Abstract
BACKGROUND AND OBJECTIVES
Saudi Arabia underwent opulence-driven socio-cultural and lifestyle changes leading to soaring rates of diabetes mellitus. This study exposes the epidemiology of abnormal glucose metabolism namely: diabetes and impaired fasting glucose (IFG) in 13 administrative regions of Saudi Arabia.
DESIGN AND SETTINGS
This is a nationwide, household, population-based cross-sectional study that was conducted through primary health care centers during the period 2007–2009.
PATIENTS AND METHODS
A nationwide, household, population-based cohort of 53 370 participants aged 0–100 years adjusted to be compatible with population census was interviewed and anthropometric measures were collected. Fasting blood sample was used to screen for IFG and diabetes.
RESULTS
The overall prevalence of abnormal glucose metabolism was 34.5%, which included 22.6% patients with IFG, 11.9% patients with diabetes, and 6.2% patients who unaware of their disease. Diabetes prevalence was 40.2% for subjects aged ≥45 years and 25.4% for those aged ≥30 years that decreased to 11.9% when the full age spectrum was considered. Type 1 diabetes prevalence was 0. 8%, contributing only to 6.6% of the total population of patients with diabetes. The top 5 regions with the highest abnormal glucose metabolism prevalence were Makkah (43.4%), Aljouf (41.7%), Eastern region (37.16%), Madinah (35.4%), and Qassim (33.7%). Urbanization, age, and obesity were behind the wide variations in diabetes and IFG prevalence in the 13 regions.
CONCLUSION
Abnormal glucose metabolism has reached an epidemic state in Saudi Arabia, where one-third of the population is affected and half of diabetic cases were unaware of their disease. This observation warrants an urgent strategy for launching diabetes primary prevention and screening programs.
Saudi Arabia is a country known for its wide geographical area and unique homogeneous socio-cultural structure with rapid population growth.1 Over the last 4 decades, this country has passed through a rapid economic development leading to urbanization reflected by life style changes. This life style change has led to soaring rates of chronic diseases, mainly diabetes mellitus, which has put a real challenge to the health system in Saudi Arabia.
Over the last 40 years, there have been many studies that looked into this medical problem from different prospective. Fatani et al in 1987, studied diabetes prevalence in the Western province only, and reported the prevalence to be 4.3% in rural areas for people aged ≥15 years, using 1980 World Health Organization (WHO) criteria.2 Al-Nuaim in 1993 calculated diabetes prevalence at a national level to be at 12.5% using 1985 WHO criteria for the age group >15 years.3 It was followed in the national survey by Al-Nozha et al in the year 2004, reporting diabetes prevalence to be at 23.7% using 1997 American Diabetes Association (ADA) criteria in a narrower age group from 30–70 years.4 Since that time, there have been no nationwide or regional studies that have investigated diabetes prevalence using the most recent diagnostic criteria.5 However, there have been many community- or hospital-based studies that reported diabetes prevalence, but these studies lacked a proper sample adjustment or case identification methodology. Other constraints of such studies were limited to either specific age, gender, or diabetes type.6–8
The significant increase in the prevalence of type 2 diabetes mellitus is attributed to the predominance of its risk factors seen in this community. Lifestyle changes, as a result of urbanization and increased affluence, have markedly increased the rates of physical inactivity and obesity.9,10 This was also coupled with socio-cultural changes that have led to high daily caloric intake reflected by the increased consumption of animal products and refined food.11,12 Increased trends in other associated diseases, mainly hypertension and hyperlipidemia, are also contributing to the ever-increasing prevalence of diabetes.13,14 Smoking is another risk factor for diabetes and is becoming even more prevalent in this society.15
Life expectancy for Saudi population has shown a significant increase reaching 75 years in the year 2011,16 which could also play a role in the increased diabetes prevalence. Ethnicity plays a major role for type 2 diabetes susceptibility, wherein Arabs are considered to have high risk of developing type 2 diabetes.17 The high prevalence of diabetes observed in Saudis could be explained on the basis of this ethnicity. Genetic factor has its add on value as a risk factor for type 2 diabetes in this society due to the high prevalence of positive family history and consanguinity rate.18,19
International Diabetes Federation Atlas in its sixth edition in the year 2013 ranked Saudi Arabia the seventh in the top 10 countries known for their high diabetes prevalence, and this position is expected to be the sixth by 2035.20 This observation was based on the previously available epidemiology studies that support this finding and warrant a nationwide study. The Saudi Abnormal Glucose Metabolism and Diabetes Impact Study (SAUDI-DM) was designed to test for the disease prevalence and related risk factors, in addition to its socio-economic impact. This study had looked into the population age at full range and involved the 13 administrative regions of Saudi Arabia in relation to the abnormal glucose metabolism epidemiological characteristics.
PATIENTS AND METHODS
Study population
SAUDI-DM is a nationwide, household population-based cross-sectional study, which was conducted through primary health care centers using a random selection method from the 13 administrative regions in Saudi Arabia during the period from 2007–2009. Trained physicians and nurses had participated in this study by recruiting Saudi national family members from every third house. All family members who were available during that visit were recruited regardless of their age, gender, or clinical status (diabetic or non-diabetic patients), excluding subjects who refused to participate or were not present during the recruitment visit.
Data collection
Every family member was interviewed and demographic data including age and gender was collected after securing a consent form. Weight, height, and waist circumference were measured for each candidate. Non-diabetic participants were asked to report after more than 10 hours overnight fasting to the primary care center for fasting venous blood sampling after being on regular diet for the last 3 days.
A total of 91 564 subjects were captured from the 13 administrative regions. Out of these, 4147 subjects were excluded because of incomplete data—981 subjects had no blood sample, 2077 subjects were without clinical data, and 1089 subjects lacked anthropometric measurements. The remaining 87 417 subjects were adjusted for normal age, gender, and residency area distribution (the residency areas were classified to urban and rural according to the definition given by the Ministry of Municipal and Rural Affairs) to be compatible with the national census of the year 2007 taking in consideration the population distribution in the 13 administrative regions as shown in Table 1. A total 34 047 subjects were excluded during this adjustment phase using the random selection feature in the SPSS (version 17.0, SPSS Inc., Chicago). This technique would eliminate any selection bias that might affect the prevalence rate. The remaining 53 370 subjects after adjustment were used to calculate the national and regional prevalence rate of abnormal glucose metabolism.
Table 1.
The sample stratification and adjustment of the total studied population for all age spectrum distributed by different administrative regions according to gender and residency area.
| Administrative regionsa | Residency areab | Gender | Total number (%)c | ||
|---|---|---|---|---|---|
| Rural (%) | Urban (%) | Men (%) | Women (%) | ||
|
| |||||
| Bahah region | 608.0 (73.4) | 220.0 (26.6) | 395.0 (47.7) | 433.0 (52.3) | 828.0 (1.6) |
| Aljouf region | 296.0 (24.0) | 938.0 (76) | 636.0 (51.5) | 598.0 (48.5) | 1234.0 (2.3) |
| Northern border | 215.0 (24.5) | 663.0 (75.5) | 456.0 (51.9) | 422.0 (48.1) | 878.0 (1.6) |
| Asir region | 2736.0 (66.8) | 1361.0 (33.2) | 1928.0 (47.1) | 2169.0 (52.9) | 4097.0 (7.7) |
| Hail region | 354.0 (24) | 1121.0 (76) | 687.0 (46.6) | 788.0 (53.4) | 1475.0 (2.8) |
| Jizan region | 1178.0 (42.8) | 1575.0 (57.2) | 1351.0 (49.1) | 1402.0 (50.9) | 2753.0 (5.2) |
| Madinah region | 1326.0 (38.7) | 2099.0 (61.3) | 1733.0 (50.6) | 1692.0 (49.4) | 3425.0 (6.4) |
| Makkah region | 1549.0 (13.3) | 10 130.0 (86.7) | 5599.0 (47.9) | 6080.0 (52.1) | 11 679.0 (21.9) |
| Najran region | 497.0 (36.6) | 860.0 (63.4) | 652.0 (48) | 705.0 (52) | 1357.0 (2.5) |
| Qassim region | 903.0 (22.7) | 3075.0 (77.3) | 1940.0 (48.8) | 2038.0 (51.2) | 3978.0 (7.5) |
| Riyadh region | 3284.0 (30.9) | 7329.0 (69.1) | 5503.0 (51.9) | 5110.0 (48.1) | 10 613.0 (19.9) |
| Eastern region | 1071.0 (11.4) | 8286.0 (88.6) | 4120.0 (44) | 5237.0 (56) | 9357.0 (17.5) |
| Tabuk region | 635.0 (37.4) | 1061.0 (62.6) | 873.0 (51.5) | 823.0 (48.5) | 1696.0 (3.2) |
|
| |||||
| Total sample | 14 652.0 (27.5) | 38 718.0 (72.5) | 25 873.0 (48.5) | 27 497.0 (51.5) | 53 370.0 (100) |
There are 13 administrative regions in Saudi Arabia distributed in 5 provinces.
The sample selected according to the national census done in Saudi Arabia by Central Department of Statistics and Information of the Ministry of Planning “Highlights Demographic Survey 1428H (2007)”.
The residency areas were classified to urban and rural according to the definition given by the Ministry of Municipal and Rural Affairs.
Participants were classified into 6 age groups that usually shared similar life style, especially diet and physical activity habits. The first group was the preschool group with age ranging from 0–6 years. The second group was from 7–18 years representing the school-age children and adolescents. The third group was from 19–24 years followed by middle age group that ranged from 25–45 years. The fifth group was from 46–65 years, whereas the last group was more than 65 years of age.
Laboratory analysis
Using vacuum blood-collecting tubes containing sodium fluoride, the fasting whole blood venous samples were collected and stored at −20°C, and transported to the central laboratory of Strategic Center for Diabetes Research in Riyadh. The blood glucose assessment was done using glucose oxidase-peroxidase methodology after separating plasma in a plain tube.
Using ADA criteria 2011,21 participants were considered normal if fasting plasma glucose (FPG) was <100 mg/dL (<5.6 mmol/L) and IFG level was between 100 mg/dL (5.6 mmol/L) and 125 mg/dL (6.9 mmol/L), whereas diabetes was considered when the level of FPG was ≥126 mg/dL (7.0 mmol/L).
Abnormal glucose metabolism cases in this survey included the known diabetic patients at the time of the survey, in addition to the newly discovered diabetic patients and IFG cases.
This study was approved by the Institutional Review Board at the College of Medicine, King Saud University.
Statistical analysis
Using SPSS, data were represented in descriptive statistics and frequency tables. A P value of <.05 was chosen as the level of significance, t test was used for continuous data, and chi-square test for categorical data. Data was represented as mean and 95% confidence interval (95% CI). The age-adjusted prevalence rate was used to study diabetes prevalence in different age groups using a 5-year interval matched with a 5-year Saudi population pyramid for the year 2007.
RESULTS
Demographic characteristics
A total number of 53 370 participants were stratified for residency areas, out of which 72.5% were living in urban areas and 27.5% in rural areas. Men participants contributed to 48.5%, whereas women contributed to 51.5%. The sample distribution showed that, the largest number of participants were from Makkah, followed by Riyadh and Eastern region, whereas the smallest were from Asir and Northern border as shown in Table 1.
Table 2 demonstrates the mean (95% CI) values for age, body mass index (BMI), waist circumference, and FPG of the total sample and for each region. The mean age for the total sample was 25.1 (24.9–25.3) years, which was lower among normal participants (24.2 [23.9–24.4] years), but significantly higher among IFG and diabetic participants (29.6 [29.1–30.1] years and 44.7 [44.2–45.3] years, respectively). The mean BMI for the total sample was 24.3 (24.2–24.4) kg/m2, which was significantly higher among both IFG and diabetic participants. The mean waist circumference for the total sample was 73.8 (73.6–74.0) cm, which was significantly higher among IFG and diabetic participants (80.7 [80.1–81.3] cm and 89.0 [88.4–89.7] cm, respectively). The mean FPG for the total sample was 5.50 (5.47–5.53) mmol/L, whereas it was 6.03 (6.02–6.04) mmol/L for IFG and 9.7 (9.57–9.89) mmol/L for diabetic participants, with a P value <.0001. The highest mean age was found in Asir (27.7 [27.1–28.3] years), whereas the lowest was found in Northern border (23.0 [21.8–24.1] years). The mean BMI was the highest in Tabuk (25.3 [24.9–25.7] kg/m2) and the lowest in Najran at 22.3 (21.9–22.8) kg/m2. The mean waist circumference was found to be the highest in Tabouk at 81.3 (80.3–82.3) cm and the lowest in Aljouf (59.8 [58.3–61.2] cm). The mean FPG was highest in Makkah (5.9 [5.86–6.02] mmol/L), but the lowest in Jizan (4.9 [4.72–4.99] mmol/L).
Table 2.
Mean (95%CI) baseline characteristics of total, normal, IFG, and diabetic precipitants aged 0 to 100 y by regions.
| Total | Bahah | Aljouf | Northern boarder | Asir | Jizan | Najran | Riyadh | Eastern | Qassim | Hail | Madinah | Makkah | Tabuk | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| Age (y) | Total | 25.1 (24.9–25.3) | 25.9 (24.6–27.4) | 23.1 (22.1–24.1) | 23.0 (21.8–24.1) | 27.7 (27.1–28.3) | 25.7 (25.0–26.4) | 23.2 (22.3–24.2) | 24.3 (23.9–24.6) | 24.6 (24.3–24.9) | 24.4 (23.9–24.9) | 25.1 (24.0–26.1) | 24.9 (24.3–25.6) | 25.9 (25.6–26.3) | 26.0 (25.3–26.8) |
| Normal | 24.2 (23.9–24.4) | 26.7 (25.1–28.4) | 23.7 (22.4–25.0) | 22.7 (21.5–23.9) | 24.5 (23.8–25.1) | 27.1 (26.0–28.1) | 22.7 (21.5–23.9) | 24.4 (23.8–24.9) | 23.5 (22.9–23.9) | 22.9 (22.2–23.6) | 24.9 (23.5–26.3) | 24.2 (23.3–25.2) | 24.8 (24.1–25.4) | 22.7 (21.8–23.7) | |
| IFG | 29.6 (29.1–30.1) | 34.6 (30.8–38.4) | 27.5 (24.9–30.1) | 29.5 (25.6–33.5) | 32.4 (30.9–33.9) | 32.2 (29.8–34.7) | 31.2 (26.3–36.2) | 29.5 (28.3–30.7) | 27.5 (26.5–28.4) | 30.4 (29.1–31.8) | 32.2 (28.8–35.6) | 32.9 (30.9–34.9) | 30.1 (29.1–31.1) | 28.5 (26.3–30.7) | |
| DM | 44.7 (44.2–45.3) | 44.6 (40.3–48.8) | 45.0 (41.6–48.5) | 48.5 (43.2–53.8) | 45.3 (43.4–47.3) | 44.2 (41.7–46.8) | 44.9 (40.7–49.2) | 47.6 (46.3–48.8) | 41.4 (40.2–42.7) | 41.9 (39.9–43.9) | 52.6 (49.4–55.8) | 48.3 (46.4–50.3) | 43.8 (42.7–45.0) | 44.9 (42.5–47.4) | |
| P value | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | |
| BMI (kg/m2) | Total | 24.3 (24.2–24.4) | 24.0 (23.4–24.6) | 24.2 (23.8–24.7) | 24.2 (23.6–24.7) | 24.2 (23.9–24.4) | 23.1 (22.8–23.4) | 22.3 (21.9–22.8) | 24.7 (24.5–24.9) | 24.9 (24.7–25.0) | 24.5 (24.3–24.8) | 22.8 (22.4–23.2) | 22.8 (22.5–23.0) | 24.5 (24.3–24.7) | 25.3 (24.9–25.7) |
| Normal | 24.0 (23.9–24.1) | 24.7 (23.8–25.6) | 25.5 (24.7–26.3) | 24.1 (23.4–24.7) | 23.2 (22.9–23.5) | 23.5 (23.0–28.1) | 22.4 (21.8–22.9) | 24.7 (24.3–25.1) | 24.4 (24.2–24.7) | 24.2 (23.8–24.6) | 23.4 (22.8–24.0) | 22.7 (22.2–23.1) | 24.3 (23.9–24.7) | 24.5 (24.0–25.0) | |
| IFG | 26.3 (26.1–26.6) | 26.9 (25.0–28.9) | 26.4 (25.3–27.6) | 27.7 (25.4–30.0) | 26.0 (25.4–26.6) | 25.2 (24.2–26.2) | 26.6 (23.1–30.0) | 26.9 (26.2–27.6) | 27.1 (26.6–27.6) | 27.0 (26.3v27.7) | 25.2 (23.8–26.5) | 25.5 (24.6–26.3) | 25.9 (25.4–26.4) | 27.7 (26.6–28.9) | |
| DM | 28.7 (28.5–28.9) | 28.3 (26.8–29.7) | 28.9 (27.7–30.1) | 30.5 (28.7–32.3) | 28.8 (28.0–29.6) | 27.1 (26.4–27.9) | 27.1 (25.7–28.5) | 29.7 (29.1–30.4) | 29.1 (28.5–29.6) | 28.8 (28.1–29.8) | 28.5 (27.3–29.7) | 27.7 (26.9–28.5) | 28 (27.7–28.6) | 29.3 (28.3–30.3) | |
| P value | <.0001 | .138 | .003 | .083 | <.0001 | .001 | .545 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .028 | |
| Waist cir. (cm) | Total | 73.8 (73.6–74.0) | 75.7 (74.1–77.3) | 59.8 (58.3–61.2) | 76.2 (74.9–77.5) | 74.3 (73.6–74.9) | 72.8 (71.9–73.7) | 72.9 (71.7–74.1) | 74.0 (73.5–74.6) | 78.6 (78.1–79.0) | 76.0 (75.3–76.7) | 74.9 (73.8–75.9) | 71.8 (71.0–72.5) | 69.7 (69.2–70.2) | 81.3 (80.3–82.3) |
| Normal | 74.7 (74.4–75.1) | 78.8 (76.9–80.8) | 60.2 (57.5–62.8) | 78.0 (76.4–79.5) | 71.1 (70.3–72.0) | 76.2 (74.8–77.5) | 72.6 (71.1–74.0) | 74.1 (73.1–75.1) | 77.7 (76.9–78.5) | 75.5 (74.5–76.5) | 76.6 (75.0–78.1) | 74.1 (72.9–75.3) | 74.5 (73.6–75.3) | 79.3 (78.0–80.7) | |
| IFG | 80.7 (80.1–81.3) | 84.2 (79.9–88.5) | 60.0 (55.1–62.8) | 84.8 (80.8–88.9) | 79.1 (77.4–80.7) | 81.2 (78.5–83.9) | 79.9 (75.0–84.8) | 80.2 (78.6–81.8) | 82.5 (81.1–83.9) | 84.7 (83.0–86.4) | 84.6 (81.3–87.9) | 82.6 (80.0–85.1) | 79.9 (78.6–81.1) | 87.6 (84.7–90.5) | |
| DM | 89.0 (88.4–89.7) | 91.2 (86.9–95.4) | 59.8 (54.3–65.3) | 95.0 (91.5–98.6) | 87.9 (85.7–90.1) | 89.2 (86.5–81.8) | 92.8 (89.1–96.4) | 92.0 (90.1–93.9) | 92.6 (91.0–94.2) | 92.7 (90.5–95.0) | 95.6 (92.4–98.8) | 90.4 (88.0–92.7) | 84.3 (83.0–85.7) | 93.6 (90.9–96.3) | |
| P value | <.0001 | .015 | .814 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .001 | |
| FPG (mmol/L) | Total | 5.5 (5.5–5.5) | 5.2 (5.0–5.3) | 5.9 (5.7–6.1) | 5.17 (5.1–5.3) | 5.4 (5.4–5.5) | 4.9 (4.7–5.0) | 5.1 (5.0–5.3) | 5.4 (5.3–5.5) | 5.6 (5.5–5.6) | 5.6 (5.5–5.6) | 5.3 (5.2–5.5) | 5.6 (5.4–5.7) | 6.0 (5.9–6.0) | 5.3 (5.1–5.4) |
| Normal | 4.6 (4.6–4.6) | 4.6 (4.5–4.7) | 4.7 (4.6–4.7) | 4.8 (4.8–4.8) | 4.7 (4.6–4.7) | 3.9 (3.8–4.0) | 4.3 (4.2–4.3) | 4.7 (4.7–4.7) | 4.76 (4.7–4.8) | 4.8 (4.8–4.9) | 4.6 (4.5–4.7) | 4.3 (4.2–4.4) | 4.8 (4.7–4.8) | 4.3 (4.2–4.4) | |
| IFG | 6.0 (6.0–6.0) | 6.0 (5.9–6.0) | 6.1 (6.1–6.2) | 6.0 (5.9–6.0) | 6.0 (6.0–6.1) | 6.1 (6.0–6.1) | 6.1 (6.0–6.2) | 6.0 (5.98–6.03) | 6.02 (5.99–6.04) | 6.0 (5.96–6.02) | 6.0 (6.0–6.1) | 6.1 (6.0–6.1) | 6.1 (6.0–6.1) | 6.07 (6.0–6.1) | |
| DM | 9.7 (9.6–9.9) | 7.9 (6.9–8.8) | 10.7 (9.8–11.7) | 8.6 (7.5–9.7) | 10.4 (9.9–10.8) | 10.3 (9.6–11.0) | 11.0 (10.0–11.9) | 9.8 (9.3–10.3) | 9.2 (8.8–9.6) | 9.2 (8.7–9.7) | 10.8 (9.6–12.0) | 10.4 (9.8–11.0) | 10.2 (9.9–10.6) | 10.0 (9.2–10.9) | |
| P value | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | |
CI: Confidence interval; IFG: impaired fasting glucose; DM: diabetes mellitus; BMI: body mass index; Waist circ.: waist circumference; FPG: fasting plasma glucose.
P value was calculated between DM and IFG.
Prevalence of diabetes and IFG
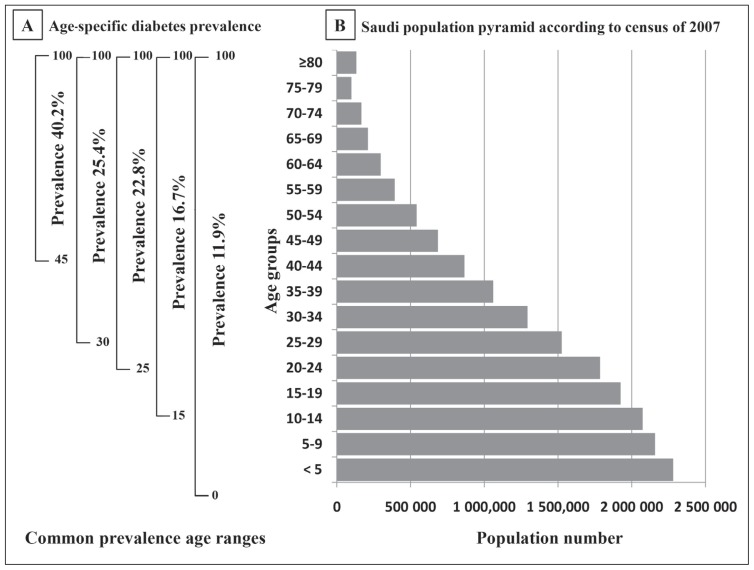
Figure 1 demonstrates the Saudi population pyramid from the Census 2007 using 5-year intervals and age-specific diabetes prevalence classified according to the commonly used age ranges. The Saudi population pyramid is following the expansive type that shows a large number of the population in the younger age groups as was observed in most developing countries as a result of high fertility rate (Figure 1B). Figure 1A demonstrates age-specific diabetes prevalence in 5 different age ranges. The overall diabetes prevalence among all age groups (0–100) was 11.9%. The diabetes prevalence increased to 16.7% for the age range between 15 and 100 years and to 22.8% for the age range between 25 and 100 years. Age range between 30 and 100 years had a diabetes prevalence rate of 25.4%, whereas it reached 40.2% when the age range was selected between 45 and 100 years.
Figure 1.
Age-specific diabetes prevalence in relation to the population pyramid according to the census 2007.
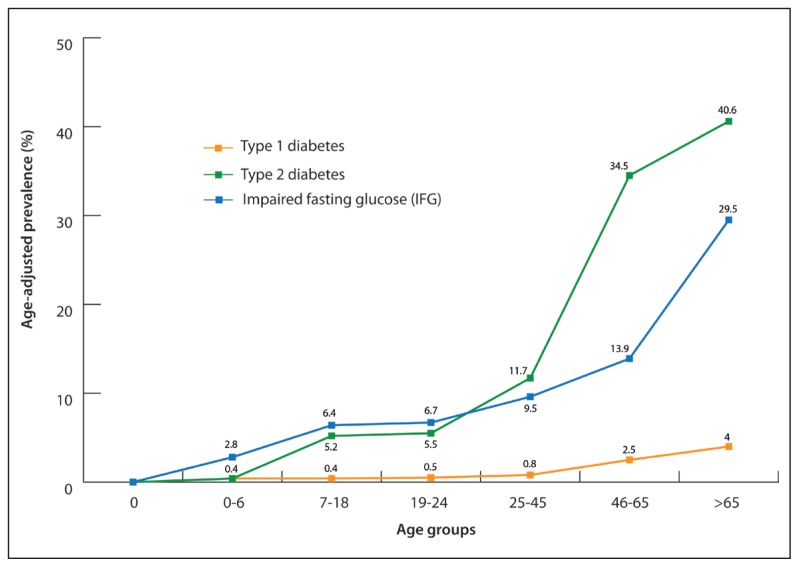
Age-adjusted prevalence of type 1 diabetes, 2 diabetes, and IFG is shown in Figure 2 according to the selected age groups. The prevalence of type 1 diabetes, type 2 diabetes, and IFG increased with age reaching its peak in the age group more than 65 years at 4.0% for type 1 diabetes, 40.6% for type 2 diabetes, and 29.5% for IFG. For children aged 0–6 years, the prevalence of type 1 diabetes was found to be 0.4%, whereas that of IFG was found to be 2.8%; however, no cases of type 2 diabetes were discovered in this age group. In the age group from 7–18 years, type 1 diabetes remained at 0.4%, whereas type 2 diabetes at 5.2% and IFG at 6.4%, which was almost similar to the age group from 19–24 years at 0.5%, 5.5%, and 6.7% for type 1 diabetes, type 2 diabetes, and IFG, respectively. Abnormal glucose metabolism increased in the age group from 25–45 years, where the prevalence of type 1 diabetes reached 0.8%, type 2 diabetes 11.7%, and IFG 9.5%. This prevalence continued to increase reaching 2.5% for type 1 diabetes, 34.5% for type 2 diabetes, and 13.9% for IFG in the age group from 46–65 years.
Figure 2.
Age-adjusted prevalence of type 1 and type 2 diabetes mellitus and impaired fasting glucose according to the age groups.
Regional distribution
Table 3 demonstrates the prevalence of different abnormal glucose metabolism, namely, type 1 diabetes, type 2 diabetes, and IFG in various administrative regions for the age group from 0–100 years. The overall prevalence of abnormal glucose metabolism was 34.5%, out of which 22.6% and 11.9% were IFG and diabetes, respectively. Among the diabetic group, the prevalence of type 1 diabetes in the total study sample was 0.8% and that of type 2 diabetes was 11.1%, divided into 4.9% known diabetic patients and 6.2% newly discovered cases.
Table 3.
Abnormal glucose metabolism prevalence according to the different types and the administrative regions for age 0 to 100 y.
| Administrative regions | Total sample Number (%) | Diabetes mellitus Number (%) | IFG Number (%) | Total abnormal glucose metabolism Number (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes | Type 2 diabetes | Total patients with diabetes | ||||||
| Known cases | New cases | Total cases | ||||||
|
| ||||||||
| Makkah region | 11 679 (21.9) | 116 (1.0) | 604 (5.2) | 1113 (9.5) | 1717 (14.7) | 1833 (15.7) | 3235 (27.7) | 5068 (43.4) |
| Aljouf region | 1234 (2.3) | 6 (0.5) | 48 (3.9) | 103 (8.3) | 151 (12.2) | 157 (12.7) | 358 (29.0) | 515 (41.7) |
| Eastern region | 9357 (17.5) | 87 (0.9) | 576 (6.2) | 481 (5.1) | 1057 (11.3) | 1144 (12.2) | 2333 (24.9) | 3477 (37.2) |
| Madinah region | 3425 (6.4) | 36 (1.1) | 176 (5.1) | 256 (7.5) | 432 (12.6) | 468 (13.7) | 743 (21.7) | 1211 (35.4) |
| Qassim region | 3978 (7.5) | 29 (0.7) | 192 (4.8) | 150 (3.8) | 342 (8.6) | 371 (9.3) | 971 (24.4) | 1342 (33.7) |
| Tabuk region | 1696 (3.2) | 22 (1.3) | 117 (6.9) | 88 (5.2) | 205 (12.1) | 227 (13.4) | 316 (18.6) | 543 (32.0) |
| Riyadh region | 10 613 (19.9) | 63 (0.6) | 456 (4.3) | 488 (4.6) | 944 (8.9) | 1007 (9.5) | 2281 (21.5) | 3288 (31.0) |
| Asir region | 4097 (7.7) | 25 (0.6) | 154 (3.8) | 289 (7.1) | 443 (10.8) | 468 (11.4) | 791 (19.3) | 1259 (30.7) |
| Bahah region | 828 (1.6) | 2 (0.2) | 69 (8.3) | 25 (3.0) | 94 (11.4) | 96 (11.6) | 144 (17.4) | 240 (29.0) |
| Jizan region | 2753 (5.2) | 9 (0.3) | 97 (3.5) | 162 (5.9) | 259 (9.4) | 268 (9.7) | 446 (16.2) | 714 (25.9) |
| Hail region | 1475 (2.8) | 9 (0.6) | 73 (5.0) | 42 (2.9) | 115 (7.8) | 124 (8.4) | 257 (17.4) | 381 (25.8) |
| Northern border | 878 (1.6) | 3 (0.3) | 22 (2.5) | 33 (3.8) | 55 (6.3) | 58 (6.6) | 107 (12.2) | 165 (18.8) |
| Najran region | 1357 (2.5) | 11 (0.8) | 53 (3.9) | 54 (4.0) | 107 (7.9) | 118 (8.7) | 101 (7.4) | 219 (16.1) |
|
| ||||||||
| Total country | 53 370 (100) | 418 (0.8) | 2637 (4.9) | 3284 (6.2) | 5921 (11.1) | 6339 (11.9) | 12 083 (22.6) | 18 422 (34.5) |
IFG: Impaired fasting glucose.
The top 5 regions pertaining to abnormal glucose metabolism were Makkah, Aljouf, Eastern region, Madinah, and Qassim with a prevalence of 43.4% 41.7%, 37.2%, 35.4%, and 33.7%, respectively. These regions also had the highest prevalence of IFG at 27.7%, 29.0%, 24.9%, 21.7%, and 24.4% respectively. Diabetes prevalence was also noted to be high at 15.7%, 12.7%, 12.2%, and 13.7% for Makkah, Aljouf, Eastern region, and Madinah, respectively, but not for Qassim, where diabetes prevalence was 9.3%. Among these top 5 regions, Madinah had the highest prevalence of type 1 diabetes at 1.1%, followed by Makkah at 1.0%, then Eastern region at 0.9%, but lower in Qassim at 0.7% and the lowest in Aliouf at 0.5%.
The other group comparatively at a lower rate of abnormal glucose metabolism included Tabuk, Riyadh, Asir, and Bahah with a prevalence rate of 32.0%, 31.0%, 30.7%, and 29.0%, respectively. Among these regions, Tabuk had the highest prevalence of diabetes, whereas Riyadh had the highest prevalence of IFG. Tabuk also demonstrated the highest prevalence rate of type 1 diabetes in the total sample (1.3%), whereas Riyadh demonstrated the lowest type 2 diabetes prevalence in this group (8.9%) but not in the total sample. Bahah region had the lowest prevalence of type 1 diabetes (0.2%) in this group and in the total sample.
The lowest prevalence of abnormal glucose metabolism was found in Najran, Northern border, Hail, and Jizan in the same order at 16.1%, 18.8%, 25.8%, and 25.9%, respectively. These regions had the lowest prevalence of IFG, down to 7.4% in Najran, and the lowest prevalence of diabetes reaching 6.6% in Northern border. Type 1 diabetes among these regions was varied being low in Jizan (0.3%) and Northern border (0.3%) and high in Najran (0.8%).
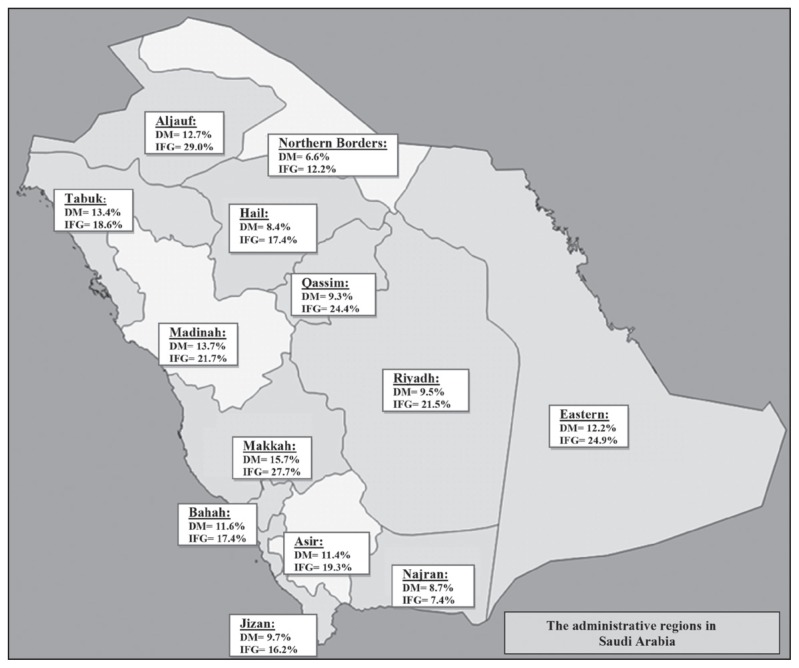
Figure 3 shows the map for different administrative regions of Saudi Arabia demonstrating diabetes and IFG prevalence in these regions. IFG prevalence was more than 20% in Aljouf, Makkah, Eastern region, Qassim, Madinah, and Riyadh in the same order, whereas it was less than 20% in Asir, Tabuk, Hail, Bahah, Jizan, Northern border and Najran. Diabetes prevalence was more than 10% in Makkah, Madinah, Tabuk, Aljouf, Eastern region, Bahah, and Asir, but lower than 10% in Jizan, Riyadh, Qassim, Najran, Hail, and Northern border in the same order.
Figure 3.
Diabetes and impaired fasting glucose prevalence in the 13 administrative regions in Saudi Arabia.
DISCUSSION
Prevalence of abnormal glucose metabolism
Saudi Arabia, in recent times, has been combating against the epidemic of type 2 diabetes mellitus, where 34.5% (11.9% diabetic patients and 22.6% IFG) in all age spectrum of the total population are considered to be suffering from either diabetes or impaired fasting glucose (IFG). It is more prevalent among the targeted age population of 30 years and above, where 1 quarter of this population is already affected with diabetes and another 1 quarter is pre-diabetics in the form of IFG, as was been shown in our previous publication.22 This finding is consistent with what Al-Nozha et al reported in 2004,4 although the use of different diagnostic criteria had its impact on the higher prevalence of IFG observed in the current study.
Age plays a significant role in the increased prevalence of diabetes, reaching 40.2% for the people aged 45 years and above in the current study. This observation was also supported by many local and international studies although the prevalence was not as high.2,23,24 Another remarkable observation that was noted from this nationwide study was the unexpected high prevalence of IFG among children at 2.8% and the high prevalence of both type 2 diabetes and IFG between the age group 7 and 18 years at 5.2% and 6.4%, respectively, which is visibly higher than what was reported from other countries.25 This could well be explained by the increased obesity rates in the study cohort accounting for 6.8% in the younger age groups, coupled with high caloric intake and low physical activity reported in this population.26–28
The IFG to diabetes ratio was found to be more than 1 among the population younger than 25 years of age, whereas it was less than 1 among older population. This transformation from IFG to diabetes could be explained on the basis of age progression as shown by other ethnicities.29 The lower IFG to diabetes ratio observed in the people older than 25 years of age could also be explained by frequent exposure to medical checkup in the older population.30,31
The Saudi population pyramid is following the expansive type, where the younger population constitutes more than 50% of the total population. As type 2 diabetes is more prevalent among the middle and old age groups, its prevalence is expected to decrease at the lower age spectrum. In the current study, the prevalence of diabetes for the population aged ≥25 years was found to be 22.8% and would decrease to 16.7% in the population aged 15 years and above. The overall prevalence of diabetes in the entire Saudi population including all age groups was 11.9%, which is considered to be high for a country with more than 50% of its population younger than 20 years of age.
Prevalence of type 1 diabetes
Type 1 diabetes accounted for just 6.6% of the total diabetic population gradually increased with the progression in age in an accumulative pattern. Its prevalence is 400 cases per 100 000 population in subjects younger than 18 years of age, which is almost 4 times higher than what was reported by Al-Herbish et al in 2008 (109.5 cases per 100 000 population).7 Type 1 diabetes prevalence in the current study has increased 10 times reaching up to 4000 cases per 100 000 population in the age group more than 65 years. This could be explained by the accumulation of cases with age coupled with missed type 1 cases that are diagnosed as type 2 in adolescents and middle-aged people. This increment could also be attributed to the better health care system that increased the life span of type 1 diabetic patients.
Regional variations
The current study showed the prevalence of abnormal glucose metabolism at a regional level with wide variations ranging from 43.4% in Makkah to 16.1% in Najran, wherein IFG was found to be the most prevalent type at 22.6%, followed by type 2 diabetes at 11.1% and type 1 diabetes at 0.8% in the total population. Makkah, Aljouf, Eastern region, and Madinah showed the highest prevalence of abnormal glucose metabolism exceeding 35%; it was mainly due to the high prevalence of IFG in these regions that had exceeded 20%. This high prevalence could be explained by the fact that they comprise older population or have higher BMI and waist circumference values when compared with the national mean values. In addition, these regions demonstrated higher mean FPG when compared with other administrative regions.
Abnormal glucose metabolism prevalence was found to be the lowest in Najran and Northern border with a prevalence rate lesser than 20%. They showed the lowest prevalence of IFG and diabetes, which could be explained by the fact that both these regions had lower mean age and BMI than the total cohort. Their mean FPG was also lower than the mean FPG for the total cohort. The remaining 7 regions had the prevalence of abnormal glucose metabolism between 25.8%% and 33.7% and diabetes between 8.4% and 13.4%.
The IFG to type 2 diabetes ratio of 2:1 in the total cohort shows that for each diabetic patient, there are 2 IFG cases. This is also similar to what was reported in Guinea and China.32,33 This ratio was found to be more than 2 in Aljouf, Eastern region, Qassim, Riyadh, and Hail, which could be explained by the increased BMI and decreased mean age in these regions.
The type 1 diabetes prevalence varied widely in each region, with the highest in Tabuk at 1.3 % and the lowest in Bahah at 0.2%. This observation was found to be different from what was earlier reported by Al-Herbish et al., where the highest prevalence was observed in Central and Southern provinces and the lowest in Northern and Eastern provinces.7 This difference could be explained on the basis of the different age groups, as his study subjects were between 0 and 19 years of age, whereas ours had no age limitation.
When looking at diabetes and IFG distribution in the 13 administrative regions, diabetes prevalence was comparatively higher in Makkah, Madinah, Tabuk, Aljouf, Bahah, Qassim, and Asir, which could be the effect of urbanization in most of these regions as shown in Table 1. On the contrary, it was lower in Jizan and Najran that have more rural areas. Urbanization also played a major role in the high prevalence of IFG in Aljouf, Makkah, Eastern region, Madinah, Qassim, and Riyadh.
Comparative analysis with other countries
To compare the current study with what was reported earlier from other countries sharing the same socio-economic and life style changes, Table 4 demonstrates diabetes prevalence in the 6 Gulf Cooperation Council (GCC) countries during the period from 1990–2009. The selected 10 studies were all countrywide epidemiological studies using different diagnostic criteria and age groups.
Table 4.
Diabetes prevalence from the country-wide epidemiological studies in the 6 GCC countries during the period between 1990 and 2009.
| Country | Authors | Year | Sample size | Age group (years) | Diagnostic criteria | Prevalence of diabetes | Ref. |
|---|---|---|---|---|---|---|---|
| Saudi Arabia | Al-Nuaim | 1997 | 13 177 | ≥15 | WHO criteria 1985 | 12.5% | [3] |
| Al-Nozha et al | 2004 | 17 232 | 30–70 | ADA criteria 1997 | 23.7% | [4] | |
| Al-Rubeaan et al | 2015 | 53 370 | ≥15 | ADA criteria 2011 | 16.7% | Current | |
| ≥30 | 25.4% | ||||||
| Kuwait | Abdella et al | 1996 | 261 387 | ≥20 | WHO criteria 1985 | 7.6% | [34] |
| Abdella et al | 1998 | 3003 | ≥20 | WHO criteria 1985 | 14.8% | [35] | |
| Oman | Asfour et al | 1995 | 5096 | ≥20 | WHO criteria 1985 | 10% | [36] |
| Al-Lawati et al | 2002 | 5838 | ≥20 | WHO criteria 1999 | 16.1% | [37] | |
| Al-Moosa et al | 2006 | 7179 | ≥20 | WHO criteria 2000 | 11.6% | [38] | |
| United Arab Emirates | Malik et al | 2005 | 5844 | ≥20 | WHO criteria 1999 | 20% | [39] |
| Bahrain | Al-Mahroos et al | 2001 | 2013 | 40–69 | WHO criteria 1985 | 30% | [40] |
| Qatar | Bener et al | 2009 | 1117 | ≥20 | WHO expert group 2006 | 16.7% | [41] |
Two studies conducted in Saudi Arabia can be compared with our current study; the first one was in 1993 for age group ≥15 years with a calculated prevalence of 12.5% using WHO 1985 criteria.3 The current study that used ADA criteria 2011 for the same cutoff age group, showed an increase in the prevalence of diabetes to 16.7% above 15 years of age. At the same time, the current study demonstrated an increased prevalence rate up to 25.4% for the age group ≥30 years when compared with the second study that was conducted in the year 2000 by Al-Nozha et al who reported a prevalence rate of 23.7% for the age group between 30 and 70 years using ADA 1997 criteria.4
Also 2 studies conducted in Kuwait, 1 in 1999 and another in 1996, both using WHO criteria 1985,34,35 demonstrated that diabetes prevalence has increased from 7.6% to 14.8% for the age group ≥20 years of age, although there was no countrywide epidemiological study in Kuwait after the year 2000 that can be compared with our findings. There was a clear increased trend in the prevalence of diabetes in this country. Oman had 3 epidemiological studies, all for people ≥20 years of age,36–38 where there was an increase in diabetes prevalence from 10% to 16.1% during the period between 1991 and 2002, which is similar to the observed trend in Saudi Arabia. However, the adoption of some diabetes prevention programs in Oman has resulted in an obvious decrease in the prevalence down to 11.6% in 2006, which proves the effectiveness of primary prevention programs in such countries. The only selected study from United Arab Emirates among multiethnic adults ≥20 years showed a prevalence of 20%,39 whereas among the age group 40 to 69 years, diabetes prevalence in Bahrain has reached 30% using WHO 1985 criteria.40 The most recent study from Qatar in 2009 has showed diabetes prevalence to be 16.7% among adults aged ≥20 years.41 Such studies from these 3 countries confirm the same observation of diabetes trend as seen in Saudi Arabia.
The rapid economic development in the GCC countries could explain the change in diabetes prevalence trend that has put 3 of them, namely, Saudi Arabia, Kuwait, and Qatar, among the top 10 countries in terms of diabetes prevalence.20
The strength of this study comes from being a countrywide household survey with a large random sample that was adjusted to be compatible with the population census, which could explain the large number of excluded subjects when we were adjusting for age, gender, residency area, and administrative region distribution. Although this study was limited by using only FPG in defining abnormal glucose metabolism cases, it is one of the recommended criteria for diabetes and IFG screening. Another limitation of the current study was the possibility that the enrolled subjects might not have adhered to our instructions of strict overnight fast, especially children and, in particular, those who were in the age group between 0 and 6 years.
In conclusion, we found that more than one-third of the total population in all age spectrums was suffering from abnormal glucose metabolism in the form of either diabetes or IFG. More than 50% of these cases were unaware of their disease and were picked up by screening. There is a large variation in IFG and diabetes prevalence in the different regions of Saudi Arabia that can be explained by urbanization, aging, and obesity effect. Although there is an increase in type 1 diabetes prevalence with age, type 2 diabetes and IFG prevalence in Saudi children and adolescents is strikingly higher than what was observed in the region or globally.
A primary prevention program in the form of lifestyle modification is sternly needed to stand against this disease epidemic and its heath and economic impact.
Acknowledgments
The authors would like to acknowledge the staff at the University Diabetes Center for their contribution to the study, and the staff of the primary care centers from the Ministry of Health involved in this study. This study was funded by the University Diabetes Center at King Saud University, Ministry of Health and the Tawuniya Company for health insurance.
Footnotes
Disclosure
None declared.
REFERENCES
- 1.Central Department of Statistics. Riyadh, Saudi Arabia: 2003. [AUTHOR: The first reference is not complete. Please provide complete bibliographic details.] [Google Scholar]
- 2.Fatani HH, Mira SA, el-Zubier AG. Prevalence of diabetes mellitus in rural Saudi Arabia. Diabetes Care. 1987 Apr;10(2):180–3. doi: 10.2337/diacare.10.2.180. [DOI] [PubMed] [Google Scholar]
- 3.Al-Nuaim AR. Prevalence of glucose intolerance in urban and rural communities in Saudi Arabia. Diabet Med. 1997 Jul;14(7):595–602. doi: 10.1002/(SICI)1096-9136(199707)14:7<595::AID-DIA377>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Al-Nozha MM, Al-Maatouq MA, Al-Mazrou YY, Al-Harthi SS, Arafah MR, Khalil MZ, Khan NB, Al-Khadra A, Al-Marzouki K, Nouh MS, Abdullah M, Attas O, Al-Shahid MS, Al-Mobeireek A. Diabetes mellitus in Saudi Arabia. Saudi Med J. 2004 Nov;25(11):1603–10. [PubMed] [Google Scholar]
- 5.Genuth S, Alberti KGMM, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003 Nov;26(11):3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 6.Alqurashi KA, Aljabri KS, Bokhari SA. Prevalence of diabetes mellitus in a Saudi community. Ann Saudi Med. 2011 Feb;31(1):19–23. doi: 10.4103/0256-4947.75773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Herbish AS, El-Mouzan MI, Al-Salloum AA, Al-Qurachi MM, Al-Omar AA. Prevalence of type 1 diabetes mellitus in Saudi Arabian children and adolescents. Saudi Med J. 2008 Sep;29(9):1285–8. [PubMed] [Google Scholar]
- 8.Al-Quwaidhi AJ, Pearce MS, Sobngwi E, Critchley JA, O’Flaherty M. Comparison of type 2 diabetes prevalence estimates in Saudi Arabia from a validated Markov model against the International Diabetes Federation and other modelling studies. Diabetes Res Clin Pract. 2014 Mar;103(3):496–503. doi: 10.1016/j.diabres.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Nozha MM, Al-Hazzaa HM, Arafah MR, Al-Khadra A, Al-Mazrou YY, Al-Maatouq MA, Khan NB, Al-Marzouki K, Al-Harthi SS, Abdullah M, Al-Shahid MS. Prevalence of physical activity and inactivity among Saudis aged 30–70 years. A population-based cross-sectional study. Saudi Med J. 2007 Apr;28(4):559–68. [PubMed] [Google Scholar]
- 10.Al-Nozha MM, Al-Mazrou YY, Al-Maatouq MA, Arafah MR, Khalil MZ, Khan NB, Al-Marzouki K, Abdullah MA, Al-Khadra AH, Al-Harthi SS, Al-Shahid MS, Al-Mobeireek A, Nouh MS. Obesity in Saudi Arabia. Saudi Med J. 2005 May;26(5):824–9. [PubMed] [Google Scholar]
- 11.Amin TT, Al-Sultan AI, Ali A. Overweight and Obesity and their Association with Dietary Habits, and Sociodemographic Characteristics Among Male Primary School Children in Al-Hassa, Kingdom of Saudi Arabia. Indian J Community Med. 2008 Jul;33(3):172–81. doi: 10.4103/0970-0218.42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahfouz AA, Abdelmoneim I, Khan MY, Daffalla AA, Diab MM, Al-Gelban KS, Moussa H. Obesity and related behaviors among adolescent school boys in Abha City, Southwestern Saudi Arabia. J Trop Pediatr. 2008 Apr;54(2):120–4. doi: 10.1093/tropej/fmm089. [DOI] [PubMed] [Google Scholar]
- 13.Al-Nozha MM, Abdullah M, Arafah MR, Khalil MZ, Khan NB, Al-Mazrou YY, Al-Maatouq MA, Al-Marzouki K, Al-Khadra A, Nouh MS, Al-Harthi SS, Al-Shahid MS, Al-Mobeireek A. Hypertension in Saudi Arabia. Saudi Med J. 2007 Jan;28(1):77–84. [PubMed] [Google Scholar]
- 14.Al-Nozha MM, Arafah MR, Al-Maatouq MA, Khalil MZ, Khan NB, Al-Marzouki K, Al-Mazrou YY, Abdullah M, Al-Khadra A, Al-Harthi SS, Al-Shahid MS, Al-Mobeireek A, Nouh MS. Hyperlipidemia in Saudi Arabia. Saudi Med J. 2008 Feb;29(2):282–7. [PubMed] [Google Scholar]
- 15.Bassiony MM. Smoking in Saudi Arabia. Saudi Med J. 2009 Jul;30(7):876–81. [PubMed] [Google Scholar]
- 16.The World Bank. Life expectancy at birth [Internet] 2011. [cited 2014 Dec 23]. Available from: http://data.worldbank.org/indicator/SP.DYN.LE00.IN?order=wbapi_data_value_2011+wbapi_data_value+wbapi_data_value-last&sort=asc,/
- 17.Al-Rubeaan K, Siddiqui K, Saeb ATM, Nazir N, Al-Naqeb D, Al-Qasim S. ACE I/D and MTHFR C677T polymorphisms are significantly associated with type 2 diabetes in Arab ethnicity: a meta-analysis. Gene. 2013 May 15;520(2):166–77. doi: 10.1016/j.gene.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Bener A, Hussain R, Teebi AS. Consanguineous marriages and their effects on common adult diseases: studies from an endogamous population. Med Princ Pract. 2007;16(4):262–7. doi: 10.1159/000102147. [DOI] [PubMed] [Google Scholar]
- 19.Al-Gazali L, Hamamy H, Al-Arrayad S. Genetic disorders in the Arab world. BMJ. 2006 Oct 21;333(7573):831–4. doi: 10.1136/bmj.38982.704931.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Diabetes Federation. IDF Diabetes Atlas [Internet] 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. Available from: http://www.idf.org/diabetesatlas. [Google Scholar]
- 21.American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care. 2011 Jan;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Rubeaan K, Al-Manaa HA, Khoja TA, Ahmad NA, Al-Sharqawi AH, Siddiqui K, Alnaqeb D, Aburisheh KH, Youssef AM, Al-Batel A, Alotaibi MS, Al-Gamdi AA. Epidemiology of abnormal glucose metabolism in a country facing its epidemic: SAUDI-DM study. J Diabetes. 2014 Sep 30; doi: 10.1111/1753-0407.12224. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, Rao PV, Yajnik CS, Prasanna Kumar KM, Nair JD Diabetes Epidemiology Study Group in India (DESI) High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia. 2001 Sep;44(9):1094–101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 24.Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003 Mar;26(3):25–731. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- 25.Clark NG, Fox KM, Grandy S SHIELD Study Group. Symptoms of diabetes and their association with the risk and presence of diabetes: findings from the Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) Diabetes Care. 2007 Nov;30(11):2868–73. doi: 10.2337/dc07-0816. [DOI] [PubMed] [Google Scholar]
- 26.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002 Aug 10;360(9331):473–82. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hazzaa HM, Abahussain NA, Al-Sobayel HI, Qahwaji DM, Musaiger AO. Physical activity, sedentary behaviors and dietary habits among Saudi adolescents relative to age, gender and region. Int J Behav Nutr Phys Act. 2011;8:140. doi: 10.1186/1479-5868-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collison KS, Zaidi MZ, Subhani SN, Al-Rubeaan K, Shoukri M, Al-Mohanna FA. Sugar-sweetened carbonated beverage consumption correlates with BMI, waist circumference, and poor dietary choices in school children. BMC Public Health. 2010;10:234. doi: 10.1186/1471-2458-10-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Hazzaa HM, Al-Rasheedi AA. Adiposity and physical activity levels among preschool children in Jeddah, Saudi Arabia. Saudi Med J. 2007 May;28(5):766–73. [PubMed] [Google Scholar]
- 30.Khambalia A, Phongsavan P, Smith BJ, Keke K, Dan L, Fitzhardinge A, Bauman AE. Prevalence and risk factors of diabetes and impaired fasting glucose in Nauru. BMC Public Health. 2011;11:719. doi: 10.1186/1471-2458-11-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esteghamati A, Etemad K, Koohpayehzadeh J, Abbasi M, Meysamie A, Noshad S, Asgari F, Mousavizadeh M, Rafei A, Khajeh E, Neishaboury M, Sheikhbahaei S, Nakhjavani M. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005–2011. Diabetes Res Clin Pract. 2014 Feb;103(2):319–27. doi: 10.1016/j.diabres.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Balde N-M, Diallo I, Balde M-D, Barry I-S, Kaba L, Diallo M-M, Kake A, Camara A, Bah D, Barry M-M, Sangare-Bah M, Maugendre D. Diabetes and impaired fasting glucose in rural and urban populations in Futa Jallon (Guinea): prevalence and associated risk factors. Diabetes Metab. 2007 Apr;33(2):114–20. doi: 10.1016/j.diabet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Hao C, Zhang C, Chen W, Shi Z. Prevalence and risk factors of diabetes and impaired fasting glucose among university applicants in Eastern China: findings from a population-based study. Diabet Med. 2014 Oct;31(10):1194–8. doi: 10.1111/dme.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdella N, Khogali M, al-Ali S, Gumaa K, Bajaj J. Known type 2 diabetes mellitus among the Kuwaiti population. A prevalence study. Acta Diabetol. 1996 Jul;33(2):145–9. doi: 10.1007/BF00569425. [DOI] [PubMed] [Google Scholar]
- 35.Abdella N, Al Arouj M, Al Nakhi A, Al Assoussi A, Moussa M. Non-insulin-dependent diabetes in Kuwait: prevalence rates and associated risk factors. Diabetes Res Clin Pract. 1998 Dec;42(3):187–96. doi: 10.1016/s0168-8227(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 36.Asfour MG, Lambourne A, Soliman A, Al-Behlani S, Al-Asfoor D, Bold A, Mahtab H, King H. High prevalence of diabetes mellitus and impaired glucose tolerance in the Sultanate of Oman: results of the 1991 national survey. Diabet Med. 1995 Dec;12(12):1122–5. doi: 10.1111/j.1464-5491.1995.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 37.Al-Lawati JA, Al Riyami AM, Mohammed AJ, Jousilahti P. Increasing prevalence of diabetes mellitus in Oman. Diabet Med. 2002 Nov;19(11):954–7. doi: 10.1046/j.1464-5491.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 38.Al-Moosa S, Allin S, Jemiai N, Al-Lawati J, Mossialos E. Diabetes and urbanization in the Omani population: an analysis of national survey data. Popul Health Metr. 2006;4:5. doi: 10.1186/1478-7954-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik M, Bakir A, Saab BA, Roglic G, King H. Glucose intolerance and associated factors in the multi-ethnic population of the United Arab Emirates: results of a national survey. Diabetes Res Clin Pract. 2005 Aug;69(2):188–95. doi: 10.1016/j.diabres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Al-Mahroos F, Al-Roomi K. Obesity among adult Bahraini population: impact of physical activity and educational level. Ann Saudi Med. 2001 Jul;21(3–4):183–7. doi: 10.5144/0256-4947.2001.183. [DOI] [PubMed] [Google Scholar]
- 41.Bener A, Zirie M, Janahi IM, Al-Hamaq AOAA, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract. 2009 Apr;84(1):99–106. doi: 10.1016/j.diabres.2009.02.003. [DOI] [PubMed] [Google Scholar]