Abstract
A 28-year-old female suffered from nephrotic syndrome after a long-term use of mercury-containing, skin-lightening cream. The blood and urinary mercury content of this patient increased with use. Renal biopsy showed minimal change disease. Her symptoms were relieved 6 months after discontinuing use of the cream and receiving sodium dimercaptosulfonate and glucocorticosteroid treatments. Proteinuria disappeared, and blood and urinary mercury levels returned to normal. Previous reports of nephrotic syndrome caused by mercury-containing, skin-lightening creams have mostly been identified as being due to membranous nephropathy. Minimal change disease has been reported in a few case reports published in the English language. Here we report a case of nephrotic syndrome with minimal change disease following exposure to a mercury-containing, skin-lightening cream. We also reviewed relevant published reports to summarize clinical features and treatments and to explore the possible mechanisms involved.
Mercury is the only heavy metal that is liquid at room temperature. It can be absorbed through the respiratory tract, digestive tract, and skin, leading to poisoning. Mercury poisoning mainly causes central nervous system and kidney damage. This kind of kidney damage most often manifests as nephrotic syndrome. It has been widely known that mercury vapor suction, filling a tooth, and intake of mercury-contaminated foods or drugs can result in nephrotic syndrome.1 However, sufficient attention has not been paid to nephrotic syndrome caused by mercury-containing cosmetics, especially skin-lightening creams. Previous reports of mercury-containing cosmetics leading to nephrotic syndrome2–7 are primarily membranous nephropathy, and there are a few reports of minimal change disease. Barr et al8 reported the first case of minimal change disease caused by a mercury-containing, skin-lightening cream in 1972, but immunofluorescence or electron microscopy data were not presented, and the possibility of membranous nephropathy was not excluded. Tang et al9 reported the first case of minimal change disease caused by mercury-containing, skin-lightening cream that was confirmed by renal biopsy in Hong Kong. Here, we report a case of nephrotic syndrome of minimal change disease in Mainland China.
CASE
A 28-year-old female patient was admitted to the hospital with complaints of pain in both limbs and facial edema for 2 weeks in January 2010. She had no history of kidney disease, diabetes, hypertension, hepatitis, or use of non-steroidal anti-inflammatory drugs. She did, however, have a history of using a homemade skin-lightening cream for 11 months that was applied in a beauty salon. The laboratory examination revealed that her white blood cell count was 6.48×109/L (normal range: 4–10×109/L), hemoglobin was 153 g/L (normal range: 110–150 g/L), the platelet count was 360×109/L (normal range: 100–300 g/L), the neutrophil rate was 65.4% (normal range: 50%–70%), and the eosinophil rate was 2.30% (normal range: 0.5%–5%). The erythrocyte sedimentation rate was normal. The serum alanine aminotransferase activity was 13.9 U/L (normal range: 0–40 U/L), the serum aspartate aminotransferase activity was 19.9 U/L (normal range: 0–45 U/L), serum total protein was 60.5 g/L (normal range: 60–80 g/L), serum albumin was 25.5 g/L (normal range: 35–50 g/L), blood urea nitrogen was 6.78 mmol/L (normal range: 1.8–7.1 mmol/L), and serum creatinine was 71.4 μmol/L (normal range: 44–133 μmol/L). The serum potassium level was 3.59 mmol/L (normal range: 3.5–5.5 mmol/L), and sodium was 138 mmol/L (normal range: 135–145 mmol/L). Serum total cholesterol was 5.01 mmol/L (normal range: 2.84–5.68 mmol/L), triglycerides were 2.53 mmol/L (normal range: 0.56–1.70 mmol/L), high-density lipoprotein cholesterol was 1.83 mmol/L (normal range: 1.14–1.91 mmol/L), and low-density lipoprotein cholesterol was 4.58 mmol/L (normal range: 1–3 mmol/L), while blood glucose levels were normal. Thyroid function and immunoglobulin content were normal, the complement C3 level was 0.60 g/L, and the C4 level was normal. Streptococcus hemolysin O antibodies, antinuclear antibodies, antineutrophil cytoplasm antibody, anti-deoxyribonucleic acid (DNA) antibody, anti-double–stranded DNA antibody, anti-glomerular basement membrane antibody, and anti-hemorrhagic fever virus antibody were negative. The 24-hour urinary excretion of protein was 8.25 g (normal <0.15 g/24 h). The urinary N-acetylβ-D-glucosaminidase (NAG) activity was 42.2 U/L (normal range: 0.3–11.5 U/L), urinary β2 microglobulin was 0.48 mg/L (normal range: 0.10–0.31 mg/L), and the urinary osmotic pressure was 484 mOsmol/L (normal <700 mOsmol/L). The abdominal ultrasound and the whole body bone scan were normal. The blood mercury concentration was 220 μmol/L (normal <50 μmol/L) and the urinary mercury concentration was 469 μmol/L (normal <50 μmol/L). The mercury concentration of the cosmetics was 6.80 mg/kg (normal < 1 mg/kg).
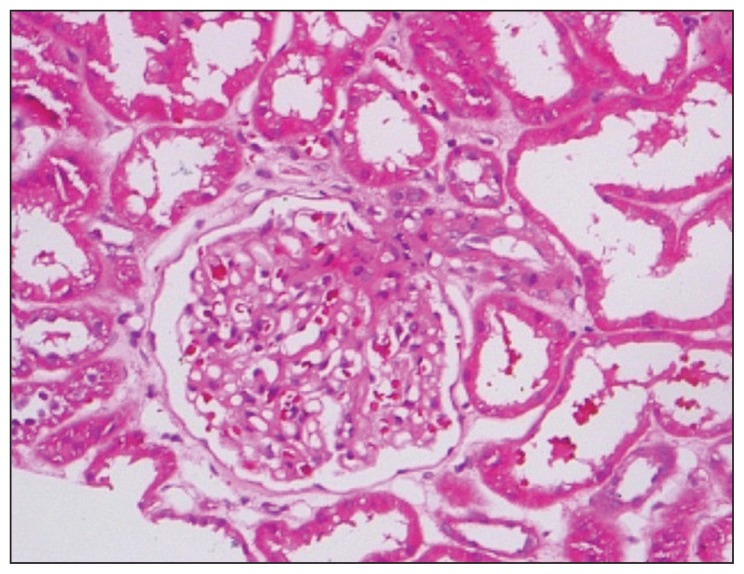
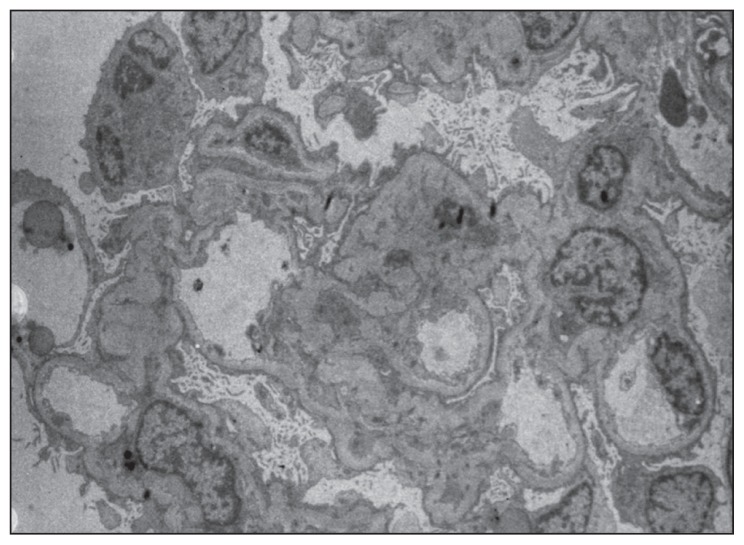
Light microscopy of a renal biopsy showed normal glomerular volume, good capillary loops, and normal glomerular structure but acute tubular necrosis in a small region of the kidneys (Figure 1). Immunofluorescence microscopy showed no deposition of immunoglobulin or complement. Diffuse podocyte process fusion and microvilli changes were observed, but no obvious basement membrane thickening, hyperplasia of mesangial cells and matrix, or electron dense regions were seen under electron microscopy (Figure 2).
Figure 1.
Histology of renal biopsy under light microscope with hematoxylin and eosin staining, magnification ×400.
Figure 2.
Histology of renal biopsy under electron microscopy, magnification ×500
The patient was given an intramuscular injection (0.25 g/d) of sodium dimercaptosulfonate (DMPS) for 3 consecutive days followed by 4 days of intermittent treatment, for a total of 2 treatment periods. She was also given prednisone (50 mg/d; patient weight=50 kg) and diuresis treatment. Her blood mercury decreased to 45.8 μmol/L and her urinary mercury was 148 μmol/L after 2 periods of mercury detoxification. Urinary protein fell to 1.62 g/24 h after 8 weeks, and thus the prednisone dose was decreased (5 mg decrement every 2 weeks). The patient was discharged after 12 weeks with serum albumin and urinary protein levels of 32 g/L and 0.63 g/24 h, respectively. She was re-examined after 6 months to measure her blood mercury, urinary mercury, urinary protein, and serum albumin levels, all of which returned to normal. Currently, the patient remains in good condition.
DISCUSSION
This patient, whose condition is described in this case study, was given a diagnosis of nephrotic syndrome associated with minimal change disease. The possibility of membranous nephropathy was excluded on the basis of renal biopsy. The patient had a long-term use of skin-lightening cream that had high content of mercury, which caused increased blood and urinary mercury and nephrotic syndrome. Her symptoms were relieved after mercury detoxification and glucocorticoid therapy.
There have been previously published reports of mercury poisoning caused by skin-lightening cream. For example, there were more than 200 cases of women poisoned by mercury after using one kind of skin-lightening cream produced by Mexico in 1995.10,11 Mercury poisoning was also reported in about 100 women in Hong Kong after the use of cosmetics made in Mainland China.12,13 Nervous system symptoms were usually observed in these reports, while renal damage was rare. Both acute and chronic mercury poisoning can affect the kidneys: acute mercury poisoning can cause renal tubular necrosis and chronic mercury poisoning can cause nephrotic syndrome, which is mostly due to membranous nephropathy.
A total of 10 cases were identified in the published reports that reported on nephrotic syndrome caused by a mercury-containing, skin-lightening cream. The clinical features of these cases are summarized in Table 1. Among the 10 cases of nephrotic syndrome we reviewed, 7 cases (70%) were diagnosed as membranous nephropathy. Barr et al8 reported that half of the patients with nephrotic syndrome caused by a skin-lightening cream were diagnosed with minimal change disease in 1972 in Kenya. However, there were no electron microscopy data for these patients, indicating that the possibility of membranous nephropathy could not be excluded. All the cases were from developing countries, and the cosmetics were mostly counterfeit or homemade products with high mercury content. The time period of cosmetic use was more than 4 months, and the longest period of use was 8 years.14
Table 1.
Clinical characteristics of nephrotic syndrome caused by skin-lightening cream.
| Cases Ref | 13 | 22 | 39 | 44 | 54 | 65 | 76 | 86 | 914 |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Age (y) | 21 | 46 | 34 | 44 | 26 | 34 | 37 | 43 | 48 |
| Sex | Female | Female | Female | Female | Female | Female | Female | Female | Female |
| Residence | Africa | Pakistan | Mainland China | Pakistan | Pakistan | Indonesia | Mainland China | Mainland China | South Africa |
| Diagnosis place | Africa | England | Hong Kong | England | England | Hong Kong | Mainland China | Mainland China | South Africa |
| Duration of cream use (mo) | 24.00 | 36.00 | 4.00 | Unspecified | Unspecified | 60.00 | 12.00 | 8.00 | 96.00 |
| Frequency of cream use | Every day | Every day | Every day | Unspecified | Unspecified | Every day | Unspecified | Unspecified | Unspecified |
| Renal pathology | MN | MN | MCD | MN | MN | MN | MN | MN | Chronic proliferative glomerulonephritis |
| 24 h Protein (g/d) | 10.00 | 2.20 | 8.35 | Unspecified | 9.00 | 4.90 | 3.82 | 3.52 | 7.00 |
| Times of urine mercury above normal (mo) | Unspecified | Unspecified | 5.74 | 3.00 | Unspecified | 75.46 | 12.50 | 2.25 | Unspecified |
| Times of blood mercury above normal (mo) | Unspecified | Unspecified | 2.48 | 5.00 | 7.76 | 3.26 | Unspecified | Unspecified | Unspecified |
| Renal function | Normal | Normal | Normal | Abnormal | Unspecified | Normal | Normal | Normal | Normal |
| Treatment | Unspecified | glucocoticoid | Mercury detoxification | Unspecified | Unspecified | Unspecified | ACEI | ARB | Unspecified |
| Outcome | Unspecified | Unspecified | Cured | Unspecified | Unspecified | Unspecified | Cured | Cured | Unspecified |
MN: Membrane nephropathy, MCD: minimal change disease,[AUTHOR: Please provide the full forms of ACEI and ARB.].
Most mercury-poisoning cases are found and diagnosed in developed countries and regions, and are associated with membranous nephropathy. This suggests that nephrotic syndrome caused by skin-lighting cream is not given enough attention by the public or physicians in developing countries and undeveloped areas, possibly because of culture, race, esthetic value or other reasons. Although membranous nephropathy caused by skin-lightening cream has been emphasized in developed countries, the potential diagnosis of minimal change disease needs to be considered again. Most of the products used by such patients are counterfeit or homemade products that are not approved by the U.S. Food and Drug Administration (FDA) and contain mercury contents that exceed allowed limits. However, we cannot consider that the creams approved by the FDA with mercury contents below the allowed limit are non-toxic.
Animal experiments revealed 2 kinds of skin-lightening creams approved by the FDA that exhibit liver, kidney, and brain toxicity, resulting in the highest mercury concentration in the kidneys.15,16 In addition, suction mercury vapor can be another cause of minimal change disease other than skin-lightening creams.17 Renal function is usually normal in patients with nephrotic syndrome caused by skin-lightening cream if there are no other concomitant diseases.
Mercury poisoning symptoms can be relieved by various treatments after discontinuing the use of skin-lightening creams. Mercury detoxification and glucocorticoid therapy are rarely used. In this case, however, the patient was cured after a 9-month mercury detoxification and glucocorticoid therapy. Tang et al9 reported another case of minimal change disease with mercury detoxification treatment and a good curative effect. The use of mercury detoxification is still controversial. Bernhoft RA1 concluded that DMPS was safe and effective and could significantly reduce the blood mercury level in animal experiments. However, it is unclear whether it can delay terminal organ damage. DMPS dose should not be too large, especially when glomerular injury is evident, because a large number of complex charged molecules will actually increase the kidney burden of mercury. The typical dose of DMPS that is administered (i.e., 0.25 g/d by intramuscular injection) is safe and has no obvious side effects.
Glucocorticoid therapy is also controversial. Only 1 case in 7 in the published reports of membranous nephropathy used glucocorticoids, but these lacked follow-up. In this case, we used glucocorticoid therapy; clinical symptoms significantly improved and the recovery time was shortened. No obvious adverse reaction occurred. Thus, minimal change disease can be responsive to glucocorticoids because the lesion is modest. DMPS combined with glucocorticoid treatment are recommended. However, the decision to use the above therapy should be made on an individual basis; however, more cases need to be studied to further verify the curative effects.
The mechanisms by which mercury-containing, skin-lightening creams cause nephrotic syndrome are still unclear. Immune pathogenesis plays an important role in this process. Mercury binds to numerous proteins forming haptens that deposit in the glomerulus and cause mesangial proliferation or membranous nephropathy. Mercury ions also have strong potential for renal tubular toxicity.18 Thus, it cannot be ruled out that an antigen released during the course of the renal tubular injury is the main cause of the glomerular immune injury. Urinary levels of both NAG and β2-MG increased; acute tubular necrosis in a portion of the kidneys was confirmed using light microscopy, suggesting that renal tubule damage may be involved in minimal change disease caused by mercury poisoning. Increases in urinary NAG and β2-MG may be considered as biomarkers of mercury poisoning in the early diagnosis of renal damage.
In conclusion, skin-lightening creams are still widely used in developing countries because of racial and cultural issues and concerns. The long-term use of mercury-containing, skin-lightening creams can result in nephrotic syndrome that is primarily characterized by membranous nephropathy. However, more attention needs to be paid to minimal change disease as a possible etiology. Nephrotic syndrome diagnosis should be considered for young and middle-aged women from developing countries who use skin-lightening products, particularly when there is a high possibility of minimal change disease.
Footnotes
Author’s Contribution
Fuyou Liu carried out the design and coordinated the study. Lin Zhang participated in manuscript preparation. Lin Sun, Chunguo Chen and Youming Peng provided expert technical assistance. All authors have read and approved the content of the manuscript.
Conflict of Interest
None.
REFERENCES
- 1.Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012;2012:460508. doi: 10.1155/2012/460508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira DB, Foster G, Savill J, Syme PD, Taylor A. Membranous nephropathy caused by mercury-containing skin lightening cream. Postgrad Med J. 1987;63(738):303–304. doi: 10.1136/pgmj.63.738.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kibukamusoke JW, Davies DR, Hutt MS. Membranous nephropathy due to skin-lightening cream. Br Med J. 1974;2(5920):646–647. doi: 10.1136/bmj.2.5920.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakera A, Lasserson D, Beck LH, Jr, Roberts IS, Winearls CG. Membranous nephropathy after use of UK-manufactured skin creams containing mercury. QJM. 2011;104(10):893–896. doi: 10.1093/qjmed/hcq209. [DOI] [PubMed] [Google Scholar]
- 5.Soo YO, Chow KM, Lam CW, Lai FM, Szeto CC, Chan MH, et al. A whitened face woman with nephrotic syndrome. Am J Kidney Dis. 2003;41(1):250–253. doi: 10.1053/ajkd.2003.50017. [DOI] [PubMed] [Google Scholar]
- 6.Li SJ, Zhang SH, Chen HP, Zeng CH, Zheng CX, Li LS, et al. Mercury-induced membranous nephropathy: clinical and pathological features. Clin J Am Soc Nephrol. 2010;5(3):439–444. doi: 10.2215/CJN.07571009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown KG, Abrahams C, Meyers AM. The nephrotic syndrome in Malawian blacks. S Afr Med J. 1977;52(7):275–278. [PubMed] [Google Scholar]
- 8.Barr RD, Rees PH, Cordy PE, Kungu A, Woodger BA, Cameron HM. Nephrotic Syndrome in Adult Africans in Nairobi. Br Med J. 1972;2(5806):131–134. doi: 10.1136/bmj.2.5806.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang HL, Chu KH, Mak YF, Lee W, Cheuk A, Yim KF, et al. Minimal change disease following exposure to mercury-containing skin lightening cream. Hong Kong Med J. 2006;12(4):316–318. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Update: Mercury poisoning associated with beauty cream—Arizona, California, New Mexico, and Texas. MMWR Morbid Mortal Wkly Rep. 1996;45(29):633–635. [PubMed] [Google Scholar]
- 11.Weldon MM, Smolinski MS, Maroufi A, Hasty BW, Gilliss DL, Boulanger LL, et al. Mercury poisoning associated with a Mexican beauty cream. West J Med. 2000;173(1):15–18. doi: 10.1136/ewjm.173.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong Kong Government Department of Health. Update on mercury poisoning investigation. News Bulletin. 2002 Jan 16; [Google Scholar]
- 13.Li AM, Chan MH, Leung TF, Cheung RC, Lam CW, Fok TF. Mercury intoxication presenting with tics. Arch Dis Child. 2000;83(2):174–175. doi: 10.1136/adc.83.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seedat YK, Simjee AE, Naidoo DV. Letter: Nephrotic syndrome due to cosmetics containing mercury. S Afr Med J. 1974;47(12):506. [PubMed] [Google Scholar]
- 15.Al-Saleh I, Khogali F, Al-Amodi M, El-Doush I, Shinwari N, Al-Baradei R. Histopathological effects of mercury in skin-lightening cream. J Environ Pathol Toxicol Oncol. 2003;22(4):287–299. doi: 10.1615/jenvpathtoxoncol.v22.i4.30. [DOI] [PubMed] [Google Scholar]
- 16.Al-Saleh I, El-Doush I, Shinwari N, Al-Baradei R, Khogali F, Al-Amodi M. Does low mercury containing skin-lightening cream (fair & lovely) affect the kidney, liver, and brain of female mice? Cutan Ocul Toxicol. 2005;24(1):11–29. doi: 10.1081/CUS-200046179. [DOI] [PubMed] [Google Scholar]
- 17.Campbell G, Leitch D, Lewington A, Dargan PI, Baker RJ. Minimal-change nephrotic syndrome due to occupational mercury vapor inhalation. Clinical Nephrology Clin Nephrol. 2009;72(3):216–219. doi: 10.5414/cnp72216. [DOI] [PubMed] [Google Scholar]
- 18.Chan TY. Inorganic mercury poisoning associated with skin-lightening cosmetic products. Clin Toxicol (Phila) 2011;49(10):886–891. doi: 10.3109/15563650.2011.626425. [DOI] [PubMed] [Google Scholar]