Abstract
BACKGROUND AND OBJECTIVES
Ependymoma is the most frequently encountered intramedullary tumor. Total surgical resection is the therapeutic modality of choice whenever possible, but carries a significant risk of morbidity. This study was designed to define prognostic factors that affect clinical outcome after surgical resection of spinal intramedullary ependymoma.
PATIENTS AND METHODS
The medical records, radiological and pathological studies of all patients with intramedullary spinal ependymomas treated surgically in one institution were reviewed retrospectively. Spinal myxopapillary ependymomas were excluded. In a multivariable regression analyis, possible prognostic factors were correlated with the 6-month postoperative neurological status using McCormick’s grading scale.
RESULTS
Surgery was performed on 17 patients (14 males, 3 females, mean age of 42±15 years) with spinal ependymoma. The cervical spine was the most common tumor location (71%). Total surgical resection of the tumor was achieved in 11 cases (65%). Intraoperative neurophysiological monitoring was used in 8 cases (47%). Postoperatively, 11 patients (65%) either improved or had no change from their preoperative neurological status. None of the 11 totally resected tumors has shown evidence of recurrence in a follow-up period range from 8–120 months (median, 33 months). Of several possible prognostic factors, the only statistically significant correlation identified with the 6-month postoperative neurological status was the preoperative McCormick grading score.
CONCLUSIONS
Preoperative neurological status was the only statistically significant factor in determining the postoperative neurological outcome of patients with spinal intramedullary ependymomas. Early diagnosis and referral for surgery to specialized centers are recommended as controllable factors in improving outcome.
Intramedullary spinal cord tumors comprise 2% to 4% of all central nervous system (CNS) tumors. They constitute 20% and 35% of all intraspinal tumors in adults and children, respectively.1–3 The majority of these tumors are glial in origin.4,5 Ependymomas are the most common intramedullary spinal cord tumors, comprising 60% of such tumors.2,3,6,7
Classical spinal ependymomas predominantly affect middle-aged adults, with an equal sex distribution.2,7–9 Pathologically, they exhibit properties of a benign histology, World Health Organization (WHO) grade II.6,10 These tumors are well circumscribed and compress rather than infiltrate surrounding spinal cord tissues. 7,8 Therefore, a gross total resection is the optimal surgical goal in the management of these tumors. In the past, surgical resection was often associated with significant postoperative neurological morbidities. Recent advances in operative techniques and intraoperative neurophysiological monitoring have made the surgical resection a relatively safe procedure.8,11–16 In this study, we aimed to describe possible prognostic factors that would affect clinical outcome after surgical resection of spinal intramedullary ependymomas.
PATIENTS AND METHODS
A retrospective analysis was conducted on all patients who underwent surgical resection for intramedullary classical spinal ependymoma in our center from 1987 to 2005. Exclusion criteria included patients with the myxopapillary subtype and patients who had surgery outside our institution. Medical charts, related images and pathological slides were all reviewed. All pre- and postoperative functional status was classified according to the McCormick Grading Scale,8 as follows:
Grade I – neurologically normal; mild focal deficit insignificantly affecting the function of the involved limb; mild spasticity; normal gait.
Grade II – sensorimotor deficit affecting the function of the involved limb; mild to moderate gait difficulty; severe pain or dysesthetic syndrome impairing the patient’s quality of life.
Grade III – more severe neurological deficit; requires cane for ambulation; significant bilateral upper-extremity impairment; may or may not function independently.
Grade IV – severe deficit; requires wheelchair, bilateral upper extremities impairment; not independent.
The pre- and postoperative McCormick grades were recorded at time of admission, discharge and at 6 months postoperatively (Table 1).
Table 1.
Clinical summary of 17 patients with intramedullary ependymoma.
| Case No. | Age (years) & sex | Duration (months) of symptoms | Symptoms | Tumor location | Surgical resection | Monitoring | Pre-op Gradea | Post-op Gradea | Grade at follow-up | Follow-up duration (months) | note |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22, M | 6 | S,M | L1-2 | subtotal | none | II | I | I | 17 | none |
| 2 | 35, F | 12 | S,M,P | C2-T1 | total | none | II | III | III | 120 | 2 operations |
| 3 | 36, M | 7 | P | C4-C7 | total | none | I | I | I | 33 | none |
| 4 | 28, M | 2 | S,P,U | T9-T11 | subtotal | SSEP | I | II | I | 54 | none |
| 5 | 56, F | 2 | S,P,M | L1-2 | total | none | III | II | II | 30 | none |
| 6 | 29, M | 3 | S,M | T10-L1 | total | MEP SSEP | II | II | II | 12 | none |
| 7 | 32, M | 14 | M,U | T5-6 | total | SSEP | II | IV | IV | 48 | DVT |
| 8 | 44, M | 48 | S,M | C3-C7 | total | none | II | IV | IV | 56 | none |
| 9 | 34, M | 12 | M,P | C2-6 | total | none | II | II | I | 96 | none |
| 10 | 50, M | 24 | S,P | C4-6 | total | MEP SSEP | I | I | I | 9 | none |
| 11 | 35, M | 48 | S,M,C | C1-6 | debulking | none | II | II | II | 33 | CSF leak |
| 12 | 74, M | 12 | S,M,P,U | C1-6 | total | MEP SSEP | II | II | I | 13 | CSF leak |
| 13 | 66, F | 12 | S,M,P,C | Medb-C1 | subtotal | none | II | II | II | 8 | none |
| 14 | 40, M | 1 | S,M | C1-2 | total | SSEP | II | IV | IV | 43 | none |
| 15 | 23, M | 8 | S,M | C2-6 | total | none | II | IV | IV | 40 | none |
| 16 | 61, M | 10 | S,M,P,C | Medb-C4 | subtotal | SSEP | IV | IV | IV | 52 | malignant |
| 17 | 43, M | 4 | S | C5-7 | subtotal | SSEP | I | I | I | 14 | none |
For symptoms, DVT: deep vein thrombosis. S: sensory, M: motor, P: pain, U: urinary incontinence, C: cranial nerves deficits. For monitoring; SSEP: somatosensory evoked potential. MEP: motor evoked potential. a: McCormick grading scale. b: Med: medulla.
Multivariate regression analyses were used to assess significance between possible prognostic factors and postoperative functional grades using the McCormick Grading Scale. Grades III and IV where grouped into one category to facilitate statistical analysis. Factors that were tested included age (<40 years versus ≥40 years), gender (male or female), location of tumor site (cervical, thoracic, lumbar), histological grading (benign or malignant), extent of tumor resection (total, subtotal or partial), the use of intraoperative monitoring somatosensory evoked potential (SSEP) alone or combined with motor evoked potential (MEP), (used or not) and preoperative McCormick grading score.
A posterior laminectomies approach with microscopic resection was used in all 17 patients. Tumors were dissected following a plane of cleavage from the surrounding normal spinal parenchyma. Central debulking of tumors was carried out as needed. Intraoperative neurophysiological monitoring, used in 8 of 17 (47.1%) patients can usually be performed in patients with no or limited neurological deficits. SSEP was used in all monitored patients and MEP in combination with SSEP in 3 patients.
RESULTS
During the defined period, 17 consecutive patients with spinal intramedullary classical ependymoma were treated surgically (1987–2005) (Table 1). There were 14 males and 3 females in the study group (M:F ratio, 4.6:1). The mean age±standard deviation of the patients was 41.65±15.1 years (range, 22–74 years). The mean duration of symptoms before surgery was 14.2±14.5 months. Motor deficit was the most common presenting symptom (13 patients, 76%), followed by sensory deficit, pain, urinary symptoms and cranial nerves deficits. One patient (patient no. 14) presented with a few days history of sudden neurological deficits secondary to hemorrhage into a tumor. Preoperatively, 4 patients (23.5%) were McCormick grade I, 11 patients (64.7%) were grade II and two patients were grade III or IV (11.8%). All patients had a preoperative MRI of the spine in their diagnostic workup. In all patients, the MRI revealed a well-defined intramedullary enhancing lesion. The extent of tumor involvement ranged from 2 to 8 vertebral heights (mean of 4). The cervical spine was the most common tumor location followed by thoracic and lumbar spine in 12, 3, 2 patients, respectively.
Total resection of the tumors, as determined by postoperative MRI, was achieved in 11 of 17 patients (64.7%) (Figure 1); 5 patients had a radical-subtotal resection (>90%) and 1 patient had a partial debulking. One patient (patient No. 2) had a second stage procedure two weeks later with total resection as a result of both procedures. Histologically, all tumors but one were benign ependymomas (WHO grade II). One patient (patient no. 16) had a malignant ependymoma histologically. Four patients received postoperative radiotherapy. Indications for radiotherapy were residual tumors present after subtotal resection and malignant pathology (1 patient). The follow-up period ranged from 8 to 120 months.
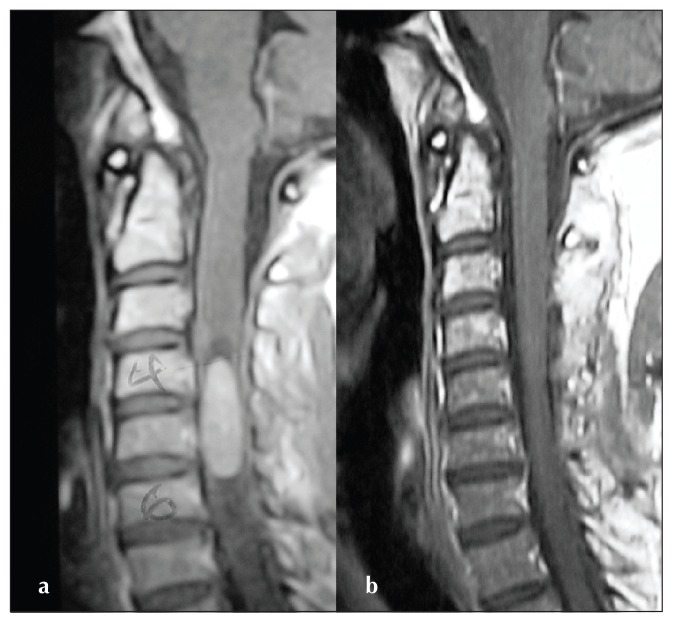
Figure 1.
Sagittal T1-weighted images of the cervical spine (patient no. 17) after gadolinium injection. a) Preoperative image demonstrating a C4-6 intramedullary ependymoma and b) postoperative image with complete resection.
All patients who originally presented with a McCormick Grade I (4 patients) continued with the same grade at 6 months postoperatively. Patients who presented with grade II (11 patients) were divided into two outcome categories, 6 patients (54.5%) ended with a favorable outcome (grade I and II), while 5 patients (45.5%) deteriorated to a higher grade. The two patients with grade III and IV remained the same postoperatively.
There was no mortality in this series. Apart from the postoperative neurological status reported in the outcome results earlier we observed 3 morbidities. Two patients (11.8%) had cerebrospinal fluid (CSF) leaks. Both patients were managed successfully with temporary lumbar drains. One patient developed deep vein thrombosis (Table 1).
A significant correlation was identified between preoperative and 6-month postoperative neurological status using McCormick’s Grading Scale (P=.042) and regression coefficient; 0.9079). The results of the regression analysis on other factors tested, such as patient age, gender, tumor site, histological grading, extend of surgical resection and the use of intraoperative monitoring found no differences that were statistically significant.
DISCUSSION
This study reports 17 consecutive cases of classical spinal ependymoma operated on in the same institute over a 18-year period by different neurosurgeons. The study provides statistical evidence that pre-operative neurological status is the only significant factor (from those studied) that determines clinical outcome. Such a relationship has been suggested but not statistically proved in previously reported series in the literature.17,18
In our study, the use of intraoperative neurophysiological monitoring was not a statistically significant prognostic factor. Explanations for this may include the limited number of patients in the study or the fact that neurophysiological monitoring is possible only in neurologically intact or almost intact patients. Those patients in this study did well with or without monitoring. The use of SSEP in monitoring spinal intramedullary surgery may be considered suboptimal as it may not predict motor outcome.19,20 In our study, neither false positive nor false negative changes were encountered. SSEP changes observed with dorsal myelotomy did not limit the monitoring process in any of our patients.
Total resection of the tumor when possible is always recommended as the treatment of choice. The extent of tumor resection was suggested as a contributing factor for long-term survival and for postoperative complications. 21,22 In our study, this was not a significant factor in the six-month functional outcome measurement. All patients with subtotal resection in our series were subjected to spinal radiation (5 patients) with no evidence of recurrences. However, none of the totally resected tumors in our series had recurrences. This makes postoperative radiation an unjustifiable option for this group of patients.23
The study failed to show statistically significant differences in outcome in relation to patient age, sex and location of the lesion, although other previous studies had suggested the impact of these factors on the outcome. 13,18 Other suggested factors in the literature, such as previous surgeries and arachnoid scarring were not observed in our study.5,24 We had only one re-operated patient and arachnoid scarring was not documented. It is interesting that motor deficits as presenting symptoms were quite high in this study. This helped in evaluating this prognostic factor independently, and indicated a statistically significant relationship. We believe that this is mainly related to delayed diagnosis and the referral system.
In conclusion, a safe total resection remains the gold standard in the management of intramedullary spinal ependymomas. Recent advances in neurosurgical techniques have helped to achieve this goal in the majority of cases. The preoperative neurological status is the single most important prognostic indicator of a favorable neurological outcome. Early diagnosis and referral to a specialized center would give patients the best chance for good outcomes. Although it was not proven in this study, use of modern surgical adjuncts like microsurgical techniques and intraoperative monitoring may provide additional safety measures and heelp achieve better tumor resection.
Acknowledgements
The authors would like to thank Musa Asyali, Ph.D. Department of Biostatistics and epidemiology, Research Centre and Mr. Nael Hasan for their dedicated efforts to make this work possible.
REFERENCES
- 1.Coxe WS. Tumors of the spinal canal in children. Am J Surg. 1961;27:62–73. [PubMed] [Google Scholar]
- 2.Helseth A, Mork SJ. Primary-intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71:842–845. doi: 10.3171/jns.1989.71.6.0842. [DOI] [PubMed] [Google Scholar]
- 3.Marker D, Weller R, Garfield J. Epidemiology of primary tumors of the brain and spinal cord: regional survey in southern England. J Neurol Neurosurg Psych. 1976;39:290–296. doi: 10.1136/jnnp.39.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidetti B, Mercuri S, Vagnozzi R. Long term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981;54:323–330. doi: 10.3171/jns.1981.54.3.0323. [DOI] [PubMed] [Google Scholar]
- 5.Hoshimaru M, Koyama T, Hashimoto N, et al. Results of microsurgical treatment for intramedullary spinal cord ependymomas. Neurosurgery. 1999;44:264269. doi: 10.1097/00006123-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Miller DC. Surgical pathology of intramedullary spinal cord neoplasms. J Neurooncol. 2000;47:189–194. doi: 10.1023/a:1006496204396. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz TH, McCormick PC. Intramedullary ependymomas. J Neurooncol. 2000;47:211218. doi: 10.1023/a:1006414405305. [DOI] [PubMed] [Google Scholar]
- 8.McCormick PC, Torres R, Post KD, et al. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523532. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 9.Nadkarni TD, Rekate HL. Pediatric intramedullary spinal cord tumors. Childs Nerv Syst. 1999;15:1728. doi: 10.1007/s003810050321. [DOI] [PubMed] [Google Scholar]
- 10.Asazuma T, Toyama Y, Suzuki N, et al. Ependymomas of the spinal cord and cauda equina: An analysis of 26 cases and a review of the literature. Spinal Cord. 1999;37:753–759. doi: 10.1038/sj.sc.3100902. [DOI] [PubMed] [Google Scholar]
- 11.Brotchi J, Noterman J, Balériaux D. Surgery of intramedullary spinal cord tumors. Acta Neurochir (Wien) 1992;116:176–178. doi: 10.1007/BF01540873. [DOI] [PubMed] [Google Scholar]
- 12.Cooper PR. Outcome after operative treatment of intramedullary spinal cord tumors in adults: Intermediate and long-term results in 51 patients. Neurosurgery. 1989;25:855–859. doi: 10.1097/00006123-198912000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Epstein FJ, Farmer J-P, Freed D. Adult intramedullary spinal cord ependymomas: The result of surgery in 38 patients. J Neurosurg. 1993;79:204–209. doi: 10.3171/jns.1993.79.2.0204. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante L, Mastronardi L, Celli P, et al. Intramedullary spinal cord ependymomas. Acta Neurochir (Wien) 1992;119:7479. doi: 10.1007/BF01541785. [DOI] [PubMed] [Google Scholar]
- 15.Hanbali F, Fourney DR, Marmor E, et al. Spinal cord ependymoma: Radical Surgical Resection and Outcome. Neurosurgery. 2002;51:1162–1174. doi: 10.1097/00006123-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Stein BM. Spinal intradural tumors. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1985. pp. 1048–1061. [Google Scholar]
- 17.Chang UK, Choe WJ, Chung SK, et al. Surgical outcome and prognostic factors of spinal intramedullary ependymoma in adults. J Neurooncol. 2002;57:133–139. doi: 10.1023/a:1015789009058. [DOI] [PubMed] [Google Scholar]
- 18.Cristante L, Herrmann HD. Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery. 1994;35:69–76. doi: 10.1227/00006123-199407000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Lesser RP, Raudzens P, Luders H, et al. Postoperative neurological deficit may occur despite unchanged intraoperative somatosensory evoked potentials. Ann Neurol. 1980;19:22–25. doi: 10.1002/ana.410190105. [DOI] [PubMed] [Google Scholar]
- 20.Whittle IR, Johnston IH, Besser M. Recording of spinal somatosensory evoked potentials for intraoperative spinal cord monitoring. J Neurosurg. 1986;64:601–612. doi: 10.3171/jns.1986.64.4.0601. [DOI] [PubMed] [Google Scholar]
- 21.Cooper PR, Epstein F. Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg. 1985;63:492–499. doi: 10.3171/jns.1985.63.4.0492. [DOI] [PubMed] [Google Scholar]
- 22.Ohata K, Takami T, Gotou T, et al. Surgical outcome of intramedullary spinal cord ependymoma. Acta Neurochir (Wien) 1999;141:341346. doi: 10.1007/s007010050309. [DOI] [PubMed] [Google Scholar]
- 23.Clover LL, Hazuka MB, Kinzie JJ. Spinal cord ependymomas treated with surgery and radiation therapy. Am J Clin Oncol. 1993;16:350353. doi: 10.1097/00000421-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery. 1994;35:865–873. doi: 10.1227/00006123-199411000-00010. [DOI] [PubMed] [Google Scholar]