Abstract
BACKGROUND AND OBJECTIVES
High incidences of acute kidney injury after cardiopulmonary bypass (CPB) were observed in previous reports. However, whether deep hypothermic circulatory arrest (DHCA) leads to more severe kidney injury than CPB without DHCA remains controversial. The aim of the study was to investigate the effects of DHCA on renal function in a novel rabbit model of using closed-thoracic DHCA.
DESIGN AND SETTINGS
Experimental study on New Zealand white rabbits performed in the Department of Cardiac Surgery, the First Affiliated Hospital of China Medical University.
METHODS
Thirty rabbits were randomly divided into 3 groups: the sham-operated group (Group A, N=10), the CPB group (Group B, N=10), and the DHCA group (Group C, N=10). Serum creatinine (Scr), blood urea nitrogen (BUN), serum cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), malondialdehyde (MDA) levels, superoxide dismutase (SOD) activities, histopathologic llesions, and apoptosis were assessed.
RESULTS
Each single rabbit in Groups B and C died during surgery. Animals received DHCA exhibited more severe kidney impairments than those received CPB and those that were sham operated. Scr and BUN concentrations at 24 and 48 hours after surgery; cystatin C and NGAL concentrations at 12, 24, and 48 hours after surgery; MDA levels, histopathological lesions, and apoptosis score of the kidneys were the highest in Group C, followed by Group B, and were the lowest in Group A (all P<.05). The activities of SOD were the lowest in Group C, followed by Group B, and were the highest in Group A (P<.05).
CONCLUSION
Our study established a simple, convenient, economical, and long-term surviving rabbit model for the study of DHCA-induced organic injury. Based on more significant kidney injuries, including elevated levels of serum cystatin C and NGAL at an early time, increased lipid peroxidation, decreased renal antioxidative ability, enhanced histological lesions, and increased tubular epithelial apoptosis from DHCA animals, we concluded that DHCA has more kidney dysfunctions than CPB without DHCA.
Deep hypothermic circulatory arrest (DHCA) is widely used for neuroprotection during complex congenital heart surgery and aortic surgeriess.1,2 Although deep DHCA has proven to be an effective technique for protecting various organs from ischemic damage as a result of circulatory arrest,3 acute kidney injury (AKI) after DHCA has a reported incidence of 5% to 50% depending on definition and patient cohort.4,5 Renal dysfunction leads to increased postoperative morbidity and mortality, and patients requiring renal replacement therapy have mortality as high as 64%.6,7 However, many previous studies have included patients undergoing aortic surgery with DHCA only, and others have featured heterogeneous patient cohorts including those undergoing emergency procedures, with a significant proportion of acute aortic dissections. Both aortic pathology and the clinical presentation of thoracic aortic emergencies significantly influence operative risk and outcome, particularly when preoperative organ malperfusion is present. These confounding effects suggest that the higher incidence of AKI after thoracic aortic surgery as compared to other types of cardiac surgery may not be a result of aortic surgery alone.8 Thus, some authors concluded that DHCA did not appear to carry the elevated risk of AKI as compared to other types of cardiac surgery.8 This study was, therefore, designed to investigate the impact of DHCA on renal function.
The previous rabbit model of DHCA was via median sternotomy9,10 and the animals could not have long-term survival because of the large trauma. As of now, no reports of closed-thoracic DHCA used in a rabbit model are available in the published studies. Our experiments indicated that closed-thoracic DHCA can be used in rabbits and make the experiment more simple, convenient, and economical.
For many decades, the diagnosis of AKI has been based on elevation of serum creatinine (Scr), elevation of blood urea nitrogen (BUN), or presence of oliguria. These traditional bio-markers, however, have several shortcomings in establishing an early and sensitive diagnosis of AKI. The absence of a more sensitive biomarker has impaired progress in this field and has had a detrimental effect on the design and outcome of AKI clinical trials. Recent studies reported that cystatin C and neutrophil gelatinase-associated lipocalin (NGAL) were independent markers for early prediction of AKI, prognosis, and mortality. The production of cystatin C or NGAL in the body is a stable process that is not altered by limitations as displayed in the preceding text. Therefore, cystatin C and NGAL may be more sensitive than Scr in predicting renal tubular injury and severity of AKI.11,12 Cystatin C and NGAL were used as bio-markers in our study for early detection of AKI induced by DHCA.
METHODS
Animals and experimental design
This animal experiment was performed in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and was approved by the China Medical University Animal Care and Use Committee. Thirty New Zealand white rabbits (both genders, 6- to 10-month old, body weight 3.0–3.5 kg) were obtained from the Experimental Animal Center of China Medical University (Shenyang, China). All animals were housed with free access to food and tap water under standard conditions with respect to temperature (22°C), humidity (60%), and day/night cycle (12 hours/12 hours) for at least 3 days before experiment. The rabbits were randomly divided into 3 groups: sham-operated group (Group A, N=10), cardiopulmonary bypass (CPB) group (Group B, N=10), and DHCA group (Group C, N=10). The time of CPB in Group B was the same as in Group C and the procedures used for Group B were the same as those used for Group C, except there was no DHCA. The time of surgery in Group A was the same as in the other 2 groups and the procedures used for Group A were the same as those used for Group B, except there was no CPB.
DHCA model
After anesthesia induction with intravenous ketamine (10 mg · kg−1), rabbits were placed in the supine position and a 4.0-mm endotracheal tube was connected to the ventilator through a tracheostomy. Anesthesia was maintained with continuous infusion of pentobarbiturate (5 mg · kg−1 · h−1) and bolus infusions of vecuronium bromide (0.5 mg · kg−1 · h−1) and ketamine (5 mg. kg−1 · h−1). Rabbits were ventilated using a pressure control ventilator. In all protocols, inspiratory time was 0.8 seconds, peak inspiratory pressure was 12 cmH2O, and positive end-expiratory pressure was 1 cmH2O. Before and after CPB, the fraction of inspired oxygen (FiO2) was 0.5, and the respiratory rate was adjusted from 30 to 40 to maintain normocapnia. During CPB, FiO2 was 0.2. After establishing CPB, a continuous positive airway pressure mode (5 cmH2O) with a fraction of inspired oxygen of 21% and the respiratory rate of 10 breaths · min−1 were applied to avoid atelectasis; however, during DHCA, the pulmonary ventilation was stopped. The left jugular vein and the right femoral artery were cannulated for obtaining blood samples, monitoring mean arterial pressure, and blood gas analysis. The right common carotid artery was catherized proximally for pumping oxygenated blood in CPB. A thin-walled catheter of 4 mm in internal diameter with 2 side holes on its tip was inserted blindly to a depth of 6 to 8 cm, reaching the inside of the right atrium via the right internal jugular vein. The left common carotid artery was catherized distally for anterograde cerebral perfusion. The distal aspect of the right common carotid artery and the proximal aspect of the left common carotid artery were clamped during systemic DHCA and antegrade cerebral perfusion via the left carotid catheter, which was added in the revised manuscript. Electrocardiographic results and nasopharynx and rectal temperatures were monitored (CSI8100, Criticare, Waukesha, Wisconsin, USA). Temperature was maintained at 37.5°C to 38.5°C before CPB with an electrical heating exchanger. Limb lead II electrocardiographic data were continuously monitored by means of subcutaneous needle electrodes. Ringer solution was used as a maintenance fluid through the ear vein. The cardiopulmonary circuit consisted of a double small roller pump (Shiley; Stockert, Munich, Germany) and a small heat exchanger connected with a thermostatic water pump (type 20–600, Jostra); a membrane oxygenator for small animals was placed 40 to 60 cm be low the level of the right atrium to effect caval drainage by gravity. A hemoconcentrator ( Jostra BC20; Maquet CP AG, Hirrlingen, Germany) was placed in the circuit with its inlet connected to the arterial line and its outlet to the venous reservoir. All connected silicone catheters were 3 mm in internal diameter to reduce the priming volume (the heat exchanger, membrane oxygenator, and catheters were provided by Xijing Medical Appliance Corp, Xian, China). All catheters were flushed with 10 U · mL−1 heparinized saline to prevent clotting during the experiment. The circuit was primed with 4% succinylated gelatin injection (B. Braun Medical [Suzhou] Co., Ltd., Suzhou, China) and Plasmalyte-A solution (Baxter International Inc., Shanghai, China), and about 150 mL donor rabbit whole blood was progressively added to the circuit during CPB to maintain hematocrit no less than 20%. In addition, heparin (200 units), dexamethasone (1 mg · kg−1), furosemide (2 mg), mannitol (1.5 g), and 5% sodium bicarbonate (20 mL) were added to the priming solution.
After an activated clotting time of longer than 480 seconds was achieved, CPB was initiated at a flow rate of 80 to 90 mL · kg−1 · min−1, which was enough to maintain a mean blood pressure of greater than 60 mm Hg most of the time. During the cooling period, the flow rate was gradually decreased. When rectal temperature was down to 30°C by using a heat exchanger and topical cooling with ice bags and a cooling blanket, a bolus of 6 to 7 mL of 10% potassium chloride was rapidly injected into the left jugular vein to induce cardiac arrest. Arrest time was defined as the point when the trace of artery blood pressure flattened. In about 30 minutes, rectal temperature was down to 16°C to 18°C. DHCA confirmed by means of asystole was maintained for 60 minutes at 16°C to 18°C. The blood flows of anterograde cerebral perfusion were 8 to 10 mL · kg−1 · min−1. CPB restarted when rewarming increased the temperature to 35°C in 30 minutes and then maintained it for 30 minutes on CPB. In the case of ventricular fibrillation, a direct current shock of 10 Ws · kg−1 (usually very effective to terminate the fibrillation with a saline-soaked paddle on the anterior area of neck and another on the inguinal region) was delivered. During rewarming, the mean arterial pressure was kept at more than 50 mm Hg with dopamine until rectal temperatures of 35°C or greater and blood flows of 60 mL · kg−1 · min−1 or greater were achieved. When hypotension occurred in weaning from CPB, infusions of extracellular fluid type were used and catecholamines (dopamine or epinephrine, or both) were given to maintain an adequate blood pressure or heart rate. Protamine (3 mg · kg−1) was given after removing the cannulas. Blood in the extracorporeal circuit was transfused over a period of 30 minutes. The rabbits were allowed to recover from anesthesia, remaining 48 hours in a controlled-environment room with food and water freely available. At the end of all experiments, rabbits were subjected to euthanasia by an overdose of pentobarbital sodium at 48 hours after surgery.
Blood, urinary, and renal sample collection
Blood samples were collected at anesthesia induction (baseline), 12, 24, and 48 hours after surgery. The kidneys were excised carefully and washed with normal saline at 48 hours after surgery. One kidney was stored in 10% formalin that was further used for histopathological studies and apoptosis assay. The other kidney was homogenized for estimating in vivo malondialdehyde (MDA) levels and renal superoxide dismutase (SOD) activities.
Assessment of renal function
Blood samples (1 mL) that were collected at 0, 12, 24, and 48 hours after surgery were placed in the refrigerator at 4°C for 20 minutes and centrifuged (6000 rpm for 3 minutes) to separate serum. These serum samples were assayed immediately or stored at –20°C until further study. Scr, BUN, serum cystatin C, and NGAL were used as indicators of impaired renal function. Scr and BUN were measured by an Auto Analyzer (Olympus, Optical Co. Ltd., Tokyo, Japan) using BUN and Scr test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the manufacturer’s instructions. Cystatin C (Rabbit cystatin C ELISA kits; Bioporto Diagnostics, Gentofte, Denmark) and NGAL (Rabbit NGAL ELISA kits; Bioporto Diagnostics, Gentofte, Denmark) assay employed the quantitative sandwich enzyme immunoassay technique.
Measurements of MDA levels and SOD activities
The concentrations of MDA and the activities of SOD were measured by commercial assay kits (Nangjin Jiancheng Bioengineering Institute, Nangjin, China). A portion of each renal tissue for SOD assay was homogenized in 0.9% NaCl solution (10% w/v) with an OMNI TH International homogenizer (The Homogenizer Company, USA). Tissue homogenates were centrifuged for 15 minutes at 18 000 g, and then the supernatants were removed for analysis. For the MDA assay, renal tissue samples were homogenized so that each gram of tissue had a 1.15% KCl solution of 9 mL. Protein levels of the homogenates were determined according to the method of Bradford.13 All of the spectrophotometric measurements were performed using a Beckman DU 530 spectrophotometer (USA). The SOD activities and the MDA levels were measured in the renal homogenates. The SOD activities were measured by reducing nitroblue tetrazolium using xanthine-xanthine oxidase system, which is a superoxide generator. Enzyme activity leading to 50% inhibition was accepted as 1 unit.14 Results were expressed as units per gram protein. Tissue MDA levels were determined spectrophotometrically according to the method described by Ohkawa.15 Results were expressed in terms of nanomoles per gram protein.
Histopathological examinations
The percentage of histological changes in the cortex and medulla were scored using a semiquantitative scale designed to evaluate the degree of tubular cell swelling, congestion, tubular dilatation, brush border loss, cytoplasmic vacuole, and nuclear loss on a 5-point scale based on the extent of involvement as described previously:16 0, normal kidney; 0.5, 10%; 1, 10% to 25%; 2, 25% to 50%; 3, 50% to 75%; and 4, 75% to 100%.
Determination of renal apoptosis
Kidney samples were fixed and collected in 4.5% neutrally buffered formaldehyde at room temperature until further processing. Dehydration, paraffin embedding, and preparation of slides were done following standard histological procedures. For assessing apoptotic cells, tissue sections were stained with the In Situ Cell Death Detection Kit (Roche Inc., Mannheim, Germany). For antigen retrieval, the slides were pretreated in citrate buffer (pH 6) in the microwave at 750 watt for 15 minutes, and endogenous peroxidase was blocked by incubation in 5% H2O2 in methanol for 15 minutes. Afterward, the TUNEL (terminal-deoxynucleoitidyl transferase-mediated nick end labeling) reaction mixture was prepared freshly, and the slides were incubated for 45 minutes in a humidified chamber. Slides were counterstained with hematoxylin-eosin and subsequently analyzed under light microscope. The results were quantified by counting the number of positively stained cells per 5 high-power fields at 400× magnification for each of the 3 areas and given as percentage.
Morphological assessment and apoptosis assay were performed by 2 pathologists who were unaware of the treatment that the animals had received.
Statistical analysis
All values were expressed as mean (SD). Statistical analysis was carried out using SPSS, version 13.0 (SPSS Inc., Chicago, IL USA). Biochemical parameters were analyzed by analysis of variance (ANOVA) followed by Bonferroni post hoc test where appropriate. Histopathologic examination was analyzed by the Kruskal-Wallis test. A value of P<.05 was considered statistically significant.
RESULTS
DHCA on serum parameters
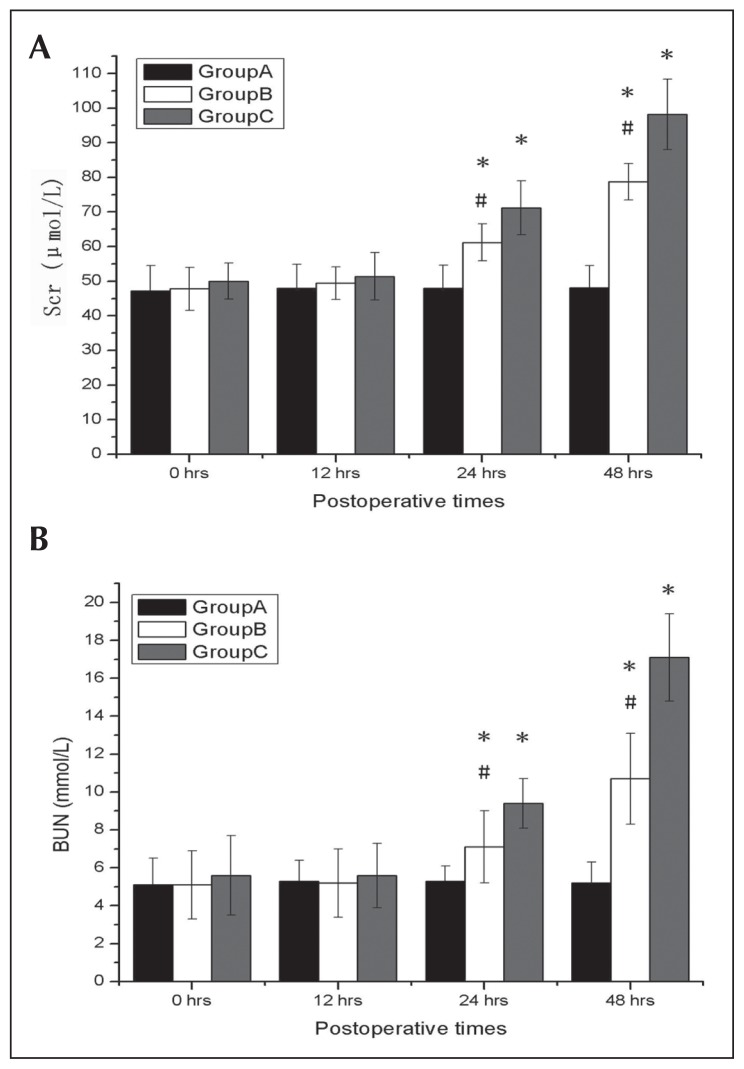
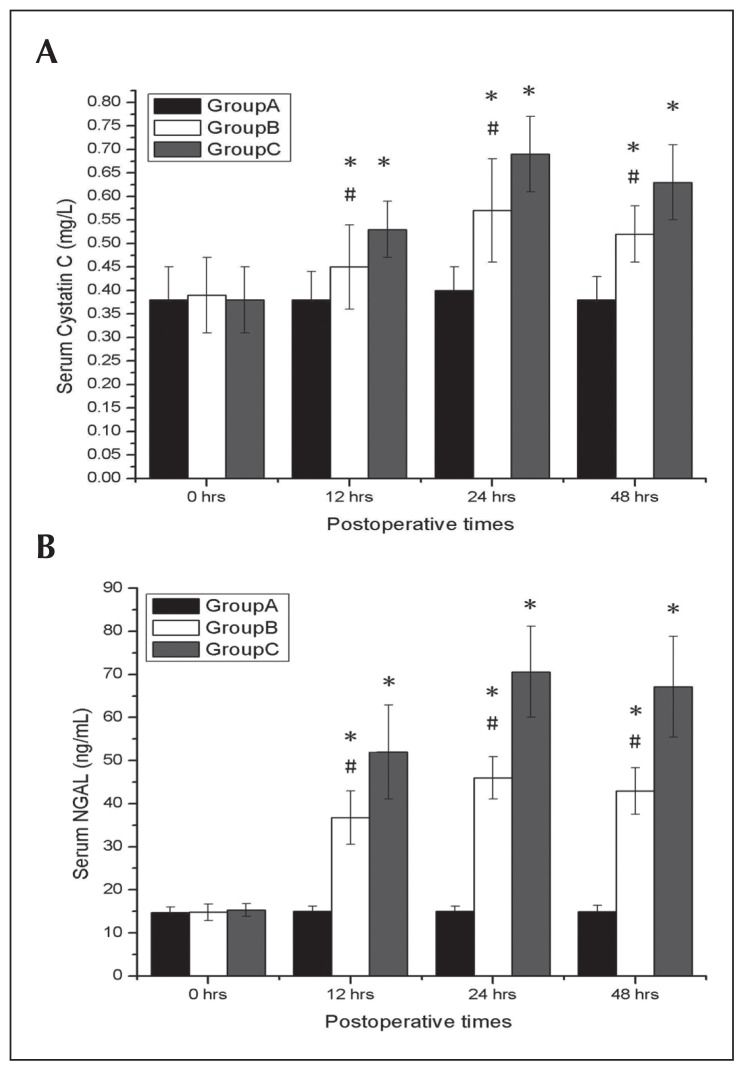
Comparing with Group A, both Groups B and C exhibited significant elevations in Scr and BUN concentrations at 24 and 48 hours and significant elevations in serum concentrations of cystatin C and NGAL at 12, 24, and 48 hours after surgery (all P<.05), which reflect a more significant degree of renal dysfunction. Group C showed increased levels of Scr and BUN at 24 and 48 hours after surgery; this group also had increased levels of serum cystatin C and NGAL at 12, 24, and 48 hours after surgery when compared with Group B (all P<.05) (Figures 1A, 1B and 2A, 2B).
Figure 1.
Effects of deep hypothermic circulatory arrest (DHCA) on renal functions. Serum creatinine (Scr) and blood urea nitrogen (BUN) at 0, 12, 24, and 48 hours after surgery from Group A, Group B, and Group C. DHCA had significant increased Scr (A) and BUN (B) after surgery. Data are expressed as mean (SD). *P<.05 compared with Group A; #P<.05 compared with Group C.
Figure 2.
Effects of deep hypothermic circulatory arrest (DHCA) on renal functions. Serum cystatin C (A) and NGAL (B) at 0, 12, 24, and 48 hours after surgery from Group A, Group B, and Group C. DHCA had significant increased serum cystatin C and NGAL after surgery. Data were expressed as mean (SD). *P<.05 compared with Group A; #P<.05 compared with Group C.
DHCA on in vivo anti-oxidant parameters
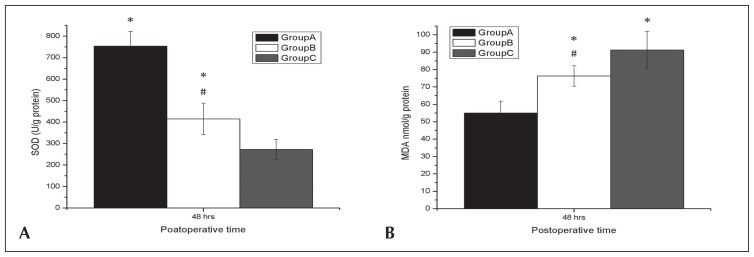
A significant decrease in the activities of SOD and a significant increase in the MDA levels were observed in both Groups B and C when compared with Group A (P<.05), which indicated more that oxidative stress occurred in both groups (P<.05). A significant decrease in the activities of SOD and a significant increase in the MDA levels were observed in Group C when compared with Group B (both P<.05) (Figures 3A, 3B).
Figure 3:
Effects of deep hypothermic circulatory arrest (DHCA) on renal functions. Superoxide dismutase (SOD) activity (A) and malondialdehyde (MDA) levels (B) at 48 hours after surgery from Groups A, B, and C. DHCA had a significant decreased SOD activity and showed significant increased MDA levels at 48 hours after surgery. Data were expressed as mean (SD). *P<.05 compared with Group A; #P<.05 compared with Group C.
DHCA on histopathological changes of kidneys
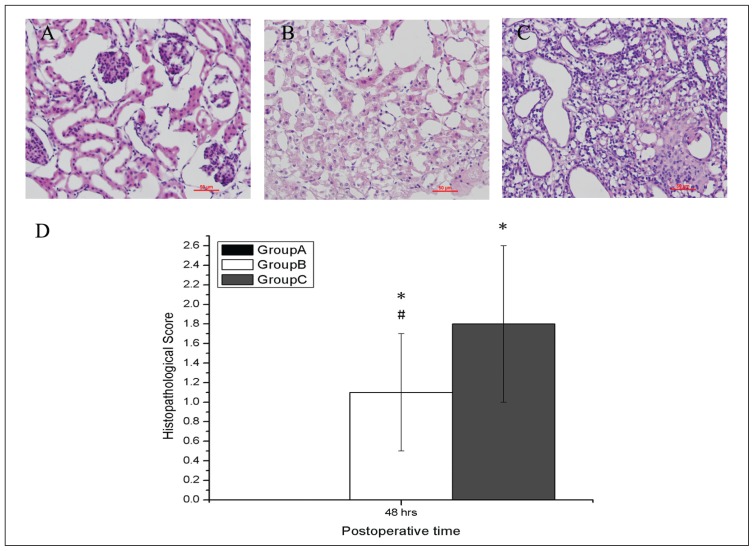
Group A did not show any morphological changes in kidneys (Figure 4A). Groups B (Figure 4B) and C (Figure 4C) showed significant widespread degeneration tubular architecture, tubular dilation, tubular cell swelling, cellular vacuolization, pyknotic nuclei, severe tubular necrosis, and luminal congestion. However, more severe damages were observed in Group C in comparison to Group B. The histopathological score of the kidneys in all groups are presented in Figure 4D. The scores of the kidneys from animals in Groups B and C were higher than those in Group A (both P<.05). However, the scores of the kidneys from animals in Group B were lower than those in Group C (P<.05).
Figure 4.
Effects of deep hypothermic circulatory arrest on renal functions. Microscopic findings of kidneys after surgery at 48 hours from Groups A, B, and C (A, B, C). Histopathologic examination was performed using hematoxylin and eosin. Semi-quantitatively graded at 400× magnification for tubular dilation and interstitial expansion with edema, and inflammatory infiltrate. Renal sections from Group C showed more severe lesions than those from Groups A and B (D). Bar=50 μm. Values represent scores (SD). *P<.05 compared with Group A; #P<.05 compared with Group C.
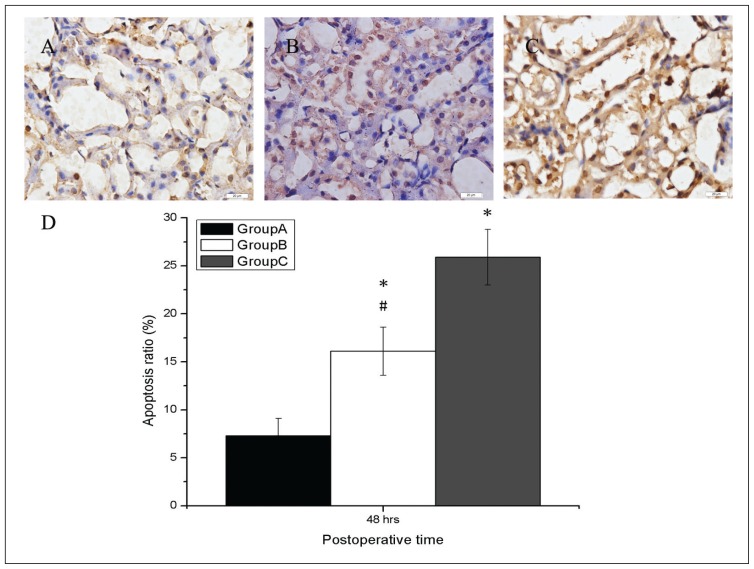
DHCA on renal apoptosis
Apoptosis-assessed morphology was seldom observed in the animals of Group A (Figure 5A). In comparison, kidneys from animals of Group B (Figure 5B) and of Group C (Figure 5C) showed extensive nuclear changes together with apoptotic cell death. The apoptosis score of the kidneys in all groups were presented in Figure 5D. The scores of the kidneys from animals in Groups B and C were higher than those in Group A (P<.05). However, the scores of the kidneys from animals in Group B were lower than those in Group C (P<.05); which indicated more severe apoptosis in the kidneys of Group C.
Figure 5.
Effects of deep hypothermic circulatory arrest on renal functions. Apoptosis-assessed morphology was rarely seen in the animals of Group A (A). In comparison, kidneys from animals that were subjected to Group B (B) and Group C (C) showed extensive nuclear changes consistent with apoptotic cell death, which was stained brown. The apoptosis score was the highest in Group C and the lowest in Group A (D). Bar=20 μm. Values represent scores (SD). *P<.05 compared with Group A; #P<.05 compared with Group C. Magnification 400×.
DISCUSSION
CPB and circulatory arrest with profound hypothermia have been widely used for cardiovascular surgery for complex congenital heart disease and aortic arch aneurysm, but CPB support may also lead to severe complications to these patients. Cardiac surgery with CPB is invariably accompanied by systemic inflammatory response and ischemia–reperfusion injury affecting multiple organs. Although during CPB and cardioplegic arrest the kidneys are under virtually continuous circulation, unlike the heart and lungs, decreased renal perfusion along with ineffective oxygen delivery system and high metabolic rate still make the kidneys vulnerable to ischemic injury.17 Cardiac surgery-associated AKI is a common and serious postoperative complication of cardiac surgery requiring CPB;18 however, the controversy is still debated that if DHCA would increase the kidney injury compared with CPB without DHCA.8,19 Although deep hypothermia has proven to be an effective technique for protecting various organs from ischemic damage as a result of circulatory arrest,3 kidney injury still may occur because of no kidney perfusion during circulatory arrest. Most studies have been focused on cerebral injury during DHCA,20 but few have addressed the kidney injury. Therefore, the aim of the study was to investigate the effects of DHCA on renal function in a rabbit model.
The advantage of our model is that closed-thoracic DHCA causes lesser trauma than the open-thoracic DHCA method, and the animal is likely to live long enough for observation of the outcome of renal function. The decisive factor for the success is the caval drainage rate that often causes failure during closed-thoracic DHCA by mismatching the arterial flow that was pumped to maintain a desired perfusion pressure. The caval drainage rate correlates highly with the inner diameter of the catheter and its position. In our study, the inner diameter of the drainage catheter was 4 mm, and there were 2 side-holes on the tip of the catheter to compensate for caval drainage in the case of plugging of the terminal hole, which can fulfill the flow capacity. The other point was that the catheter must be inserted carefully into the right atrium with closely monitoring electrocardiogram and arterial pressure; otherwise, a sufficient caval drainage rate cannot be obtained. We have met 2 cases in which the catheter insertion suddenly stopped the circulation and the animals were dead. This could have been caused by the catheter occluding the tricuspid valve. When cardiac arrhythmia or hypotension occurs, it should be considered that the tip of the catheter has reached the right atrium and the insertion should be stopped immediately.
Rabbits that underwent CPB showed characteristic signs of renal dysfunction. The decline in renal function after CPB was reflected in the results, showing increased levels of Scr and BUN at 24 and 48 hours after surgery. The levels of Scr and BUN at 24 and 48 hours after surgery were significantly higher in Group C than in Group B in our data, which also showed that DHCA would increase the kidney injury compared with CPB without DHCA. However, in the case of Scr, many factors that modify Scr concentration are not linked to renal injury, such as muscular activity, body weight, age, gender, race, and protein intake. In addition, the elevation of Scr usually occurs from 48 to 72 hours after the kidney injury has occurred; thus, an early diagnosis based on creatinine elevation is unlikely to be feasible.21 BUN is also an insensitive AKI marker because its concentration may be altered by non-renal factors, such as a high-protein diet, glucocorticoid therapy, or trauma.22 The production of cystatin C and NGAL in the body is not altered by limitations as displayed in the preceding text; cystatin C and NGAL are more sensitive than Scr in predicting renal tubular injury and severity of AKI, and these are independent markers for early prediction of AKI.11,12 According to our result, the level of Scr and BUN increased at a later time (24 and 48 hours) after surgery, whereas the level of serum cystatin C and NGAL significantly elevated at an eary time (12 hours) after surgery in Groups B and C. We found CPB and/or DHCT increased serum cystatin C and NGAL at an early time (12 hours) and Scr and BUN at a later time (24 and 48 hours) after surgery. We also inferred that DHCA would increase the kidney injury compared with CPB without DHCA at an early time after surgery.
MDA is one of the products of free-radical chain reaction and lipid peroxidation. The changes in tissue MDA content reflect changes in the quantity of polyunsaturated fatty acid reductions during ischemia reperfusion injury. Consequently, the increase of MDA content indicates a lowering of the membrane fluidity and impairment of the normal membrane structure of the mitochondria. SOD is an important anti-oxidase that scavenges oxygen-free radicals and protects mitochondria against damage caused by potentially cytotoxic reactions. Usually anti-oxidative ability of the tissue is evaluated by MDA content and SOD activity. Our present study showed that both Groups B and C resulted in an increase of MDA content and a decrease of SOD activity in the kidney tissue. These results indicated that CPB may relate to aggravation in the endogenous antioxidant system and increased lipid peroxidation due to nitric oxide scavenging property. In this study, we also found that the MDA levels were higher and the SOD activities were lower in the renal tissues in Group C in comparison to Group B. These findings demonstrated that DHCA could increase lipid peroxidation and decrease renal antioxidative ability of a rabbit kidney.
Our study also showed that DHCA significantly increased a severe damage of renal tissue morphology and apoptosis contributing to more function impairment, tubular damage, tubular cell necrosis, tubular cell apoptosis, and worse histopathological structure. The histopathological and the apoptosis scores of the kidney were the highest in Group C, followed by Group B, and were the lowest in Group A, which supports the biochemical parameters evaluated in the present study. These evidences indicate that DHCA increased the kidney injury compared with CPB without DHCA. In a recent meta-analysis, the rate of renal failure was lower in off-pump patients.23 A recent meta-analysis of randomized and observational studies carried out by Nigwekar24 showed that patients undergoing off-pump surgery were prone to renal injury and failure despite the different AKI definitions used in the enrolled studies. As it is evident, the published studies reflect controversial evidence regarding renal impairment following on-pump and off-pump surgeries. The results of our study showed sham-operated rabbits in Group A, where CPB was avoided, confirmed that off-pump surgery might reduce the detrimental effect of CPB on renal function. The role of an extracorporeal circulation device in the development of renal injury is not entirely clear, but the possible reason is that CPB is associated with the development of systemic inflammatory response, endothelial damage, and subsequent tissue edema and organ dysfunction.
Limitation of study
We did not use norepinephrine for blood pressure control, and it would have avoided the ongoing debate of the effect of dopamine on renal blood flow (although there are no indications about its beneficial renal effect on human conditions, not sure about rabbits). Gender differences in cardiac surgery are a well-described phenomenon. Women have higher morbidity and mortality after cardiac surgery compared with men. However, both male and female rabbits were used in our study, which is commendable given the possibility that the impact of CPB and DHCA on the kidney may differ based on sex. Another limitation concerns the lack of continuous monitoring about the long-term renal function after DHCA.
In conclusion, our study established a simple, convenient, economical and long-term surviving rabbit model for the study of DHCT-induced organic injury. Based on more significant kidney injuries, especially in the elevations of the levels of serum cystatin C and NGAL at an early time, increased lipid peroxidation, decreased renal antioxidative ability, enhanced histological lesions, and increased tubular epithelial apoptosis from DHCA animals, we conclude that DHCA has more kidney dysfunctions than CPB without DHCA.
REFERENCES
- 1.Ergin MA, Galla JD, Lansman sL, et al. Hypothermic circulatory arrest in surgerys on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg. 1994;107:788–97. [PubMed] [Google Scholar]
- 2.Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–64. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 3.Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg. 1975;70:1051–63. [PubMed] [Google Scholar]
- 4.Mora Mangano CT, Neville MJ, Hsu PH, et al. Aprotinin, blood loss, and renal dysfunction in deep hypothermic circulatory arrest. Circulation. 2001;104(12 Suppl 1):1276–81. doi: 10.1161/hc37t1.094702. [DOI] [PubMed] [Google Scholar]
- 5.Mori Y, Sato N, Kobayashi Y, et al. Acute kidney injury during aortic arch surgery under deep hypothermic circulatory arrest. J Anesth. 2011;25:799–804. doi: 10.1007/s00540-011-1210-8. [DOI] [PubMed] [Google Scholar]
- 6.Augoustides JG, Pochettino A, Ochroch EA, et al. Clinical predictors for prolonged intensive care unit stay in adults undergoing thoracic aortic surgery requiring deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2006;20:8–13. doi: 10.1053/j.jvca.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Leacche M, Winkelmayer WC, Paul S, et al. Predicting survival in patients requiring renal replacement therapy after cardiac surgery. Ann Thorac Surg. 2006;81:1385–92. doi: 10.1016/j.athoracsur.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Englberger L, Suri RM, Greason KL, et al. Deep hypothermic circulatory arrest is not a risk factor for acute kidney injury in thoracic aortic surgery. J Thorac Cardiovasc Surg. 2011;141:552–8. doi: 10.1016/j.jtcvs.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Yang J, Long C, et al. Hyperoxia management during deep hypothermia for cerebral protection in circulatory arrest rabbit model. ASAIO J. 2012;58:330–6. doi: 10.1097/MAT.0b013e318251dfab. [DOI] [PubMed] [Google Scholar]
- 10.Pan X, Sun L, Ma W, et al. Overactivation of poly(adenosine phosphate-ribose) polymerase 1 and molecular events in neuronal injury after deep hypothermic circulatory arrest: study in a rabbit model. J Thorac Cardiovasc Surg. 2007;134:1227–33. doi: 10.1016/j.jtcvs.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 11.Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–69. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–28. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 13.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Oberley LW, Ying Li. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Hu L, Yang C, Zhao T, et al. Erythropoietin ameliorates renal ischemia and reperfusion injury via inhibiting tubulointerstitial inflammation. J Surg Res. 2012;176:260–6. doi: 10.1016/j.jss.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 17.Choi YS, Shim JK, Kim JC, et al. Effect of remote ischemic preconditioning on renal dysfunction after complex valvular heart surgery: A randomized controlled trial. J Thorac Cardiovasc Surg. 2011;142:148–54. doi: 10.1016/j.jtcvs.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Mao H, Katz N, Ariyanon W, et al. Cardiac surgery-associated acute kidney injury. Blood Purif. 2014;37(Suppl 2):34–50. doi: 10.1159/000361062. [DOI] [PubMed] [Google Scholar]
- 19.Roh GU, Lee JW, Nam SB, et al. Incidence and risk factors of acute kidney injury after thoracic aortic surgery for acute dissection. Ann Thorac Surg. 2012;94:766–71. doi: 10.1016/j.athoracsur.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 20.Pan XD, Sun LZ, Ma WG, et al. Neuroprotective effect of deep hypothermic circulatory arrest with low priming volume: study in a rabbit model. Thorac Cardiovasc Surg. 2008;56:133–9. doi: 10.1055/s-2007-989458. [DOI] [PubMed] [Google Scholar]
- 21.Bagshaw SM, Gibney RT. Conventional markers of kidney function. Crit Care Med. 2008;36:S152–88. doi: 10.1097/CCM.0b013e318168c613. [DOI] [PubMed] [Google Scholar]
- 22.Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008;3:481–90. doi: 10.2215/CJN.03520807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reston JT, Tregear SJ, Turkelson CM. Meta-analysis of short-term and mid-term outcomes following off-pump coronary artery bypass grafting. Ann Thorac Surg. 2003;76:1510–15. doi: 10.1016/s0003-4975(03)01195-0. [DOI] [PubMed] [Google Scholar]
- 24.Nigwekar SU, Kandula P, Hix JK, et al. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized and observational studies. Am J Kidney Dis. 2009;54:413–23. doi: 10.1053/j.ajkd.2009.01.267. [DOI] [PubMed] [Google Scholar]