Abstract
BACKGROUND AND OBJECTIVE
The combination of alfentanil-propofol or remifentanil-propofol provides adequate conditions for tracheal intubation without neuromuscular blocking drugs in most patients, but hypotension can occur during induction of anesthesia with propofol. We compared clinically acceptable intubating conditions and cardiovascular responses to induction and endotracheal intubation in patients receiving either alfentanil 40 μg/kg or remifentanil 2, 3 or 4 μg/kg, followed by thiopental 5 mg/kg.
PATIENTS AND METHODS
In a randomized trial, 80 patients were assigned in equal numbers to one of four groups: remifentanil 2, 3, or 4 μg/kg (groups R2, R3, R4, respectively) or alfentanil 40 μg/kg (group A40). In each group, the injection was given over 90 seconds followed by thiopental 5 mg/kg. Ninety seconds after the administration of thiopental, laryngoscopy and intubation were attempted. Intubating conditions were assessed as excellent, satisfactory, fair, or unsatisfactory. Arterial blood pressure and heart rate changes accompanying the four induction techniques were also recorded.
RESULTS
Overall conditions at intubation were significantly better (P<.05), and the frequency of excellent conditions was significantly higher (P<.05) in the R4 or A40 group compared with the R2 or R3 group. Intubating conditions were not significantly different between group R4 and A40 (P>.05). The highest dose of remifentanil (4μg/kg) resulted in an 18.7% decrease in mean arterial pressure (MAP) after induction of anesthesia compared with a 16.4% decrease in MAP with alfentanil 40 μg/kg (difference not statistically significant).
CONCLUSION
The administration of remifentanil 4 μg/kg or alfentanil 40 μg/kg before thiopental 5 mg/kg provided good to excellent conditions for endotracheal intubation with acceptable hemodynamic changes.
Tracheal intubation is usually facilitated by administration of a muscle relaxant to supplement drugs given for the induction of anesthesia. Over the past few years, several factors have led researchers to consider omitting neuromuscular blocking agents for tracheal intubation. The driving forces were the apparent ability of propofol to blunt responses to tracheal stimulation1 and the availability of the rapidly acting opioids, alfentanil and remifentanil. In addition, the inappropriate use of neuromuscular blocking agents was thought to be involved in problems such as awareness, residual paralysis and allergy. Therefore, many studies focused on the possibility of performing tracheal intubation without the use of neuromuscular blocking agents. The challenge was to find the correct choice and dose of induction agent and opioid drug to produce adequate intubating conditions without cardiovascular side effects. The trachea can be successfully intubated without neuromuscular blocking drugs after the alfentanil-propofol induction sequence in most patients with normal anatomy.2–4 In the study by Jabbour-Khoury and colleagues,5 excellent intubating conditions were found in 72% of American Society of Anesthesiologists (ASA) physical status I or II patients receiving propofol 3 mg/kg in combination with alfentanil 20 μg/kg, but mean arterial blood pressure (MAP) in the alfentanil group decreased to 72±28 mm Hg (mean±standard deviation), which indicates that in 16% of ASA physical status I or II patients, MAP may reach 44 mm Hg (72 minus 28) or less.
Remifentanil is a new opioid agent with a speed of onset of effect similar to that of alfentanil, but with a shorter half-life as it undergoes rapid hydrolysis by non-specific blood and tissue esterases.6 Remifentanil as part of an induction regimen effectively attenuates the hemodynamic response to laryngoscopy and tracheal intubation.7–8 Although the trachea can be reliably intubated without a neuromuscular block in patients who have received remifentanil followed by propofol, 9 hypotension during the induction of anesthesia with propofol can occur.10,11 Alexander et al12 reported MAP values less than 45 mm Hg in 27% of patients receiving 4 or 5 μg/kg remifentanil in combination with propofol. With a lower dose of remifentanil (3 μg/kg) intubating conditions were poor in 30% of cases. In other words, when neuromuscular blocking agents are not used, there is a high risk of either poor intubating conditions or hypotension. The decreases in MAP and heart rate values may be well tolerated in healthy, well-hydrated patients, but this technique cannot be recommended for sick, hypovolemic or debilitated patients, the elderly, or those with clinically significant cardiovascular or cerebrovascular disease. The results of the Erhan et al13 study indicated that propofol might be a better hypnotic agent for tracheal intubation, but at the risk of hypotension when for omitting neuromuscular blocking agents. Since 1948, thiopental alone has been used to facilitate endotracheal intubation. 14 Furthermore, thiopental, rather than propofol, may provide more favorable intubating conditions.15 Durmus and colleagues16 showed that remifentanil 4 μg/kg administered before thiopental 5 mg/kg provided excellent or satisfactory intubation conditions with acceptable hemodynamic changes. With respect to the intubating conditions and cardiovascular responses there was no previous information on equipotency between alfentanil and remifentanil. Experience obtained from pilot studies gave us reason to choose three distinct doses of remifentanil to be compared with alfentanil before induction of anesthesia with thiopental, the dose of which was chosen on the basis of previous results. A larger dose of alfentanil might have provided as favorable conditions for intubation as remifentanil 4 mg/kg. However, data from prospective studies comparing intubation conditions after remifentanil-thiopental with alfentanil-thiopental combination are lacking so we designed this randomized, double-blind study to compare clinically acceptable intubating conditions and cardiovascular responses to induction and endotracheal intubation in premedicated patients receiving either alfentanil 40 μg/kg or remifentanil 2, 3 or 4 μg/kg, followed by thiopental 5 mg/kg.
PATIENTS AND METHODS
After obtaining approval from our ethics committee and written informed consent from the patients, we enrolled 80 ASA physical status I–II patients aged 18 to 60 years scheduled for elective surgery into this randomized, double-blind study. Exclusion criteria included a history of drug or alcohol abuse, gastroesophageal reflux or hiatus hernia, cardiovascular disease, reactive airway disease, a body mass index of 30 or more, administration of sedative or narcotic drugs in the previous 24 hours, renal or hepatic impairment, and predicted difficulty in intubation or airway maintenance. All patients were premedicated with midazolam 0.03 mg/kg IV approximately 5 minutes before the induction of anesthesia. All patients were prehydrated with physiological saline (0.9%) 7 mL/kg before induction of anesthesia. Patients were randomly assigned to receive remifentanil 2, 3, or 4 μg/kg (groups R2, R3, R4, respectively, n=20 per group) or alfentanil 40 μg/kg (group A40, n=20) by means of individually prepared envelopes. Remifentanil and alfentanil syringes were prepared by an independent anesthesiologist in a total volume of 10 mL with 0.9% saline. Therefore, all anesthesia personnel were blinded to the dose of remifentanil. Baseline heart rates (HR), systolic arterial blood pressure (SAP), diastolic arterial blood pressure (DAP), and mean arterial pressures (MAP) were recorded. After preoxygenation for 2 minutes, the study drug was then injected over 90 seconds by an assistant behind a drape so that the anesthesiologist performing the intubation was blinded to drug doses. Sixty seconds later, thiopental 5 mg/kg was given over 40 seconds. When the patient became unconscious, judged by the loss of the response to command and the loss of the eyelash reflex, manual ventilation of the lungs by a facemask was started. Forty-five seconds after thiopental, the patient’s postinduction vital signs were recorded. Ninety seconds after the thiopental administration, laryngoscopy and intubation were attempted by the same experienced anesthesiologist using a Macintosh 3 laryngoscope blade and a 7.0- or 8.0-mm endotracheal tube (for women and men, respectively). The endotracheal tube cuff was inflated slowly. The anesthesiologist performing the intubation assessed and scored the condition of each patient at laryngoscopy and tracheal intubation using these criteria7: (a) excellent defined by flaccid relaxation of jaw muscles, mouth wide open, good cord visualization, cord well separated-abducted, and no bucking at intubation; (b) satisfactory defined by mouth easily opened, jaw muscles well relaxed, good cord visualization, slight cord movement when touched but abducted, and minimal bucking at intubation; (c) fair defined by conditions less favorable, jaw muscles not well relaxed, cord visualization fair but allowing intubation, and bucking on intubation; (d) unsatisfactory defined by poor relaxation of the jaw and resistance to opening the mouth, poor cord visualization or none, cord abducted if viewed, superior pharyngeal constrictor muscle activity, and patient unable to be intubated or, if intubated, marked bucking and body movement. Patients who could not be intubated on the first attempt were given succinylcholine 1 mg/kg intravenously, and intubation was completed. Adverse events such as laryngospasm, bronchospasm or chest wall rigidity, indicated by difficulty in ventilating the lungs, and the administration of further drugs were also recorded. Monitors included an automated arterial pressure cuff, electrocardiogram, peripheral pulse oximeter and capnometer. Control values for arterial pressure, heart rate and peripheral oxygen saturation (SpO2) were obtained before induction. Then the measurements were performed 45 seconds after the bolus dose of thiopental was given (postinduction) and 1, 3, 5, 10, and 15 minutes after the intubation. Hypotension (MAP <25% from baseline for 60 seconds) was treated with ephedrine 5–10 mg IV and bradycardia (HR <50 bpm for 60 seconds if hypotension occurred) was treated with atropine 20 μg/kg IV. Anaesthesia was maintained with 0.5–1% isoflurane and 66% nitrous oxide in oxygen, and the lungs were ventilated to normocapnia.
Based on a pilot study, a sample size of 20 was required for an 80% chance of demonstrating a significant difference (P<.05) between groups. Parametric data were analyzed by one-way analysis of variance. Two-way analysis (group vs. time) of variance was used to test for differences in hemodynamic data among groups. Differences from baseline within groups were evaluated using the paired-sample t-test. A chi-square test or Fisher’s exact test, when appropriate, was used for nonparametric data. Bonferroni correction was performed for multiple comparisons. Significance was defined as P<.05.
RESULTS
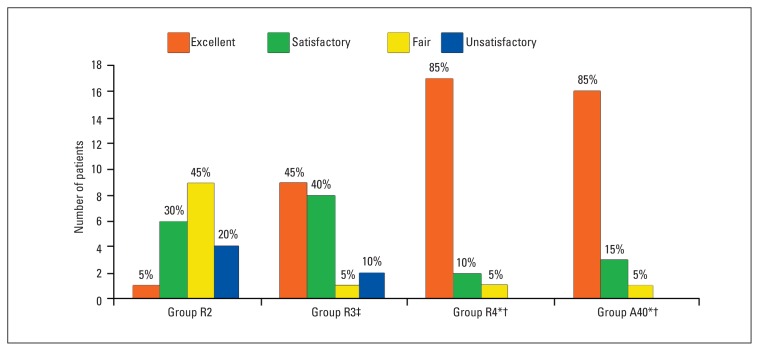
Demographic variables of patients and duration of operation were comparable among the four groups (Table 1). Tracheal intubation conditions in the different groups are shown in Figure 1. There was a significant improvement in intubation conditions between groups R2 and R3, R2 and R4, and between R3 and R4 (P<.05). Intubation conditions were not significantly different between group R4 and A40 (P>.05). Among all patients, 3 from group R2, 1 from group R3, and 1 from group A40 could not be intubated (P>.05), but after the administration of 1.5 mg/kg of succinylcholine, intubation was easily performed.
Table 1.
Patient characteristics by study group.
| R2 | R3 | R4 | A40 | |
|---|---|---|---|---|
| No. of patients | 20 | 20 | 20 | 20 |
| Sex (female/male) | 8/12 | 11/9 | 9/11 | 7/13 |
| ASA (I/II) | 11/9 | 14/6 | 16/4 | 12/8 |
| Age (y) | 30.2 (7.1) | 30.8 (6.2) | 35.2 (7.4) | 33.5 (10.7) |
| Weight (kg) | 64.1 (9.5) | 68.1 (10.6) | 63.7 (6.0) | 63.6 (8.3) |
| Height (cm) | 164.6 (7.8) | 167.7 (5.4) | 167.2 (6.1) | 165.1 (6.6) |
| Surgical time (min) | 79.5 (11.7) | 81.2 (9.3) | 79.7 (11.2) | 78.5 (13.8) |
Data are presented as either number of patients or as mean±SD. No significant difference among groups.
Figure 1.
Distribution of intubation conditions in the patient groups. There was a significant improvement in intubation conditions between groups R2 and R3, R2 and R4, and R3 and R4 (P<.05). Intubation conditions were not significantly different between groups R4 and A40. *P<.01 vs. R2, †P<.05 vs R3, ‡P<.01 vs. R2.
During induction of general anesthesia, no patient manifested clinically significant opioid-induced rigidity (P>.05). Coughing was observed in 3, 2, 1, and 1 patient in group R2, R3, R4, and A40, respectively (P>.05). Movement at intubation was observed in 8 patients from group R2, 2 patients from group R3, 1 patient from group R4, and 1 patient from group A40 (P<.01).
The baseline values in HR, SAP, DAP, and MAP were comparable between the groups. Compared with the baseline levels, the decrease in HR, SAP, DAP, and MAP were significant in all groups after anesthetic induction and endotracheal intubation (P<.05). Immediately after intubation, heart rate did not change in all groups when compared with the postinduction values. Immediately after intubation, SAP increased significantly only in group R2 (P<.05), whereas DAP and MAP increased significantly in all groups (P<.05) when compared with the postinduction values. The differences between the groups were not significant. All hemodynamic variables and differences among groups and within groups are shown in Table 2. There was no significant difference between group R4 and A40 with respect to HR, SAP, DAP, and MAP in all times of measurement. MAP decreased 18.7% in group R4 after induction of anesthesia compared with a 16.4% decrease in group A40 (difference not statistically significant). Bradycardia, which was treated by atropine, was observed in 2 patients from group R4 and 1 patient from group A40 (P>.05). Hypotension was observed in 1, 2, and 1 patients in groups R3, R4, and A40, respectively (P>.05), which was treated by ephedrine. Peripheral oxygen saturation remained at preinduction values (97–98%) in all groups throughout the investigation.
Table 2.
Hemodynamic data in each patient group at baseline and after induction of anesthesia.
| Pressure | Heart rate (bpm) | Systolic arterial blood pressure (mm Hg) | Diastolic arterial blood pressure (mm Hg) | Mean arterial blood pressure (mm Hg) |
|---|---|---|---|---|
| Group R2 | ||||
| Baseline | 90.6±2.1 | 126.3±3.6 | 76.0±4.6 | 92.8±3.1 |
| After induction | 83.8±2.6 | 114.0±3.1 | 66.4±3.2 | 82.3±2.2 |
| 1 min | 82.3±3.3 | 119.1±4.6 | 70.4±5.9 | 87.0±4.5 |
| 3 min | 81.9±4.9 | 118.4±4.5 | 69.3±4.0 | 85.6±3.2 |
| 5 min | 80.1±4.6 | 115.3±5.6 | 68.1±6.1 | 83.8±5.3 |
| 10 min | 78.5±5.5 | 114.6±5.0 | 66.0±4.1 | 82.2±3.2 |
| 15 min | 78.7±3.7 | 114.9±4.8 | 65.7±3.7 | 82.1±2.2 |
| Group R3 | ||||
| Baseline | 90.1±2.8 | 124.5±3.6 | 75.3±4.2 | 91.7±3.0 |
| After induction | 84.0±2.7 | 113.3±2.7 | 65.5±2.6 | 81.5±2.0 |
| 1 min | 83.8±5.1 | 112.1±3.3* | 70.8±3.9 | 84.6±2.5 |
| 3 min | 79.3±4.6 | 108.4±3.5* | 66.9±4.0 | 80.7±3.1* |
| 5 min | 77.2±4.7 | 106.5±3.8* | 65.8±2.3 | 79.4±2.3* |
| 10 min | 74.6±4.1 | 106.5±4.5* | 63.6±2.1 | 78.0±2.2* |
| 15 min | 74.2±2.2* | 107.0±3.2* | 63.1±3.0 | 77.7±2.2* |
| Group R4 | ||||
| Baseline | 91.7±2.2 | 125.5±4.7 | 74.0±6.0 | 91.2±5.0 |
| After induction | 83.9±3.0 | 108.1±3.2†§ | 59.4±4.7†§ | 75.7±3.7†§ |
| 1 min | 81.3±5.1 | 108.0±2.5†§ | 64.5±2.8†§ | 79.0±2.1†§ |
| 3 min | 77.3±5.6 | 103.7±4.2†§ | 64.0±2.9† | 77.3±2.4†§ |
| 5 min | 76.4±4.3 | 101.3±6.5†§ | 65.0±4.7 | 77.0±3.7† |
| 10 min | 76.1±4.8 | 102.0±2.4†§ | 63.0±5.1 | 75.8±3.7† |
| 15 min | 74.3±3.7† | 101.6±2.4†§ | 63.0±5.1 | 75.8±3.7† |
| Group A40 | ||||
| Baseline | 91.6±3.1 | 126.0±4.4 | 75.0±3.2 | 92.0±2.9 |
| After induction | 85.1±3.2 | 110.3±3.8‡# | 61.0±2.6‡# | 77.3±2.1‡# |
| 1 min | 85.3±5.4 | 109.2±5.8‡ | 65.8±2.1‡# | 80.3±3.0‡# |
| 3 min | 78.1±5.4 | 105.6±5.4‡ | 65.5±2.4‡ | 79.0±2.4‡ |
| 5 min | 77.7±4.8 | 103.3±5.2‡ | 65.7±6.1 | 78.3±5.0‡ |
| 10 min | 77.0±4.8 | 104.5±4.2± | 64.3±2.9 | 77.7±2.5‡ |
| 15 min | 76.8±5.0 | 103.4±3.4‡# | 63.5±6.0 | 76.8±4.0‡ |
All hemodynamic variables decreased to statistically significant levels after the induction of anesthesia compared with the baseline levels within groups. Data are presented as mean±SD. Statistically significant differences * between group R2 and R3, † between group R2 and R4, ‡ between group R2 and A40,§ between group R3 and R4, # between group R3 and A40.
DISCUSSION
The results of this study suggest that thiopental 5 mg/kg administered with remifentanil 4 μg/kg or alfentanil 40 μg/kg provide excellent or satisfactory intubation conditions with acceptable hemodynamic responses in healthy, premedicated patients with favorable airway anatomy. Remifentanil 4 μg/kg or alfentanil 40 μg/kg offered a significant improvement in overall conditions for intubation compared with remifentanil 2 or 3 μg/kg. Three patients (15%) could not be intubated with remifentanil 2 μg/kg, but increasing the dose to 3 or 4 μg/kg or administrating alfentanil 40 μg/kg led to satisfactory or excellent intubating conditions in 95%, 100%, and 95% of patients, respectively.
Durmus and colleagues16 compared different doses of remifentanil in combination with thiopental 5 mg/kg and reported that remifentanil 4 μg/kg provided satisfactory intubating conditions more reliably than remifentanil 2–3 μg/kg. Kleomola and colleagues,9 in a study that included a standard comparator group receiving alfentanil, reported that administration of remifentanil 4 μg/kg in combination with propofol 2.5 mg/kg offered significant improvements in overall conditions compared with alfentanil 30 μg/kg. They found that tracheal intubation was judged impossible in 20%, 25% and 5% of patients receiving alfentanil 30 μg/kg, remifentanil 3 μg/kg or remifentanil 4 μg/kg, respectively. In contrast, in our study, tracheal intubation was possible in 95% and 100% patients given alfentanil 40 μg/kg or remifentanil 4 μg/kg before thiopental 5 mg/kg, in which the higher dose suggested by Scheller and colleagues4 was used. In Kleomola et al, excellent intubating conditions were significantly more frequent in patients receiving remifentanil 4 μg/kg compared with alfentanil 30μg/kg (55% versus 20%). In our study, excellent intubating conditions were seen in 85% of patients receiving remifentanil 4 μg/kg and 80% of patients receiving alfentanil 40 μg/kg. This shows that induction of anesthesia with 40 μg/kg alfentanil before the usual dosage of thiopental probably provides more satisfactory intubation conditions compared with 40 μg/kg alfentanil before propofol. In a study by Jabbour-Khoury et al,17 72% of patients receiving alfentanil 20 μg/kg before propofol 3 mg/kg had excellent intubating conditions. MAP decreased to a low level of 44 mm Hg or less in some patients. In our results, MAP decreased maximally to 75.8±3.7 and 76.8±4.0 in group R4 and A40, respectively. Hypotension is common with large doses of opioids. In a study by Alexander and colleagues,12 27% of patients receiving 4 or 5 μg/kg remifentanil before propofol had a MAP less than 45 mm Hg. Thirty percent of patients receiving a lower dosage of remifentanil (3 μg/kg) had poor intubating conditions. In our study, there was no patient with a MAP <45 mm Hg. Increasing the dose of remifentanil provides acceptable intubation conditions for premedicated patients, but results in an 18% decrease in MAP.
In our study, which included use of thiopental instead of propofol as induction agent, bradycardia or hypotension was observed in two patients from group R4 and one patient from group A40. The bradycardia and hypotension were treated by atropine or ephedrine, respectively, while atropine was not used before injection of study drugs. All these studies were conducted in healthy patients scheduled for elective surgery. Propofol is superior to barbiturates in decreasing muscle tone and abolishing laryngeal responses to tracheal intubation or to laryngeal mask insertion.18,19 The possible development of severe hypotension is a limiting factor for using propofol,10,11 whereas thiopental may provide more favorable intubation conditions according to a study by Hovorka et al,15 while causing less hemodynamic decrease. In the present study, the induction of anesthesia with thiopental 5 mg/kg and remifentanil 2–4 μg/kg or alfentanil 40 μg/kg resulted in significant decreases in HR, SAP, DAP, and MAP values. Increasing the dose of remifentanil provided acceptable intubation conditions for premedicated patients, but resulted in a 14.3%, 22.4%, and 18.7% decrease in SAP, DAP, and MAP, respectively. These hemodynamic changes can be seen with any inhaled or IV induction regimen. Alfentanil 40 μg/kg, compared with remifentanil 4 μg/kg, caused a 12.7%, 18.7%, 16.4% decrease in SAP, DAP, and MAP.
In addition to acceptable intubation conditions, the usual increase in cardiovascular responses after tracheal intubation was not observed in any group in this study. With regard to cardiovascular parameters, there was dissimilarity between the changes caused by the different drug combinations both after induction and intubation. In two previous studies,2 the mean reductions after anesthetic induction with propofol combined with either alfentanil or remifentanil was 19% to 23% in systolic and 23% to 29% in diastolic arterial pressure. In contrast, in our study, the mean reductions after anesthetic induction with thiopental combined with either remifentanil 4 μg/kg or alfentanil 40 μg/kg was 13.6% and 12.5% in SAP, and 19.6% and 18.7% in DAP, respectively. The reduction in MAP was 17% in group R4 and 15.9% in group A40. The decrease in arterial pressure following remifentanil-propofol or alfentanil-propofol combination might not be well tolerated in less healthy patients such as the elderly, compromised patients, or in patients with clinically significant cardiovascular or cerebrovascular disease. If we want to achieve adequate intubating conditions with less hemodynamic change, thiopental seems superior to propofol.
Generally, muscle rigidity may be associated with rapid infusions of large doses of potent opioids.20 After remifentanil, such muscle rigidity was demonstrated in 11% to 32% of patients with target concentrations escalating from 2.0 to 16.0 ng/mL.21 However, none of our midazolam premedicated patients exhibited signs of opioid-induced muscular rigidity such as stiff chest. The lungs of all patients could be easily ventilated via mask. Our results are in agreement with those obtained previously in which drug combination and dosage were similar to ours.4 After alfentanil, no muscular rigidity was observed, which is similar to the Scheller et al study.4 The absence of signs indicating opioid-induced muscular rigidity in our patients might be due to the rather moderate injection rate of the opioids. Nevertheless, it has been suggested that the incidence and severity are dependent not only on the dose but also the rate of administration.20,22 Further, there is evidence that pretreatment with a benzodiazepine is effective in preventing opioid-induced muscle rigidity.23
In the present study, we did not use atropine before injection of study drugs. HR was consistently reduced in the period after induction with alfentanil and the varying doses of remifentanil. This decrease necessitated treatment only in 2 patients from group R4 and 1 patient from group A40. Note that the routine use of atropine to mask the side effects of high-dose remifentanil also has its own side effects because of the blockade of muscarinic cholinergic receptors in the periphery and in the central nervous system. Peripheral effects, e.g. a dry mouth, may be irritating, but atropine has a mild antisialagogue effect compared with glycopyttolate and scopolamine.24 Central nervous system side effects have been seen with relatively large doses of atropine, e.g. 1–2 mg.24
Our results confirm better control of hemodynamic changes following induction with thiopental compared with propofol. It is important to avoid the side effects of anesthesia (e.g., postoperative myalgias, nausea and vomiting) that may delay the discharge or return to routine activities of living of the day-surgery patient. It is also advantageous in cases where tracheal intubation is necessary, but neuromuscular block is not required, to facilitate surgical access. Avoiding muscle relaxants when they are not required for the planned procedure may help to achieve this objective. For example, the clinician might avoid succinylcholine and its potential to cause associated myalgias,25 as well as its less common, more serious complications (e.g., masseter spasm, malignant hyperthermia, cardiac rhythm disturbances). When nondepolarizing muscle relaxants are not used, the clinician may also avoid the complications of their antagonism, including the potential to increase postoperative nausea and vomiting.26 The use and misuse of muscle relaxants is often thought to contribute to awareness. The administration of muscle relaxants during balanced anaesthesia is often routine, albeit often unnecessary, and it removes important motor signs of awareness. Limiting the use of muscle relaxants to appropriate indications and not using them as substitutes for anesthesia, or to prevent movement in all patients, should help one to prevent awareness.27 Grant and colleagues28 suggested that this technique could also be advantageous in the event of a prolonged difficult intubation that was predicted or unexpected, since it could allow assessment of the airway by laryngoscopy. The decision whether or not to awaken the patient or proceed could then be made. It should also be noted that tracheal intubation without neuromuscular block can be hazardous in some situations. If laryngoscopy and intubation are attempted under inadequate conditions (e.g., poor jaw relaxation, closed vocal cords), trauma to the airway or inadequate ventilation can result. In addition, remifentanil can result in severe bradycardia, muscle rigidity, apnea, and an increased incidence of postoperative nausea and vomiting.29,30 We conclude that the administration of remifentanil 4 μg/kg or alfentanil 40 μg/kg before thiopental 5 mg/kg provided good to excellent conditions for endotracheal intubation with acceptable hemodynamic changes. The combination totally prevented the cardiovascular intubation response. The technique may be appropriate when neuromuscular blockade is undesirable or not required for the planned surgical procedure.
REFERENCES
- 1.McKeating K, Bali IM, Dundee JW. The effects of thiopentone and propofol on upper airway integrity. Anaesthesia. 1988;43:638–40. doi: 10.1111/j.1365-2044.1988.tb04146.x. [DOI] [PubMed] [Google Scholar]
- 2.Saarnivaara L, Klemola U-M. Injection pain, intubating conditions and cardiovascular changes following induction of anaesthesia with propofol alone or in combination with alftentanil. Acta Anaesthesiol Scand. 1991;35:19–23. doi: 10.1111/j.1399-6576.1991.tb03235.x. [DOI] [PubMed] [Google Scholar]
- 3.Hiller A, Klemola U-M, Saarnivaara L. Tracheal intubation after induction of anaesthesia with propofol, alfentanil and lidocaine without neuromuscular blocking drugs in children. Acta Anaesthesiol Scand. 1993;37:725–729. doi: 10.1111/j.1399-6576.1993.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 4.Scheller MS, Zornow MH, Saidman LJ. Tracheal intubation without the use of muscle relaxants: a technique using propofol and varying doses of alfentanil. Anesth Analg. 1992;75:788–793. doi: 10.1213/00000539-199211000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Jabbour-Khoury SI, Dabbous AS, Rizk LB, et al. Alfentanil-lidocaine-propofol vs fentanyl-lidocainepropofol for tracheal intubation without the use of muscle relaxants. Can J Anesth. 2003;50:116–20. doi: 10.1007/BF03017841. [DOI] [PubMed] [Google Scholar]
- 6.Egan TD, Minto CF, Herman DJ, Barr J, Muir KT, Shafer SL. Remifentanil versus alfentanil: Comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology. 1996;84:821–833. doi: 10.1097/00000542-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 7.O’Hare R, McAtamney D, Mirakhur RK, Hughes D, Carabine U. Bolus of remifentanil for control of haemodynamic response to tracheal intubation during rapid sequence induction of anaesthesia. Br J Anaesth. 1999;82:283–285. doi: 10.1093/bja/82.2.283. [DOI] [PubMed] [Google Scholar]
- 8.Song D, Whitten CW, White PF. Use of remifentanil during anesthetic induction: A comparison with fentanyl in the ambulatory setting. Anesth Analg. 1999;88:734–736. doi: 10.1097/00000539-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Klemola UM, Mennander S, Saarnivaara L. Tracheal intubation without use of muscle relaxants: remifentanil or alfentanil in combination with propofol. Acta Anaesthesiol Scand. 2000;44:65–9. doi: 10.1034/j.1399-6576.2000.440419.x. [DOI] [PubMed] [Google Scholar]
- 10.Purcell-Jones G, Yates A, Baker JR, James IG. Comparison of the induction characteristics of thiopentone and propofol in children. Br J Anaesth. 1987;59:1431–6. doi: 10.1093/bja/59.11.1431. [DOI] [PubMed] [Google Scholar]
- 11.Hogue CW, Bowdle TA, O’Leary C, et al. A multicenter evaluation of total intravenous anesthesia with remifentanil and propofol for elective inpatient surgery. Anesth Analg. 1996;83:279–85. doi: 10.1097/00000539-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Alexander R, Olufolabi AJ, Booth J, El-Moalem HE, Glass PS. Dosing study of remifentanil and propofol for tracheal intubation without the use of muscle relaxants. Anaesthesia. 1999;54:1037–40. doi: 10.1046/j.1365-2044.1999.00904.x. [DOI] [PubMed] [Google Scholar]
- 13.Erhan E, Ugur G, Gunusen I, Alper I, Ozyar B. Propofol - not thiopental or etomidate - with remifentanil provides adequate intubating conditions in the absence of neuromuscular blockade. Can J Anesth. 2003;50:108–15. doi: 10.1007/BF03017840. [DOI] [PubMed] [Google Scholar]
- 14.Lewis CB. Endotracheal intubation under thiopental. Anaesthesia. 1948;3:113. doi: 10.1111/j.1365-2044.1948.tb06759.x. [DOI] [PubMed] [Google Scholar]
- 15.Hovorka J, Honkovaara P, Kortilla K. Tracheal intubation after induction of anaesthesia with thiopentone or propofol without muscle relaxants. Acta Anaesthesiol Scand. 1991;35:326–8. doi: 10.1111/j.1399-6576.1991.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 16.Durmus M, Ender G, Kadir BA, et al. Remifentanil With Thiopental for Tracheal Intubation Without Muscle Relaxants. Anesth Analg. 2003;96:1336–9. doi: 10.1213/01.ANE.0000061222.81081.71. [DOI] [PubMed] [Google Scholar]
- 17.Jabbour-Khoury SI, Dabbous AS, Rizk LB, et al. Alfentanil-lidocaine-propofol vs fentanyl-lidocainepropofol for tracheal intubation without the use of muscle relaxants. Can J Anesth. 2003;50:116–20. doi: 10.1007/BF03017841. [DOI] [PubMed] [Google Scholar]
- 18.Brown GW, Patel N, Ellis FR. Comparison of propofol and thiopentone for laryngeal mask insertion. Anaesthesia. 1991;46:771–2. doi: 10.1111/j.1365-2044.1991.tb09776.x. [DOI] [PubMed] [Google Scholar]
- 19.Stevens J, Vercovo MV, Harris K, et al. Tracheal intubation using alfentanil and no muscle relaxant: is the choice of hypnotic important. Anesth Analg. 1997;84:1222–6. doi: 10.1097/00000539-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JP, Rowbotham DJ. Remifentanil - an opioid for the 21st century. Br J Anaesth. 1996;76:341–343. doi: 10.1093/bja/76.3.341. [DOI] [PubMed] [Google Scholar]
- 21.Lang E, Kapila A, Shlugman D, Hoke JF, Sebel PS, Glass PSA. Reduction of isoflurane minimal alveolar concentration by remifentanil. Anesthesiology. 1996;85:721–728. doi: 10.1097/00000542-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Jhaveri R, Joshi P, Batenhorst R, Baughman V, Glass PS. Dose comparison of remifentanil and alfentanil for loss of consciousness. Anesthesiology. 1997;87:253–259. doi: 10.1097/00000542-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Sanford TJ, Jr, Weinger MB, Smith NT, Benthuysen JL, Head N, Silver H, et al. Pretreatment with sedative-hypnotics, but not with nondepolarizing muscle relaxants, attenuates alfentanil- induced muscle rigidity. J Clin Anesth. 1994;6:473–480. doi: 10.1016/0952-8180(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 24.Moss J, Renz CL. The autonomic nervous system. In: Miller RD, editor. Anesthesia. 5th edn. Pennsylvania, USA: Churchill Livingstone; 2000. pp. 564–565. [Google Scholar]
- 25.Trepanier CA, Brousseau C, Lacerte L. Myalgia in outpatient surgery: comparison of atracurium and succinylcholine. Can J Anaesth. 1988;35:255–8. doi: 10.1007/BF03010619. [DOI] [PubMed] [Google Scholar]
- 26.Ding Y, Fredman B, White PF. Use of mivacurium during laparoscopic surgery: effect of reversal drugs on postoperative recovery. Anesth Analg. 1994;78:450–4. doi: 10.1213/00000539-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Bailey PL, Egan TD, Stanley TH. Intravenous opioid anaesthetics. In: Miller RD, editor. Anesthesia. 5th edn. Pennsylvania, USA: Chutchill Livingstone; 2000. pp. 273–376. [Google Scholar]
- 28.Grant S, Noble S, Woods A, Murdoch J, Davidson JAH. Assessment of intubacing conditions in adults aftet induction with propofol and varying doses of remifentanii. Br J Anaesthesia. 1998;81:540–543. doi: 10.1093/bja/81.4.540. [DOI] [PubMed] [Google Scholar]
- 29.Avramov MN, Smith I, White PF. Interactions between midazolam and remifentanil during monitored anesthesia care. Anesthesiology. 1996;85:1283–9. doi: 10.1097/00000542-199612000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Bowdle TA, Camporesi EM, Maysick L, et al. A multicenter evaluation of remifentanil for early postoperative analgesia. Anesth Analg. 1996;83:1292–7. doi: 10.1097/00000539-199612000-00028. [DOI] [PubMed] [Google Scholar]