |
| Constant | 56.133 | 4.281 | - |
R2=0.242;
Not significant;
SE = Standard error
Table 4.
Multiple linear regression model to predict HRQOL from MCS for diabetes patients.
| Variables* | Regression coefficient (B) | SE B | P value |
|---|---|---|---|
| Income | 1.575 | 0.801 | .051** |
| Family history | −2.799 | 1.205 | .021 |
| Self-monitor of urine | 1.809 | 0.763 | .091** |
| Obesity | −4.110 | 1.690 | .061** |
| FBS level | −3.873 | 1.511 | .011 |
| Constant | 46.704 | 2.486 | - |
R2= 0.151;
Not significant;
SE=Standard error
DISCUSSION
In this study, most of the type 2 diabetic patients (98.2%) were more than 30 years old, which was similar to the patients in the Al-Arabi study in 1996.18 The Al-Arabi study assessed diabetes-related distress and psychological adjustment in diabetic patients registered in PHC centers in the Al-Khobar area. The mean duration for the cases was 8.6±7.1 years, which was similar to the Al-Arabi study (8.8±6.1 years).19 About half of the cases and 28.2% of the controls were illiterate, which was similar to the Al-Arabi study (52.2% vs. 30.1%). There were a significantly greater number of subjects with a low educational level in cases (49.5%) than in controls (28.2%). In addition, 22.7% of diabetics had low income. The low socioeconomic status and low educational level could explain the associated poor metabolic control and the presence of complications and therefore the poor HRQOL. These results were consistent with the results of Larsson et al.20
Our study showed decreased HRQOL in type 2 diabetic patients compared to controls. This result was consistent with the study of Hänninen et al, who reported that the mean scores of the six SF-20 dimensions were 11% to 27% lower in type 2 diabetes patients than in the controls (P<.01).21 The mean PCS and MCS were significantly lower in type 2 diabetic patients. The Hänninen et al study, a cross-sectional study conducted in one healthcare center, also showed no association between HRQOL and gender, age or marital status.21 The authors stated that the cause of the decrease in HRQOL in type 2 diabetics was mainly due to large vessel and microvascular complications. However, because the study was cross-sectional, it could not establish a causal relationship. In addition, the results were not generalizable. In our study, 156 (72.6%) of the cases had complications. The decrease in the HRQOL could have been related to the effect of these complications on physical and mental functions of the patients.
Our study also showed a decreased HRQOL in females compared to males. The explanation for this observation may be that females were more obese which by itself impairs HRQOL, as noted by Murray, who reported that obese patients in general tend to have decreased HRQOL compared with those who are not obese.22 Females diabetics had lower HRQOL and more visits to the emergency room and hospital admissions than male diabetics. Emergency visits and hospital admissions were usually due to hyperglycemia and increased symptoms of diabetes. Increased symptoms of diabetes were found to be associated with decreased HRQOL as reported by Pfalzgraf et al.23 This was also reported by Hemingway et al24 who found that women scored lower than men in every age group by the SF-36 in non-diabetic patients. In our study, males had higher scores than females, which was also reported by Lloyd et al, who reported that men consistently produced higher mean scores on the SF-36 than women, in 1233 type 2 diabetic patients who were not using insulin.25 The duration of diabetes was not a significant factor in our study and this was consistent with the results of Lloyd et al who reported that the duration of diabetes was not a significant factor in any of the SF-36 domains.25
The decreased HRQOL in uncontrolled type 2 diabetic patients in our study could be explained by the significantly higher mean BMI (30.4±5.9 kg/m2) in uncontrolled patients compared with the controlled group (27±3.6 kg/m2). About 51% of uncontrolled patients had a BMI≤30, which was significantly higher than controlled patients (26.2%). This result was consistent with the result of the Hill-Briggs et al study, which showed that obesity reduced HRQOL in urban African-Americans with type 2 diabetes.15 Uncontrolled diabetes can lead to more hospital admissions and more complications and this can lead to low HRQOL in diabetic patients, as reported by Glasgow et al 1997.26
As age increased, there was a decrease in HRQOL in our study, but this was not significant. In comparison, Glasgow et al reported that lower income, older age and being female, more diabetes complications, more morbid illnesses and lower physical activity led to low HRQOL in diabetic patients.26 HRQOL significantly improved with exercise longer than 30 minutes, which was consistent with the result reported by Kirk et al.27 They found that exercise of 30 minutes for 3 days or more each week produced positive changes in most subscales of SF-36, and this was significantly more with the mental subscale.
As the frequency of the emergency room visits increased, there was a decrease in HRQOL but this was not significant. Emergency room visits may be for reasons other than diabetes, which could explain this finding. As hospital admissions increased, there was a decrease in HRQOL, a difference just statistically significant. Hospital admission was usually due to hyperglycemia and increased symptoms of diabetes. Increased symptoms of diabetes are associated with a decreased HRQOL as reported by Pfalzgraf et al.23 The findings of this study were supported by Goddijn et al28 who reported that disappearance of hyperglycemia after treatment and glycemic control were associated with improvement in HRQOL, particularly in physical functioning, social functioning, vitality and health change with the SF-36 scale. The Goddijn et al28 study was a prospective cohort study with 94 patients. They switched some patients to insulin therapy and education by a diabetic specialist, which can improve glycemic control. Education could have contributed to the improvement in HRQOL in their patients (the Hawthorne effect).
As the number of complications increased, there was a decrease in HRQOL in our study. This result was consistent with the Glasgow et al study.26 Although Cheng et al reported that there was a trend for total quality of life scores to increase with more complications in Chinese elderly type 2 diabetic patients,29 the results of this study showed a higher prevalence of complications than the study of Lloyd et al.25 Our study showed a higher prevalence of coronary artery disease (CAD), hypertension, retinopathy, and peripheral neuropathy, as well as erectile dysfunction and loss of libido among diabetic patients. Lloyd et al showed that CAD was 8%, hypertension 46%, retinopathy 8%, and peripheral neuropathy 12%. Lloyd et al also showed that the presence of mild diabetic complications had a significant impact on HRQOL.25 This could be due to a decrease in health awareness and health education in patients in the Al-Khobar area.
In our study, patients with a high income scored a higher HRQOL than those with a low income, which was not significant (P=.051). This result is consistent with the study of Murray et al who reported an increase in MCS with rising income.22 This increase is usually because rising income is associated with better socioeconomic status, which was associated with better metabolic control as stated in Larsson et al.20 In our study, patients who had a family history of diabetes scored a significantly lower HRQOL in MCS than patients without a family history of diabetes. The possible reason was that in our study 41.7% of the patients who were uncontrolled had a family history of diabetes and uncontrolled status was associated with a decrease in HRQOL. This may reflect a lack of health education among diabetic patients in this study. Patients who self-monitored urine glucose scored a higher HRQOL than those who did not, but the difference was not statistically significant. Most of the studies on HRQOL did not mention self-monitoring of urine as a variable that affects HRQOL.
Obese patients scored a lower HRQOL than non-obese patients in our study, but the difference was not statistically significant. Fontaine et al reported that obese persons were significantly more impaired on the bodily pain, general health and vitality scores of the SF-36.16 Also, Lean et al reported that obesity impaired HRQOL.30 Sturm et al observed that obesity affects HRQOL using the SF-12 questionnaire.31 The possible reason for this difference between Lean et al and Sturm et al could be that most of the patients who had obesity had associated chronic disease. Sturm et al found that when controlling for demographic variables, obesity was associated with more chronic conditions and worse physical HRQOL. In our study as uncontrolled FPG level increased, MCS for HRQOL significantly decreased, which is in agreement with Smide et al.32 They reported that Tanzanian patients with poor glycemic control had significantly poorer reported health in the mental health domain by using the SF-36 measure. The impact of FPG on HRQOL appears to be because of its important role in the development of diabetic complications.32 In our study, there was no relation between use of oral hypoglycemic drugs and HRQOL. This same result was also reported by De Visser et al.33 A limitation of De Visser et al was the loss of follow-up on 59 of 248 participants, using the SF-36 and Disease Specific Diabetes Health Profile (DHP). Davis et al also reported that generic instruments such as the SF-36 are less sensitive than the diabetes quality of life questionnaire to therapy-related and lifestyle issues.34 In this study there was no association between home blood glucose monitoring and HRQOL. A similar result using SF-20 was reported by Hännian et al who reported that regular blood-glucose monitoring at home had no association with good HRQOL in type 2 diabetic patients.35 The Al-Arabi study19 was similar to our study in that it comprised an Al-Khobar population as cases and controls. Also, most uncontrolled diabetics were of low educational level, especially housewives. There may be poor access to health education about this disease through sources such as TV programs, pamphlets and brochures.
In conclusion, this study found that the HRQOL of type 2 diabetic patients was significantly lower than that of a matched control group in both MCS and PCS on the SF-12 scale. Females had significantly worse HRQOL than males. Uncontrolled diabetic patients had significantly poorer HRQOL than controlled diabetic patients. Factors that decrease PCS in HRQOL in type 2 diabetes were male patients with frequent admissions to the hospital due to hyperglycemia, exercise time less than 30 minutes in duration and an increased number of complications. Factors that decreased MCS in HRQOL in type 2 diabetes were a family history of diabetes and a high FPG level.
PHC doctors need to be informed about HRQOL in type 2 diabetic patients and the factors that affect it, so they can improve their knowledge in managing their patients. They need to be provided with updated knowledge in this field of medical practice. There is a need for continuous medical education programs for doctors in the PHC centers and hospitals about diabetes and the importance of involving nurses in this field to reach a better HRQOL and better control for diabetes.
Acknowledgments
I want to thank my advisors.
Appendix 1. English version of the SF-12 Health Survey questionnaire.8
Appendix 2. Arabic translation of the SF-12 Health Survey questionnaire
REFERENCES
- 1.El-Hazmi M, Warsy A, Al-Swailem A, Al-Swailem A, Sulaimani R. Diabetes mellitus as a health problem in Saudi Arabia. East Mediterr Health J. 1998;4(1):58–67. [Google Scholar]
- 2.Testa M. Quality-of-Life Assessment in Diabetes Research: Interpreting the Magnitude and Meaning of Treatment Effects. Diabetes Spectrum. 2000;13:29. [Google Scholar]
- 3.Polonsky W. Understanding and assessing diabetes-specific quality of life. Diabetes Spectr. 2000;13:36. [Google Scholar]
- 4.Rubin R. Diabetes and quality of life. Diabetes Spectr. 2000;13:21. [Google Scholar]
- 5.Luscomble F. Health-related quality of life measurement in type 2 diabetes. Value in Health. 2000;3(1):15–28. doi: 10.1046/j.1524-4733.2000.36032.x. [DOI] [PubMed] [Google Scholar]
- 6.Franciosi M, Pellegrini F, De Berardis G, Belfiglio M, Cavaliere D, Nardo B, et al. The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients. Diabetes Care. 2001;24(11):1870–1877. doi: 10.2337/diacare.24.11.1870. [DOI] [PubMed] [Google Scholar]
- 7.Palitzsch K, Volmer T, Abrams C, Schoelmerich J. The impact of diabetes risk factors on the quality of life of patients with type 2 diabetes. Diabetes. 2000;49(5):390. [Google Scholar]
- 8.Ware JE, Kos M, Keller SD. SF-12: How to score the SF-12 Physical and Mental Health Summary Scales. 3rd Ed. Lincoln: Quality Metric Inc; 1998. pp. 29–59. [Google Scholar]
- 9.EL-Hazmi M, Warsy AS, Al Swilem AR, AL-Swilem AM, AL-Sulimanni R, AL-Meshari A. Diabetes mellitus and impaired glucose tolerance in Saudi Arabia. Ann Saudi Med. 1996;16(4):381–385. doi: 10.5144/0256-4947.1996.381. [DOI] [PubMed] [Google Scholar]
- 10.Daniel W. Biostatistics: A Foundation for Analysis in the Health Sciences. 5th Ed. New York: John Wiley & Sons, Inc; 1991. pp. 154–158. [Google Scholar]
- 11.Ware J. SF-36 Health survey update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 12.Ware J, Kosinski M. SF-36: Physical and Mental Health Summary Scales: Manual for Users of Version 1. 2nd Ed. Lincoln. RI: Quality Metric Inc; 2001. [Google Scholar]
- 13.Gandek B, Ware J, Aaronson N, Apolone, Bjorner J, Brazier J, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries_results from the medical outcomes study. J Clin Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 14.ADA (American Diabetes Association) Guidelines for glycemic control in people with diabetes. Reprinted with permission from the American Diabetes Association. Clinical practice recommendations 2001. Diabetes Care. 2001;24(1):S34. [Google Scholar]
- 15.Hill-Briggs F, Gary T, Hill M, Bone L, Brancati F. Health-related quality of life in urban African- Americans with type 2 diabetes. J Gen Intern Med. 2002;17(6):412–9. doi: 10.1046/j.1525-1497.2002.11002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontaine KR, Bartlett SJ, Barofsky I. Health-related quality of life among obese persons seeking and not currently seeking treatment. Int J Eat Disord. 2000;27(1):101–5. doi: 10.1002/(sici)1098-108x(200001)27:1<101::aid-eat12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Mazzeo R, Tanaka H. Exercise prescription for the elderly current recommendations. Sports Med. 2001;31(11):809–818. doi: 10.2165/00007256-200131110-00003. [DOI] [PubMed] [Google Scholar]
- 18.SPSS-Win. SPSS Release 10.0.1. USA: 1999. Statistical package for social sciences (computer program) [Google Scholar]
- 19.Al-Arabi A. Dissertation. Al-Khobar, KSA: King Faisal niversity; 1996. Assessment of Diabetes-Related Distress and Psychosocial Adjustment. [Google Scholar]
- 20.Larsson D, Lager I, Nilsson P. Socio-economic characteristics and quality of life in diabetes mellitus- relation to metabolic control. Scand J Public Health. 1999;2:101–105. [PubMed] [Google Scholar]
- 21.Hanninen J, Takala J, Keinanen-Kiukaanniemi S. Quality of life in NIDDM patients assessed with the SF-20 questionnaire. Diabetes Res Clin Pract. 1998;42(1):17–27. doi: 10.1016/s0168-8227(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 22.Murray F. Body mass index and quality of life in a survey of primary care patients. J Fam Pract. 2000;49(8):734–737. [PubMed] [Google Scholar]
- 23.Pfalzgraf A. The relationship of diabetes symptoms and health-related quality of life. Value in Health. 2001;4(2):58. [Google Scholar]
- 24.Hemingway H, Nicholson A, Marmot M. The Impact of Socioeconomic Status on Health Functioning as Assessed by the SF-36 Questionnaire: The Whitehall II Study. American. doi: 10.2105/ajph.87.9.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd A, Sawyer W, Hopkinson P. Impact of long-term complications on quality of life in patients with type 2 Diabetes not using insulin. Value in Health. 2001;4(5):392–400. doi: 10.1046/j.1524-4733.2001.45029.x. [DOI] [PubMed] [Google Scholar]
- 26.Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care. 1997;20(4):562–567. doi: 10.2337/diacare.20.4.562. [DOI] [PubMed] [Google Scholar]
- 27.Kirk A, Higgins L, Hughes A, Fishert B, Mutrie N, Hillis S, et al. A randomized, controlled trial to study the effect of exercise consultation on the promotion of physical activity in people with Type 2 diabetes: a pilot study. Diabetes UK Diabetes Med. 2001;18:877–882. doi: 10.1046/j.0742-3071.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 28.Goddijn P, Bilo H, Feskens E, Groenier K, Zees K, Jong B. Longitudenal study on glycemic control and quality of life in patients with type 2 diabetes mellitus referred for intensified control. Br Diabetic Assoc Diabetic Med. 1999;16:23–30. doi: 10.1046/j.1464-5491.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheng A, Tsui E, Hanely A, Zinman B. Developing a quality of life measure for Chinese patients with diabetes. Diabetes Res Clin Pract. 1999;46:259–267. doi: 10.1016/s0168-8227(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 30.Lean M, Han T, Seidell J. Impairment of health and quality of life using new US federal guidelines for the identification of obesity. Arch Intern Med. 1999;159(8):837–843. doi: 10.1001/archinte.159.8.837. [DOI] [PubMed] [Google Scholar]
- 31.Sturm R, Wells KB. Does obesity contribute as much to morbidity as poverty or smoking? Public Health. 2001;115(3):229–235. doi: 10.1038/sj/ph/1900764. [DOI] [PubMed] [Google Scholar]
- 32.Smide B, Lukwale J, Msoka A, Wikblad K. Self-reported health and glycaemic control in Tanzanian and Swedish diabetic patients. J Adv Nurs. 2002;37(2):182–191. doi: 10.1046/j.1365-2648.2002.02072.x. [DOI] [PubMed] [Google Scholar]
- 33.De Visser C, Bilo H, Groenier K, De Visser W, De Jong B. The influence of cardiovascular disease on quality of life in type 2 diabetics. Quality Life Res. 2002;11:249–261. doi: 10.1023/a:1015287825660. [DOI] [PubMed] [Google Scholar]
- 34.Davis T, Clifford R, Davis W. Effect of insulin therapy on quality of life in type 2 diabetes mellitus: The Fremantle Diabetes Study. Diabetes Res Clin Pract. 2001;52:63–71. doi: 10.1016/s0168-8227(00)00245-x. [DOI] [PubMed] [Google Scholar]
- 35.Hänninen J, Takala J, Nen-Kiukaanniemi S. Good continuity of care may improve quality of life in Type 2 diabetes. Diabetes Res Clin Pract. 2001;51:21–27. doi: 10.1016/s0168-8227(00)00198-4. [DOI] [PubMed] [Google Scholar]