Abstract
Background
Circulating-free DNA (cfDNA) is under investigation as a liquid biopsy of cancer for early detection, monitoring disease progression and therapeutic response. This systematic review of the primary cfDNA literature aims to identify and evaluate factors that influence recovery of cfDNA, and to outline evidence-based recommendations for standardization of methods.
Methods
A search of the Ovid and Cochrane databases was undertaken in May 2018 to obtain relevant literature on cfDNA isolation and quantification. Retrieved titles and abstracts were reviewed by two authors. The factors evaluated include choice of specimen type (plasma or serum); time-to-processing of whole blood; blood specimen tube; centrifugation protocol (speed, time, temperature and number of spins); and methods of cfDNA isolation and quantification.
Findings
Of 4,172 articles identified through the database search, 52 proceeded to full-text review and 37 met the criteria for inclusion. A quantitative analysis was not possible, due to significant heterogeneity in methodological approaches between studies. Therefore, included data was tabulated and a textual qualitative synthesis approach was taken.
Interpretation
This is the first systematic review of methodological factors that influence recovery and quantification of cfDNA, enabling recommendations to be made that will support standardization of methodological approaches towards development of blood-based cancer tests.
Keyword: Oncology
1. Introduction
In healthy individuals, circulating-free DNA (cfDNA) originates from apoptosis of nucleated cells and is found at low levels in blood (Stroun et al., 2001) but can increase following exercise (Fatouros et al., 2010). In malignancy, the tumor derived fraction of total cfDNA, termed circulating tumor DNA (ctDNA), can derive from tumor cells by a combination of apoptosis, necrosis and active secretion (Stroun et al. 2001; Jahr et al., 2001), and from disseminated tumor cells in micrometastatic deposits (Shaw et al., 2012; González-Masiá et al., 2013). Numerous studies have demonstrated the potential of genetic and epigenetic alterations in ctDNA as tumor-specific biomarkers to facilitate detection of resistance and relapse, and for prediction of response to treatment (Alix-Panabières and Pantel, 2016; Heitzer et al., 2015; Diaz et al., 2016).
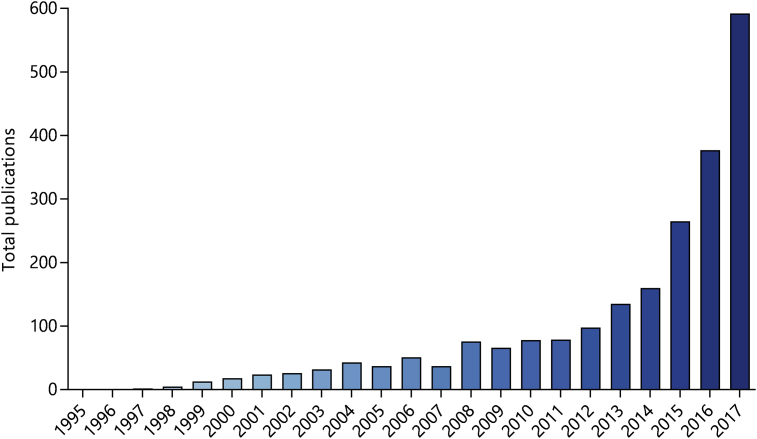
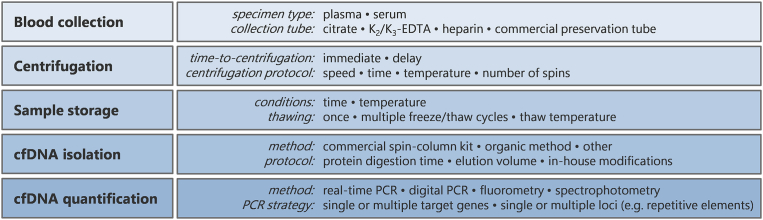
The number of cfDNA-related publications has risen rapidly since the mid-1990s (Fig. 1 and Supplemental Table S1). However, the community is yet to agree on a consensus for the isolation and analysis of cfDNA, thus limiting comparison between studies. This issue was highlighted at the consensus meeting on cfDNA, hosted by the Experimental Cancer Medicine Centre (ECMC) Network and the National Cancer Research Institute Biomarkers and Imaging Clinical Studies Group (UK). It was agreed that a systematic review would be of use to the cfDNA community to identify and evaluate the methodological factors that influence quality and yield of cfDNA (ECMC, 2014). In support of this, there is wide variation in the methods used to isolate plasma from whole blood and to extract cfDNA (Fig. 2). We randomly sampled 50 primary research articles published in 2015 relating to cfDNA in cancer and found deficiencies in reporting of methodology. Sixteen articles provided no information regarding plasma/serum isolation and a further 19 mentioned centrifugation but did not state the speed or number of centrifugation steps. Lastly, 18 articles provided no information on cfDNA quantification prior to downstream analysis (Supplemental Table S2). Therefore, we carried out a systematic review of the cancer cfDNA literature with the aim of providing a series of methodological recommendations to promote optimal and reproducible processing and analysis of cfDNA. After scoping the literature, we determined there was sufficient information to examine the following factors: (1) choice of specimen type (plasma or serum); (2) time-to-processing of whole blood and choice of blood specimen tube; (3) centrifugation speed, number of spins and centrifugation time of whole blood; (4) cfDNA isolation method; and (5) cfDNA quantification method. The search was updated in May 2018 to ensure inclusion of recent publications.
Fig. 1.
Number of publications by year relating to cfDNA in cancer between 1995 and 2017. The search was conducted in PubMed using search terms combined with Boolean operators, as follows: (ccfDNA OR cfDNA OR cfNA OR ctDNA OR cell-free DNA OR cell free DNA OR cell-free serum DNA OR circulating DNA OR circulating free DNA OR circulating-free DNA OR circulating nucleic acids OR circulating tumor DNA OR free DNA OR free tumor DNA OR plasma DNA OR plasma tumor DNA OR ptDNA OR serum DNA OR serum cell-free) AND (cancer OR neoplas* OR tumor). Publications relating to cell-free DNA in non-blood analytes (e.g. urine and ascites) and cell culture media were not included. A yearly breakdown of publication types is presented in Supplemental Table S1.
Fig. 2.
Methodological variables in processing and analysis of cfDNA.
2. Materials and methods
This systematic review was guided by the criteria of the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) (Supplemental Table S3) (Moher et al., 2010).
2.1. Literature search strategy
An initial scoping search in Ovid (Embase and Medline) was carried out to assess for appropriateness, and relevant publications were identified from which keywords were obtained. A comprehensive publication search was conducted in the PubMed, Ovid (Embase and Medline) and Cochrane library databases prior to July 28, 2017 (updated to May 5, 2018) without limits for language, publication type or publication date. Keywords were chosen to reflect the concepts of ‘circulating-free DNA’, ‘cancer’ and ‘methodology’, with provision for alternative spellings, synonyms and abbreviations for circulating-free DNA. Keywords were combined using Boolean operators and translated into database-specific syntax, and were searched in title and abstract only. The final search strategy was as follows: ((ccfDNA OR cfDNA OR cfNA OR ctDNA OR cell-free DNA OR cell free DNA OR cell-free serum DNA OR circulating DNA OR circulating free DNA OR circulating-free DNA OR circulating nucleic acids OR circulating tumo?r DNA OR free DNA OR free tumo?r DNA OR plasma DNA OR plasma tumo?r DNA OR ptDNA OR serum DNA OR serum cell-free) AND (cancer OR neoplas* OR tumo?r) AND (process* OR isolat* OR centrifug* OR spin* OR extract* OR quanti* OR sensitiv* OR specific* OR measur*)). Question marks and asterisks represent optional wildcards and truncations, respectively. As an example, the search strategy for Ovid (Embase and Medline) is shown in Table 1. All returned studies were reviewed by title and abstract by two authors. Additional studies were identified through a manual search of bibliographies in included studies and relevant narrative reviews. Authors of the following publications were contacted by email for further information: Chiminqgi et al., 2007; Holdenrieder et al., 2005; Morgan et al., 2012; Swinkels et al., 2003; and Taback et al., 2004. Conference abstracts were excluded due to insufficient detail for valid assessment.
Table 1.
Literature search strategy for Ovid (Embase and Medline).
| ID | Search |
|---|---|
| 1 | (ccfDNA OR cfDNA OR cfNA OR ctDNA) [kw,ti,ab] |
| 2 | (cell-free DNA OR cell-free serum OR cell free DNA OR cell free serum) [kw,ti,ab] |
| 3 | (circulating DNA OR circulating free DNA OR circulating-free DNA OR circulating nucleic acids OR circulating tumo?r DNA) [kw,ti,ab] |
| 4 | (free DNA OR free tumo?r DNA) [kw,ti,ab] |
| 5 | (plasma DNA OR plasma tumo?r DNA OR ptDNA) [kw,ti,ab] |
| 6 | (serum DNA OR serum cell-free OR serum cell free) [kw,ti,ab] |
| 7 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 |
| 8 | (neoplasms) [sh,ti,ab] |
| 9 | (centrif* OR process* OR spin*) [kw,ti,ab] |
| 10 | (extract* OR isolate*) [kw,ti,ab] |
| 11 | (quanti* OR measure*) [kw,ti,ab] |
| 12 | (sensitiv* OR specific*) [kw,ti,ab] |
| 13 | 9 AND 10 AND 11 AND 12 |
| 14 | 7 AND 8 AND 13 |
ab, abstract word; kw, keyword; ti, title word; sh, subject heading (i.e. MeSH); ?, wildcard; *, truncation.
2.2. Selection criteria
Studies investigating the effect of methodological factors on quality and/or yield of cfDNA proceeded to full-text review. The criteria for inclusion were as follows: (1) provided a direct comparison between two or more protocols in processing/analysis of circulating-free DNA; (2) n ≥ 5 for any comparative study; and (3) absolute values stated (or available through author contact) for quantitative data. The criteria for exclusion were as follows: (1) n < 5 for any comparative study; (2) only pregnant female subjects; (3) qualitative data or insufficient data available (despite further information provided by author contact) for valid analysis; (4) specific research questions not addressed; (5) unsuitable publication types, such as commentaries, editorials and review articles without data; and (6) duplicate publication. All included studies (n = 36) and 320 randomly selected publications, approximately 10% of the total, were verified for eligibility by a second independent reviewer (LJM). Included studies were also reviewed to check for additional references not generated by the data mining process.
2.3. Data extraction and analysis
The following data were independently extracted into an electronic table by two reviewers: first author, year of publication, number of subjects in each group, health status of subjects, gender of subjects (if stated), cfDNA isolation method, cfDNA quantification method, technical details of protocol and mean (with standard deviation and/or range if stated) of any measurements. Where data were presented graphically but not specified numerically, individual data points were estimated by two observers by interpolating graphs. A quantitative analysis was not deemed possible, due to significant heterogeneity in methodological approaches between studies. Therefore, included data was tabulated and a textual qualitative synthesis approach was taken.
3. Results
3.1. Literature search and study characteristics
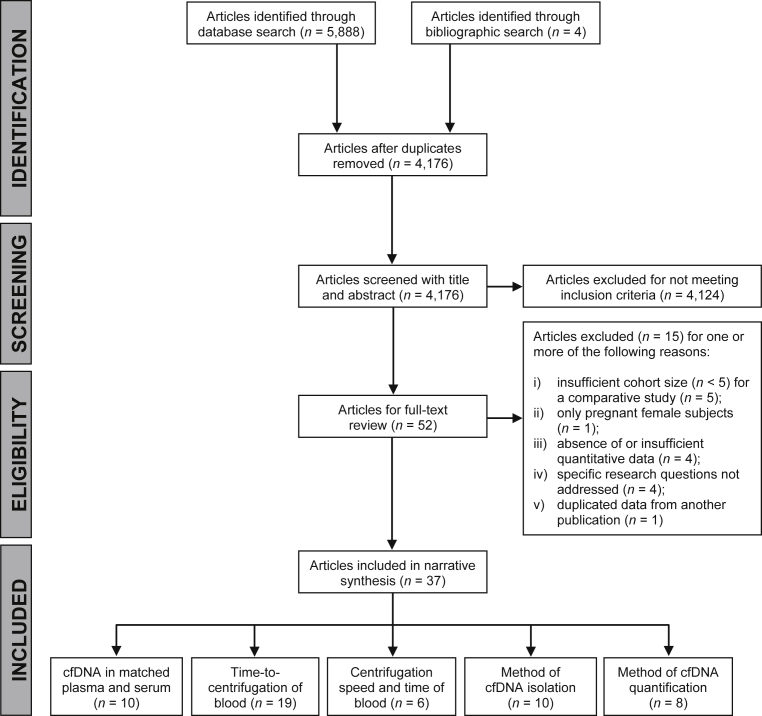
The literature search and inclusion/exclusion process is shown in Fig. 3. A total of 5,888 articles were identified through database searching and 4,172 articles remained after duplicated articles were removed. All articles were screened with title and abstract against the inclusion and exclusion criteria, and 48 articles proceeded to full-text review. The review process identified a further 4 articles. Of the 52 articles that were reviewed by full-text, 15 were excluded for one or more of the following reasons: (i) insufficient cohort size (n < 5) for a comparative study (n = 5); (ii) only pregnant female subjects (n = 1); (iii) insufficient quantitative data (n = 4); (iv) specific research questions not addressed (n = 4); and (v) duplicated data from another publication (n = 1). Studies with only pregnant female subjects were not included, as the biological characteristics of fetal cfDNA may not be comparable to those for tumor-derived cfDNA. Due to the broad search strategy adopted, a large number of publications were retrieved.
Fig. 3.
PRISMA flow diagram of literature search and inclusion/exclusion process.
A total of 37 articles met all inclusion criteria and no exclusion criteria, and were included in the narrative synthesis (Table 2). Studies were published between 2002 and 2018, and all articles were written in English. Twelve studies recruited healthy subjects only, 17 recruited patients with cancer only, and 6 recruited both. One study recruited patients with suspected cancer and one study combined patients with different health statuses into a single cohort for analysis. Cohort size ranged between 5 and 156 subjects.
Table 2.
Summary characteristics of included studies.
| Author (et al) | Year | Subjects | cfDNA |
|
|---|---|---|---|---|
| Isolation method | Quantification method | |||
| Barták | 2018 | Cancer; benign; healthy | Multiple commercial kits | FL |
| Board | 2008 | Cancer; healthy | QIAamp® Virus Spin Kit | qPCR |
| Chan | 2005 | Cancer | QIAamp® DBM Kit | qPCR |
| Chiminqgi | 2007 | Cancer; healthy | QIAamp® Blood Kit | qPCR, FL |
| Denis | 2015 | Cancer | iPrep™ PureLink® Virus Kit | qPCR |
| Devonshire | 2014 | Healthy | Multiple commercial kits | dPCR, qPCR |
| Diaz | 2016 | Healthy | QIAamp® DNA Blood Mini Kit | qPCR |
| Fleischhacker | 2011 | Cancer; benign disease | Multiple commercial kits | qPCR |
| Fong | 2009 | Cancer | Multiple commercial kits | qPCR, FL |
| Garcia | 2017 | Cancer | QIAamp® CNA Kit | qPCR, FL |
| Jung | 2003 | Healthy | NucleoSpin® Blood Kit | qPCR |
| Kadam | 2012 | Cancer | QIAamp® DBM Kit | qPCR |
| Kang | 2016 | Cancer | QIAamp® CNA Kit | dPCR |
| Kloten | 2017 | Cancer; healthy | Multiple commercial kits | dPCR |
| Lam | 2004 | Healthy | QIAamp® DBM Kit | qPCR |
| Lee | 2001 | Healthy | HIV Monitor Assay | qPCR |
| Lui (a) | 2002 | Healthy | QIAamp® Blood Kit | qPCR |
| Lui (b) | 2002 | Healthy | QIAamp® Blood Kit | qPCR |
| Mauger | 2015 | Cancer | Multiple commercial kits | qPCR, FL |
| Markus | 2018 | Healthy | QIAamp® CNA Kit | dPCR |
| Mazurek | 2013 | Cancer | Multiple commercial kits | qPCR |
| Morgan | 2012 | Cancer | QIAamp® CNA Kit | qPCR |
| Norton | 2013 | Healthy | QIAamp® CNA Kit | dPCR |
| Page | 2013 | Healthy | Multiple commercial kits | qPCR |
| Park | 2012 | Cancer | QIAamp® UltraSens Virus Kit | qPCR |
| Parpart-Li | 2017 | Cancer | QIAamp® CNA Kit | dPCR, FL |
| Ramachandran | 2013 | Suspected cancer | QIAamp® UltraSens Virus Kit | qPCR, FL, SP |
| Rothwell | 2015 | Healthy | QIAamp® CNA Kit | qPCR |
| Sherwood | 2016 | Cancer | QIAamp® CNA Kit | qPCR |
| Swinkels | 2003 | Healthy | QIAamp® Blood Kit | qPCR |
| Szpechcinski | 2008 | Cancer | QIAamp® DBM Kit | qPCR, FL |
| Taback | 2004 | Cancer | QIAamp® extraction Kit | FL |
| Thijssen | 2002 | Cancer; healthy | QIAquick® PCR Purification Kit | qPCR, FL |
| Toro | 2015 | Cancer | QIAamp® CNA Kit | dPCR |
| Umetani | 2006 | Cancer | None | qPCR |
| van Dessel | 2017 | Cancer | QIAamp® CNA Kit | FL |
| Van Ginkel | 2017 | Cancer; healthy | Multiple commercial kits | FL |
CNA, Circulating Nucleic Acid; DBM, DNA Blood Mini; dPCR, digital polymerase chain reaction; FL, fluorometry; qPCR, quantitative real-time polymerase chain reaction; SP, spectrophotometry.
Ten studies investigated cfDNA levels in matched plasma and serum, 19 studies investigated the effect of time delay post-venepuncture on cfDNA levels in plasma using different collection tubes, 6 studies investigated the effect of centrifugation speed and time on cfDNA levels in plasma, 10 studies compared efficiency of cfDNA extraction between different commercially available kits, and 8 studies compared methods of cfDNA quantification. Several different methods were used to quantify cfDNA. Polymerase chain reaction (PCR) was the most commonly used method, with 27 studies using quantitative real-time PCR (qPCR) and 7 studies using digital PCR (dPCR). Ten studies used a fluorescent DNA-binding dye (fluorometry), and one study used ultraviolet spectrophotometry. Eight studies utilized two or more quantification methods. The cfDNA concentration was expressed as either ng/mL or haploid genome equivalents (copies)/mL. Two studies reported total amounts per plasma/serum sample, one study used cell equivalents/mL and one study used copies/μL. To permit a direct comparison between studies in this review, haploid genome equivalents (copies) were multiplied by 3.3 × 10−3 to give ng. Lastly two qPCR-based studies reported cycle threshold (CT) values only which could not be interpolated to give the cfDNA concentration.
3.2. Choice of specimen type (plasma or serum)
Ten studies investigated yield of cfDNA from matched plasma and serum samples. Total cfDNA levels were between 1.63 and 11.09-fold higher in serum than in plasma of healthy controls and patients with cancer (Table 3). Two studies that met the inclusion criteria also assessed integrity of cfDNA in plasma and serum (Chan et al., 2005; Holdenrieder et al., 2008). Using a ratio of 201 bp/105 bp amplicons, cfDNA isolated from serum was found to have significantly higher integrity than that from plasma (50% vs 33%, respectively). This difference in integrity between specimens was greater with a 356 bp/105 bp ratio, at 26% vs 12% for serum and plasma, respectively, thus supporting the presence of high molecular weight DNA from hematopoietic cells in serum (Chan et al., 2005). Although KRAS allelic frequencies were much lower than expected in serum samples compared to plasma (Kloten et al., 2017), serum was still a useful source for recovery of ctDNA, as plasma and serum were comparable in terms of sensitivity (31% and 25%, respectively) and specificity (97% and 100%, respectively) (Morgan et al., 2012).
Table 3.
Extracted data from studies addressing blood specimen type. Where data were expressed as copies or haploid genome equivalents per mL, values were multiplied by 0.0033 ng to reach ng/mL. †, median values. *, values were estimated by reading off a graph, #, data for two different extraction methods were combined in the original paper.
| Author (et al) | Subjects (n) | Isolation method | Quantification method | Mean serum (ng/mL) | Mean plasma (ng/mL) | Ratio serum: plasma |
|---|---|---|---|---|---|---|
| Board | Healthy (10) | QIAamp® Virus Spin Kit | qPCR (AAT, 77 bp) | 24.65 | 5.07 | 4.86 |
| Chan | Healthy (27) | QIAamp® DBM Kit | qPCR (LEP, 105 bp) | 3.2* | 2.0* | 1.63 |
| Jung | Healthy (10) | NucleoSpin® Blood Kit | qPCR (HBB, 110 bp) | 8.3* | 4.0* | 2.08 |
| Kloten | Healthy (8) | Maxwell RSC | qPCR (KRAS) | 2300 | 450 | 4.18 |
| Cancer (50) | ccfDNA Plasma kit and QIAamp® CNA Kit | |||||
| Lee | Healthy (6) | HIV Monitor Assay | qPCR (HLA-DQA1, 242 bp) | 33* | 9.9* | 3.33 |
| Park | Cancer (130) | QIAamp® UltraSens Virus Kit | qPCR (ALU repeat, 81 bp) | 755.9 | 68.7 | 11.00 |
| Taback | Cancer (10) | QIAamp® Extraction Kit | PicoGreen® | 1059† | 259† | 4.09 |
| Thijssen | Healthy (28) | QIAquick® PCR Purification Kit | PicoGreen® | 12.9 | 4.8 | 2.69 |
| Cancer (26) | 47.6 | 10.6 | 4.49 | |||
| Umetani | Cancer (24) | None | qPCR (ALU repeat, 115 bp) | 970 | 180 | 5.39 |
| van Ginkel | Healthy (25) | QIAamp® CNA Kit and MagNA Pure LC DNA Isolation Kit | dPCR (BRAF, 91 bp)# | 673.2 | 60.72 | 11.09 |
3.3. Time-to-centrifugation of blood and blood specimen tube
The effect of time delay between venepuncture and centrifugation of blood on cfDNA levels has been reported by several groups, with the general observation that cfDNA levels increase over time prior to centrifugation of EDTA-stabilized blood (Page et al., 2011). To overcome the need for immediate blood processing, specialized blood collection tubes are available that contain a preservative agent to prevent lysis of peripheral leukocytes and permit storage of whole blood at room temperature for several days prior to centrifugation; these include the Cell-Free DNA BCT® (Streck) and PAXgene® Blood DNA Tube (Qiagen). Nineteen studies investigated the effect of time-to-centrifugation of blood on levels of cfDNA over time points ranging up to 14 days (Table 4). Eight studies investigated EDTA tubes only, 2 studies investigated a single specialized tube, 8 studies compared EDTA tubes with one or more specialized tubes (Cell-Free DNA BCT®, CellSave and PAXgene® Blood DNA Tube), and one study compared two specialized tubes. Table 4 summarizes the included studies and shows the stability of blood, which was defined as the longest time post-venepuncture at which cfDNA levels remained stable relative to at baseline levels (i.e. immediate centrifugation of blood), as determined by the Student's t-test on paired plasma samples.
Table 4.
Extracted data from studies addressing time-to-processing of blood. Stability is defined as the longest time point post-venepuncture in which cfDNA levels were not significantly elevated relative to at baseline (i.e. immediate processing of blood). All blood was stored at room temperature prior to processing unless indicated with a cross (†), indicating separate experiments were conducted on samples stored at both room temperature and 4 °C. d, days.
| Author (et al) | Subjects (n) | Isolation method | Quantification method | Tube type | Time points (hours) | Stability (hours) |
|---|---|---|---|---|---|---|
| Board | Healthy (10) | QIAamp® Virus Spin Kit | qPCR (AAT, 77 bp) | EDTA | 0, 2, 4, 8, 24 | 24 |
| Chan | Healthy (27) | QIAamp® DNA Blood Mini Kit | qPCR (LEP, 105 bp) | EDTA† | 0, 6, 24 | 6† |
| Garcia | Cancer (7) | QIAamp® Circulating Nucleic Acid Kit | qPCR (hTERT, 62 bp) | EDTA† | 4, 24 | 24 |
| Jung | Healthy (10) | NucleoSpin® Blood Kit | qPCR (HBB, 110 bp) | EDTA† | 0, 2, 4, 8 | 8† |
| Lam | Healthy (10) | QIAamp® Blood Kit | qPCR (HBB, 102 bp) | EDTA | 0, 2, 6, 24 | 6 |
| Lui | Healthy (8) | QIAamp® Blood Kit | qPCR (HBB, 102 bp) | EDTA | 0, 3, 6 | 6 |
| Page | Healthy (5) | QIAamp® DNA Blood Mini Kit | qPCR (GAPDH, 96 bp) | EDTA | 2, 6 | 6 |
| Denis | BRAFV600E-positive Melanoma (9) | iPrep™ PureLink® Virus Kit | therascreen®BRAF RGQ PCR Kit | EDTA | 2, 4d, 7d, 10d | 2 |
| Cell-Free DNA BCT® | 2, 4d, 7d, 10d | 10d | ||||
| Diaz | Healthy (60) | QIAamp® DNA Blood Mini Kit | qPCR (LINE-1, 96 bp) | Cell-Free DNA BCT® | 2, 3d, 5d | 5d |
| Kadam | Metastatic cancer (10) | QIAamp® DNA Blood Mini Kit | qPCR (ACTB, 182 bp) | EDTA | 0, 24 | 24 |
| NucleoSpin® Plasma XS Kit | Cell-Free DNA BCT® | 0, 24 | 24 | |||
| EDTA | 0, 24 | 0 | ||||
| Cell-Free DNA BCT® | 0, 24 | 0 | ||||
| Kang | Metastatic cancer (6) | QIAamp® Circulating Nucleic Acid Kit | dPCR (PIK3CA and TP53) | EDTA† | 0, 6, 48 | 6† |
| Cell-Free DNA BCT®† | 0, 6, 48 | 48† | ||||
| CellSave | 0, 6, 48 | 48 | ||||
| Markus | Healthy (23) | QIAamp® Circulating Nucleic Acid Kit | dPCR (9 single-copy loci) | Cell-Free DNA BCT®† | 24, 48 | 48 |
| Norton | Healthy (12) | QIAamp® Circulating Nucleic Acid Kit | qPCR (HBB, 136 bp) | EDTA | 0, 24, 48, 72, 7d, 14d | 24 |
| Cell-Free DNA BCT® | 0, 24, 48, 72, 7d, 14d | 7d | ||||
| Parpart-Li | Cancer (9) | QIAamp® Circulating Nucleic Acid Kit | dPCR (proprietary BRAF, EGFR and KRAS assays) | EDTA | 0, 24, 72, 5d, 7d | 0 |
| Cell-Free DNA BCT® | 0, 24, 72, 5d, 7d | 7d | ||||
| Rothwell | Healthy (20) | QIAamp® Circulating Nucleic Acid Kit | qPCR (RPPH1, 87 bp) | EDTA | 4, 4d | 4 |
| CellSave | 4, 4d | 4d | ||||
| Sherwood | Lung cancer (20) | QIAamp® Circulating Nucleic Acid Kit | qPCR (RPPH1, 87 bp) | EDTA | 2, 72 | 2 |
| Cell-Free DNA BCT® | 2, 72 | 2 | ||||
| Toro | Metastatic breast cancer (9) | QIAamp® Circulating Nucleic Acid Kit | dPCR (proprietary PIK3CA assays) | Cell-Free DNA BCT® | 24, 7d | 7d |
| PAXgene® Blood DNA Tube | 24, 7d | 24 | ||||
| van Dessel | Cancer (16) | QIAamp® Circulating Nucleic Acid Kit | qPCR (β actin, 136 bp) | EDTA | 1, 24, 96 | 24 |
| Cell-Free DNA BCT® | 1, 24, 96 | 96 | ||||
| CellSave | 1, 24, 96 | 96 | ||||
| van Ginkel | Healthy (6) | Quick-cfDNA™ serum & plasma Kit (Zymo Research) | dPCR (RRP30, 98 bp) | EDTA | 0, 3, 6, 24 | 24 |
Of the studies that investigated EDTA tubes, 10 studies included a baseline of 0 hours (i.e. immediate centrifugation) and the minimum stability was 6 hours in these studies. Two studies did not assess stability beyond 6 hours and in both studies cfDNA was stable for this time. Concerning longer times to processing, 6 of 9 studies that included a 24 hour time point reported stability for this duration.
Ten studies used the Cell-Free DNA BCT®, 2 studies used the CellSave tube (CellSearch) and 1 study used the PAXgene® Blood DNA Tube. The Cell-Free DNA BCT® is reported to stabilize cfDNA for up to 14 days post-venepuncture. In 8 of the 10 studies that used the Cell-Free DNA BCT®, cfDNA was stable up to the last time point (24 hours–10 days). One study showed a significant increase in cfDNA levels after 72 hours (relative to 2 hours), and another study demonstrated stability for up to 7 days but not 14 days. Lastly, one study measured ctDNA, where there was a trend at day 3 towards a decrease in ctDNA levels in both BCT® and EDTA tubes (Parpart-Li et al., 2017).
In the 2 studies that used CellSave tubes, cfDNA was stable up to the last time point (48 hours and 4 days). One study used the PAXgene® Blood DNA Tube and assessed cfDNA levels after 24 hours and 7 days. Here cfDNA levels were significantly elevated after 7 days.
3.4. Centrifugation protocol: speed, time, temperature and number of spins
Six studies investigated the effect of centrifugation speed (range, 400g–16,000g) on cfDNA recovery from EDTA plasma, and 5 of these studies also investigated the addition of a second centrifugation step (Table 5). A second centrifugation step had no significant effect on DNA yield apart from in one study where this was associated with a significant reduction, suggesting that differences may be due to removal of plasma without disturbing the buffy layer after the first spin. Lui et al. (2002) observed no significant differences in DNA yield with single centrifugations of 10 minutes at 400g, 800g, 1,500g, 3,000g and 16,000g. Similarly, Page et al. (2013) reported no significant difference in DNA yields at 1,000g, 2,000g and 10,000g for 10 minutes. However, Swinkels et al. (2003) reported that after an initial centrifugation at 800g for 10 minutes, a second centrifugation step at 16,000g for 10 minutes significantly reduced DNA yield. Interestingly, Sherwood et al. (2016) found that double centrifugation at 2,000g for 10 mins did not decrease DNA yield relative to single centrifugation of blood processed 2 hours post-collection, but the second step significantly reduced DNA yield in blood processed 72 hours post-collection. This suggests that contaminating nuclear DNA was present at 72 hours due to lysis of white blood cells that was not efficiently pelleted by a single centrifugation step. Lastly, van Ginkel et al. (2017) also incorporated a freeze thaw step between the first and second centrifugation steps, but this did not affect recovery.
Table 5.
Extracted data from studies addressing centrifugation speed and number of spins. ×2 indicates two centrifugations at the given speed, with removal of plasma from the cellular fraction or debris before the second centrifugation. *second centrifugation was conducted following freezing, then post-thawing of sample.
| Author (et al) | Subjects (n) | Isolation method | PCR assay | First speed (g) | Second speed (g) | Effect on cfDNA yield |
|---|---|---|---|---|---|---|
| Lui (a) | Healthy (14) | QIAamp® Blood Kit | HBB, 102 bp | 3,000 | 16,000 | No significance |
| (3,000) ×2 | 16,000 | |||||
| (3,000) ×2 | (16,000) ×2 | |||||
| Page | Healthy (5) | QIAamp® DNA Blood Mini Kit | GAPDH, 96 bp | 1,000 | 1,000 | No significance |
| 1,000 | 2,000 | |||||
| 1,000 | 10,000 | |||||
| Sherwood | Healthy (5) | QIAamp® Circulating Nucleic Acid Kit | RPPH1, 87 bp | 2,000 | None | No significance |
| 2,000 | 2,000 | |||||
| Lui (b) | Healthy (10) | QIAamp® Blood Kit | HBB, 102 bp | 400 | None | No significance |
| 800 | None | |||||
| 1,500 | None | |||||
| 3,000 | None | |||||
| 16,000 | None | |||||
| Swinkels | Healthy (10) | QIAamp® Blood Kit | HBB, 81 bp | 800 | None | Significantly lower yield after second spin (p = 0.01) |
| 800 | 16,000 | |||||
| van Ginkel | Healthy (6) | Quick-cfDNA™ serum & plasma Kit (Zymo Research) | dPCR (RRP30, 98 bp) | 800 | None | |
| 800 | 11,000 | No significance | ||||
| 800 | 11,000* |
Four studies (Chan et al., 2005; Garcia et al., 2017; Jung et al., 2003; Kang et al., 2016) investigated the effects of storage temperature of unprocessed blood in EDTA tubes and reported no significant influence of temperature on resulting cfDNA levels.
3.5. Methods of cfDNA isolation and quantification
Methods of cfDNA isolation reported varied from in-house protocols to the use of commercially available kits. Of these, the QIAamp® Circulating Nucleic Acid Kit (Qiagen) was most widely used (Tables 6 and 7). Page et al. (2013) showed that this kit led to the highest yield of total cfDNA. qPCR was the most widely used method for cfDNA quantification, while fluorometry (e.g. PicoGreen® and Qubit®) was also widely used as a cost-effective, rapid and simple method. Ramachandran et al. (2013) also compared qPCR with fluorometry and UV spectrophotometry (Table 7). Total cfDNA levels were significantly correlated in all studies that compared qPCR and fluorometry (PicoGreen® or Qubit®) (r ≥ 0.72; P < 0.05), except for in one study (Mauger et al., 2015). Ramachandran et al. (2013) reported that cfDNA levels quantified by qPCR were not significantly correlated with UV spectrophotometry (r = 0.23) (not included in Table 7) and van Ginkel showed positive correlation between dPCR and fluorometry by Qubit (r = 0.98; P < 0.0001) but not UV spectrophotometry (Nanodrop).
Table 6.
Summary of studies comparing cfDNA isolation methods. *pooled plasma samples. FL, fluorometry.
| Author (et al) | Subjects (n) | Isolation methods | Quantification method |
|---|---|---|---|
| Board | Healthy (5) | QIAamp® Mini Blood Kit | qPCR (AAT, 77 bp) |
| GENFIND™ Blood & Serum Genomic DNA Isolation Kit | |||
| QIAamp® Virus Spin Kit | |||
| ChargeSwitch® gDNA Serum Kit | |||
| Devonshire | Healthy (5)* | QIAamp® Circulating Nucleic Acid Kit | qPCR (TERT, 79 bp; ALUJ, various length) |
| NucleoSpin® Plasma XS Kit | |||
| FitAmp® Plasma/Serum DNA Isolation Kit | |||
| QIAamp® DNA Blood Mini Kit | |||
| Fleischhacker | Cancer (36), benign disease (8) | QIAamp® DNA Blood Mini Kit | qPCR (ERV, 135 bp; GAPDH, 92 bp; HBB, 101 bp) |
| NucleoSpin® Plasma F Kit | |||
| MagaPure® LC DNA Isolation Kit | |||
| Fong | Healthy (5)* | QIAamp® DNA Blood Midi Kit | FL, qPCR (CHD1, 68 bp, ACTB, 115 bp) |
| Puregene DNA Purification System Cell and Tissue Kit | |||
| ChargeSwitch® gDNA Serum Kit | |||
| ZR Serum DNA Kit™ | |||
| Kloten | Healthy (8) Cancer (50) |
Maxwell RSC | qPCR (KRAS) |
| ccfDNA Plasma kKt and QIAamp | |||
| Circulating Free Nucleic Acid Kit | |||
| Mauger | Cancer (4), healthy (1) | QIAamp® Circulating Nucleic Acid Kit | qPCR (KPN, 55 bp) |
| Norgen Plasma/Serum Cell-Free Circulating DNA | |||
| Norgen Plasma/Serum Circulating DNA Purification Mini Kit | |||
| NucleoSpin® Plasma XS Kit | |||
| Chemagic NA Extraction Kit | |||
| Mazurek | Cancer (10) | QIAamp® DNA Blood Mini Kit | qPCR (TERT, 98 bp) |
| QIAamp® Circulating Nucleic Acid Kit | |||
| Sherlock AX kit | |||
| Page | Healthy (1) | QIAamp® DNA Blood Mini Kit QIAamp® Circulating Nucleic Acid Kit NucleoSpin® Plasma XS Kit FitAmp® Plasma/Serum DNA Isolation Kit |
qPCR (GAPDH, 96 bp, ACTBL2 67 bp and HPRT1 64 bp) |
| Sorber | Cancer (10) | QIAamp® Circulating Nucleic Acid Kit | FL |
| PME free-circulating DNA Extraction Kit | |||
| Maxwell® RSC ccfDNA Plasma Kit | |||
| EpiQuikTM Circulating Cell-Free DNA Isolation Kit | |||
| NEXTprep-MagTM cfDNA Isolation Kit v1 | |||
| NEXTprep-MagTM cfDNA Isolation Kit v2 | |||
| van Ginkel | Healthy (4) | QIAamp® Circulating Nucleic Acid Kit | dPCR (BRAF, 91 bp) |
| PME free-circulating DNA Extraction Kit | |||
| Quick-cfDNA™ Serum & Plasma Kit | |||
Table 7.
Extracted data from studies comparing cfDNA quantitation method. *quantitative data not available.
| Author (et al) | Subjects (n) | Isolation method | Quantification methods | Results |
|---|---|---|---|---|
| Chiminqgi | Healthy (58) Colorectal (87) and prostate (93) cancer |
QIAamp® Blood Kit | PicoGreen® dsDNA Kit (qPCR HBB, 102 bp; PPIA, 105 bp) | PicoGreen correlated with qPCR targeting HBB (r = 0.915) and PPIA (r = 0.81). |
| Devonshire | Healthy (17) | QIAamp® Circulating Nucleic Acid Kit | qPCR and dPCR (ERV3, 135 bp; TERT, 79 bp, RPPH1, 64 bp) | Good agreement between the qPCR and droplet dPCR measurements of copy number* |
| Garcia | Cancer (7) | QIAamp® Circulating Nucleic Acid Kit | qPCR (hTERT, 62 bp), FL | Good agreement between qPCR and Qubit FL (r2 = 0.85; P < 0.00) |
| Mauger | Healthy (2) Colon (2), pancreatic (2), lung (2) and breast (2) cancers |
Norgen Plasma/Serum Circulating DNA Purification Mini Kit QIAamp® Circulating Nucleic Acid Kit |
Qubit® dsDNA HS assay; qPCR (DHFRP2, 102 bp; KPN, 102 bp) | Qubit did not correlate with qPCR assays targeting KPN (r = 0.04; P = 0.91) and DHFRP2 (r = 0.14; P = 0.70). Qubit correlated with qPCR assays targeting KPN (r = 0.75; P = 0.01) and DHFRP2 (r = 0.72; P = 0.02). |
| Ramachandran | Healthy (156) and prostate cancer (85) | QIAamp® UltraSens Virus Kit | PicoGreen® dsDNA Kit; qPCR (GSTP1, 95 bp); UV spectrophotometry | PicoGreen correlated with qPCR (r = 0.8552; P < 0.0001). UV absorbance weakly correlated with PicoGreen (r = 0.2376) and qPCR (r = 0.2274). |
| Szpechcinski | Lung cancer (10) | QIAamp® DNA Blood Midi Kit | PicoGreen® dsDNA Kit; qPCR (ACTB, 99 bp) by SYBR® Green and TaqMan® | PicoGreen correlated with SYBR® Green qPCR (r = 0.87; P < 0.0001) and TaqMan® qPCR (r = 0.94; P < 0.0001). SYBR® Green and TaqMan® qPCR were correlated (r = 0.96; P < 0.0001). |
| Thijssen | Colorectal cancer (26) | Isopropanol precipitation followed by QIAquick® PCR Purification Kit | PicoGreen®; dsDNA Kit qPCR (ALB, 81 bp) | PicoGreen correlated with qPCR (P < 0.05). |
| van Ginkel | Healthy (33 – Nanodrop) and Healthy (78 – Qubit) | Multiple commercial kits | dPCR vs Nanodrop and Qubit respectively | dPCR positively correlated with Qubit (r2 = 0.98; P < 0.0001). Nanodrop did not correlate with dPCR (r2 = 0.04; P = 0.81). |
4. Discussion
We systematically reviewed the cfDNA literature in order to investigate the influence of preanalytical and analytical factors on quality and/or yield of cfDNA for subsequent molecular analysis of ctDNA. Of 4,176 articles retrieved by database and bibliography searching, 37 articles were identified that met all inclusion criteria and were included in the review. Given the heterogeneity observed across the included studies, we took a narrative approach to data synthesis and tabulated the relevant data to examine the following factors: (1) choice of specimen type (plasma or serum); (2) time-to-processing of whole blood and choice of blood specimen tube; (3) centrifugation speed, time, temperature and number of spins of whole blood; (4) cfDNA isolation method; and (5) cfDNA quantification method. Table 8 summarizes the evidence-based methodological recommendations to promote best practice in processing and analysis of cfDNA.
Table 8.
Evidence-based methodological recommendations for processing and analysis of cfDNA. *insufficient data available to comment on other methods (e.g. digital PCR).
| Methodological factor | Recommendation | Justification |
|---|---|---|
| Blood specimen type | Plasma for analytical techniques that are sensitive to a wild-type DNA background; plasma or serum for all other techniques. | cfDNA levels in serum appear significantly greater than in plasma due to contamination from genomic DNA. |
| Storage conditions of blood and time to processing | Store EDTA blood at either room temperature or 4 °C and process within 6 hours. Specialist tubes should adhere to manufacturers' guidelines. | Storage of blood at room temperature does not influence cfDNA yield relative to storage at room temperature. cfDNA levels increase in EDTA blood processed after 6 hours. |
| Centrifugation speed and time of blood | Spin blood and then re-spin plasma prior to storage for cfDNA isolation (double spin). Centrifugation speed and temperature (room temperature or 4 °C) is not critical. | Plasma can be contaminated with cells from the buffy layer. The second spin helps to minimize the potential for contamination of plasma with cells from the buffy layer. |
| Method of cfDNA isolation and quantification | Follow manufacturers recommendation for cfDNA isolation. Quantify by qPCR or fluorometry. |
Quantification of cfDNA shows good agreement by qPCR, dPCR and fluorometry.* |
Ten of the included studies assessed cfDNA levels in paired plasma and serum samples from healthy subjects and/or patients with cancer. All 10 studies (Table 3) reported a significant increase in cfDNA levels in serum relative to plasma, likely due to lysis of white blood cells in serum during clotting. In support of this, one study also compared DNA integrity, which was higher in serum than plasma, confirming likely release of DNA from white blood cells (Chan et al., 2005). Accordingly, use of plasma is preferable for applications where detection of tumor-specific alterations may be masked by extraneous wild-type DNA, and the limit of detection of a specific variant may be compromised.
Immediate processing of blood by centrifugation may not always be possible in the clinical setting; for example, a centrifuge may not be available on-site or blood samples may be taken over a period of time during a clinic. Consistent with the notion of DNA contamination in serum, the included studies reported a general increase in cfDNA levels over time in unprocessed serum and EDTA blood, albeit at a slower rate. There was significant heterogeneity in study design, with studies measuring cfDNA levels at intervals of several hours to several days, and with some studies omitting a baseline time point (i.e. immediate processing).
In this review, the term ‘stability’ is used to refer to the longest time point post-venepuncture in which cfDNA levels did not significantly change relative to at baseline (i.e. the first time point in each study). Nineteen studies investigated the effect of a delay in blood processing on yield of cfDNA from plasma. Nine studies used ETDA tubes only, 8 studies compared EDTA tubes with one or more specialized cfDNA preservation tubes (Cell-Free DNA BCT®, CellSave and PAXgene® Blood DNA Tube) and 1 study compared two specialized tubes. All studies using EDTA tubes reported stability of cfDNA for at least 6 hours, and up to 24 hours in several studies (Board et al., 2008; Kadam et al., 2012; Norton et al., 2013; van Dessel et al., 2017; van Ginkel et al., 2017). Several studies (Denis et al., 2015; Rothwell et al., 2016; Sherwood et al., 2016; Toro et al., 2015; van Dessel et al., 2017) did not process blood immediately, instead using 1, 2 or 4 hours as the first time point. However, based on data from the 10 studies that processed blood immediately, it was unlikely that cfDNA levels increased in blood over 1, 2 and 4 hours post-venepuncture. Hence, up to 4 hour time points were considered a suitable baseline. Four studies stored paired blood samples at room temperature and 4 °C, but did not observe any significant influence of temperature on cfDNA yield. Based on the minimum period of cfDNA stability reported across these studies, it is preferable that EDTA blood is processed ideally within 6 hours of venepuncture and stored either at room temperature or 4 °C during this time. While the Cell-Free DNA BCT® is reported to stabilize blood for up to 14 days post-venepuncture, only one study in this review included time points that extended to 14 days; and blood was reported to be stable for up to 7 days, but not 14 days. Another study reported stability for up to 10 days and the other studies that assessed the Cell-Free DNA BCT® reported stability up to the last time point (ranging from 24 hours to 7 days). Seven days is therefore a sensible maximum time to processing for samples from Cell-Free DNA BCT® tubes.
Protocols for isolation of plasma from whole blood must ensure the resultant plasma is completely acellular to prevent contamination with genomic DNA. This review included 6 studies that compared the effect of centrifugation speed (range, 400–16,000g) and the number of centrifugation steps (1–4) on cfDNA yield from plasma. The use of a second centrifugation step is common practice due to possible disturbance of cells in the buffy layer on removal of plasma. This is supported by one study that used a second centrifugation step of 16,000g after a lower 800g first step that reported a significant decrease in cfDNA yield relative to the single step alone (P = 0.01) (Swinkels et al., 2003). A double-centrifugation protocol is desirable to minimize the potential for contamination of plasma with cells in the buffy layer such that carry over of cells from the first centrifugation will be pelleted in the second centrifugation. However, centrifugation speed and temperature are not critical as these did not influence cfDNA recovery.
Methods of cfDNA isolation vary with different commercially available kits. All require adherence to the protocol to minimize variability in recovery of cfDNA. There are also a number of validated automated platforms available, which minimise hands-on time and should aid in standardization, although none were tested in the studies evaluated in this review. Among the methods analyzed in these studies, the Qiagen QIAamp® CNA Kit and DNA Blood Mini Kit were the most widely used methods. Quantification of cfDNA was predominantly carried out by either qPCR or fluorometry, which showed good agreement. Where qPCR is not available, fluorometry is a quick and cost-effective method of cfDNA quantification, within the limits of the particular assay. An advantage of PCR-based quantification is that it can help to assess the likelihood of successful downstream analysis by NGS, qPCR or digital PCR (dPCR).
Based on this systematic review, we have summarized evidence-based methodological recommendations to promote best practice in processing and analysis of cfDNA (Table 8). This review is therefore an important step in enabling research groups to increase comparability between cfDNA analyses, helping to facilitate translation of cfDNA into multi-center clinical trials as a step towards implementation in the clinic. To ensure rapid dissemination of the results and to reach an analytical consensus in the field, the use of uniform units should also be considered for adoption by the scientific community.
Declarations
Author contribution statement
Jacqui Shaw, Ricky Trigg, Luke Martinson, Sonya Parpart-Li: Conceived and designed the analysis; Analyzed and interpreted the data; Contributed analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Experimental Cancer Medicine Centre Network (ECMC, 2016). Ricky Trigg was supported by an MRC PhD Studentship. Luke Martinson was supported by a Cancer Research UK PhD studentship.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Alix-Panabières C., Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016 May;6(5):479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- Barták B.K., Kalmár A., Galamb O., Wichmann B., Nagy Z.B., Tulassay Z., Dank M., Igaz P., Molnár B. Blood collection and cell-free DNA isolation methods influence the sensitivity of liquid biopsy analysis for colorectal cancer detection. Pathol. Oncol. Res. 2018 Jan 27 doi: 10.1007/s12253-018-0382-z. [DOI] [PubMed] [Google Scholar]
- Board R.E., Williams V.S., Knight L., Shaw J., Greystoke A., Ranson M., Dive C., Blackhall F.H., Hughes A. Isolation and extraction of circulating tumor DNA from patients with small cell lung cancer. Ann. N. Y. Acad. Sci. 2008 Aug;1137:98–107. doi: 10.1196/annals.1448.020. [DOI] [PubMed] [Google Scholar]
- Chan K.C., Yeung S.W., Lui W.B., Rainer T.H., Lo Y.M. Effects of preanalytical factors on the molecular size of cell-free DNA in blood. Clin. Chem. 2005 Apr;51(4):781–784. doi: 10.1373/clinchem.2004.046219. Epub 2005 Feb 11. [DOI] [PubMed] [Google Scholar]
- Chiminqgi M., Moutereau S., Pernet P., Conti M., Barbu V., Lemant J., Sacko M., Vaubourdolle M., Loric S. Specific real-time PCR vs. fluorescent dyes for serum free DNA quantification. Clin. Chem. Lab. Med. 2007;45(8):993–995. doi: 10.1515/CCLM.2007.191. [DOI] [PubMed] [Google Scholar]
- Denis M.G., Knol A.C., Théoleyre S., Vallée A., Dréno B. Efficient detection of BRAF mutation in plasma of patients after long-term storage of blood in cell-free DNA blood collection tubes. Clin. Chem. 2015 Jun;61(6):886–888. doi: 10.1373/clinchem.2015.238352. [DOI] [PubMed] [Google Scholar]
- Devonshire A.S., Whale A.S., Gutteridge A., Jones G., Cowen S., Foy C.A., Huggett J.F. Towards standardisation of cell-free DNA measurement in plasma: controls for extraction efficiency, fragment size bias and quantification. Anal. Bioanal. Chem. 2014 Oct;406(26):6499–6512. doi: 10.1007/s00216-014-7835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz L.A., Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J. Clin. Oncol. 2014 Feb 20;32(6):579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M.I., Nocon A., Mehnert D.H., Fredebohm J., Diehl F., Holtrup F. Performance of Streck cfDNA blood collection tubes for liquid biopsy testing. PLoS One. 2016 Nov 10;11(11) doi: 10.1371/journal.pone.0166354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECMC 2014. http://www.ecmcnetwork.org.uk/sites/default/files/cfDNA%20consensus%20Meeting%20Report%2024Nov14%20Final_0.pdf
- Fatouros I.G., Jamurtas A.Z., Nikolaidis M.G., Destouni A., Michailidis Y., Vrettou C., Douroudos, Avloniti A., Chatzinikolaou A., Taxildaris K., Kanavakis E., Papassotiriou I., Kouretas D. Time of sampling is crucial for measurement of cell-free plasma DNA following acute aseptic inflammation induced by exercise. Clin. Biochem. 2010;43(16–17):1368–1370. doi: 10.1016/j.clinbiochem.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Fleischhacker M., Schmidt B., Weickmann S., Fersching D.M., Leszinski G.S., Siegele B., Stötzer O.J., Nagel D., Holdenrieder S. Methods for isolation of cell-free plasma DNA strongly affect DNA yield. Clin. Chim. Acta. 2011 Nov 20;412(23–24):2085–2088. doi: 10.1016/j.cca.2011.07.011. Epub 2011 Jul 23. [DOI] [PubMed] [Google Scholar]
- Fong S.L., Zhang J.T., Lim C.K., Eu K.W., Liu Y. Comparison of 7 methods for extracting cell-free DNA from serum samples of colorectal cancer patients. Clin. Chem. 2009 Mar;55(3):587–589. doi: 10.1373/clinchem.2008.110122. [DOI] [PubMed] [Google Scholar]
- Garcia J., Dusserre E., Cheynet V., Bringuier P.P., Brengle-Pesce K., Wozny A.S., Rodriguez-Lafrasse C., Freyer G., Brevet M., Payen L., Couraud S. Evaluation of pre-analytical conditions and comparison of the performance of several digital PCR assays for the detection of major EGFR mutations in circulating DNA from non-small cell lung cancers: the CIRCAN_0 study. Oncotarget. 2017 Sept Sep 21;8(50):87980–87996. doi: 10.18632/oncotarget.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Masiá J.A., García-Olmo D., García-Olmo D.C. Circulating nucleic acids in plasma and serum (CNAPS): applications in oncology. OncoTargets Ther. 2013 Jul 8;6:819–832. doi: 10.2147/OTT.S44668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzer E., Ulz P., Geigl J.B. Circulating tumor DNA as a liquid biopsy for cancer. Clin. Chem. 2015 Jan;61(1):112–123. doi: 10.1373/clinchem.2014.222679. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S., Stieber P., Chan L.Y., Geiger S., Kremer A., Nagel D., Lo Y.M. Cell-free DNA in serum and plasma: comparison of ELISA and quantitative PCR. Clin. Chem. 2005 Aug;51(8):1544–1546. doi: 10.1373/clinchem.2005.049320. [DOI] [PubMed] [Google Scholar]
- Holdenrieder S., Burges A., Reich O., Spelsberg F.W., Stieber P. DNA integrity in plasma and serum of patients with malignant and benign diseases. Ann. N. Y. Acad. Sci. 2008 Aug;1137:162–170. doi: 10.1196/annals.1448.013. [DOI] [PubMed] [Google Scholar]
- Jahr S., Hentze H., Englisch S., Hardt D., Fackelmayer F.O., Hesch R.D., Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61(4):1659–1665. [PubMed] [Google Scholar]
- Jung M., Klotzek S., Lewandowski M., Fleischhacker M., Jung K. Changes in concentration of DNA in serum and plasma during storage of blood samples. Clin. Chem. 2003 Jun;49(6 Pt 1):1028–1029. doi: 10.1373/49.6.1028. [DOI] [PubMed] [Google Scholar]
- Kadam S.K., Farmen M., Brandt J.T. Quantitative measurement of cell-free plasma DNA and applications for detecting tumor genetic variation and promoter methylation in a clinical setting. J. Mol. Diagn. 2012 Jul;14(4):346–356. doi: 10.1016/j.jmoldx.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Kang Q., Henry N.L., Paoletti C., Jiang H., Vats P., Chinnaiyan A.M., Hayes D.F., Merajver S.D., Rae J.M., Tewari M. Comparative analysis of circulating tumor DNA stability in K3EDTA, Streck, and CellSave blood collection tubes. Clin. Biochem. 2016 Dec;49(18):1354–1360. doi: 10.1016/j.clinbiochem.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Kloten V., Rüchel N., Brüchle N.O., Gasthaus J., Freudenmacher N., Steib F., Mijnes J., Eschenbruch J., Binnebösel M., Knüchel R., Dahl E. Liquid biopsy in colon cancer: comparison of different circulating DNA extraction systems following absolute quantification of KRAS mutations using Intplex allele-specific PCR. Oncotarget. 2017 Sep 21;8(49):86253–86263. doi: 10.18632/oncotarget.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam N.Y., Rainer T.H., Chiu R.W., Lo Y.M. EDTA is a better anticoagulant than heparin or citrate for delayed blood processing for plasma DNA analysis. Clin. Chem. 2004 Jan;50(1):256–257. doi: 10.1373/clinchem.2003.026013. [DOI] [PubMed] [Google Scholar]
- Lee T.H., Montalvo L., Chrebtow V., Busch M.P. Quantitation of genomic DNA in plasma and serum samples: higher concentrations of genomic DNA found in serum than in plasma. Transfusion. 2001 Feb;41(2):276–282. doi: 10.1046/j.1537-2995.2001.41020276.x. [DOI] [PubMed] [Google Scholar]
- Lui Y.Y., Chik K.W., Chiu R.W., Ho C.Y., Lam C.W., Lo Y.M. Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin. Chem. 2002 Mar;48(3):421–427. [PubMed] [Google Scholar]
- Markus H., Contente-Cuomo T., Farooq M., Liang W.S., Borad M.J., Sivakumar S., Gollins S., Tran N.L., Dhruv H.D., Berens M.E., Bryce A., Sekulic A., Ribas A., Trent J.M., Lorusso P.M., Murtaza M. Evaluation of pre-analytical factors affecting plasma DNA analysis. Sci. Rep. 2018;8(1):7375. doi: 10.1038/s41598-018-25810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauger F., Dulary C., Daviaud C., Deleuze J.F., Tost J. Comprehensive evaluation of methods to isolate, quantify, and characterize circulating cell-free DNA from small volumes of plasma. Anal. Bioanal. Chem. 2015 Sep;407(22):6873–6878. doi: 10.1007/s00216-015-8846-4. [DOI] [PubMed] [Google Scholar]
- Mazurek A.M., Fiszer-Kierzkowska A., Rutkowski T., Składowski K., Pierzyna M., Scieglińska D., Woźniak G., Głowacki G., Kawczyński R., Małusecka E. Optimization of circulating cell-free DNA recovery for KRAS mutation and HPV detection in plasma. Cancer Biomark. 2013;13(5):385–394. doi: 10.3233/CBM-130371. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. Epub 2010 Feb 18. Erratum in: Int J Surg. 2010;8(8):658. [DOI] [PubMed] [Google Scholar]
- Morgan S.R., Whiteley J., Donald E., Smith J., Eisenberg M.T., Kallam E., Kam-Morgan L. Comparison of KRAS mutation assessment in tumor DNA and circulating free DNA in plasma and serum samples. Clin. Med. Insights Pathol. 2012;5:15–22. doi: 10.4137/CPath.S8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S.E., Lechner J.M., Williams T., Fernando M.R. A stabilizing reagent prevents cell-free DNA contamination by cellular DNA in plasma during blood sample storage and shipping as determined by digital PCR. Clin. Biochem. 2013 Oct;46(15):1561–1565. doi: 10.1016/j.clinbiochem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Page K., Guttery D.S., Zahra N., Primrose L., Elshaw S.R., Pringle J.H., Blighe K., Marchese S.D., Hills A., Woodley L., Stebbing J., Coombes R.C., Shaw J.A. Influence of plasma processing on recovery and analysis of circulating nucleic acids. PLoS One. 2013 Oct 18;8(10) doi: 10.1371/journal.pone.0077963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K., Hava N., Ward B., Brown J., Guttery D.S., Ruangpratheep C., Blighe K., Sharma A., Walker R.A., Coombes R.C., Shaw J.A. Detection of HER2 amplification in circulating free DNA in patients with breast cancer. Br. J. Cancer. 2011 Apr 12;104(8):1342–1348. doi: 10.1038/bjc.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.L., Kim H.J., Choi B.Y., Lee H.C., Jang H.R., Song K.S., Noh S.M., Kim S.Y., Han D.S., Kim Y.S. Quantitative analysis of cell-free DNA in the plasma of gastric cancer patients. Oncol. Lett. 2012 Apr 1;3(4):921–926. doi: 10.3892/ol.2012.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpart-Li S., Bartlett B., Popoli M., Adleff V., Tucker L., Steinberg R., Georgiadis A., Phallen J., Brahmer J., Azad N., Browner I., Laheru D., Velculescu V.E., Sausen M., Diaz L.A., Jr. The effect of preservative and temperature on the analysis of circulating tumor DNA. Clin. Cancer Res. 2017 May 15;23(10):2471–2477. doi: 10.1158/1078-0432.CCR-16-1691. [DOI] [PubMed] [Google Scholar]
- Ramachandran K., Speer C.G., Fiddy S., Reis I.M., Singal R. Free circulating DNA as a biomarker of prostate cancer: comparison of quantitation methods. Anticancer Res. 2013 Oct;33(10):4521–4529. [PubMed] [Google Scholar]
- Rothwell D.G., Smith N., Morris D., Leong H.S., Li Y., Hollebecque A., Ayub M., Carter L., Antonello J., Franklin L., Miller C., Blackhall F., Dive C., Brady G. Genetic profiling of tumours using both circulating free DNA and circulating tumour cells isolated from the same preserved whole blood sample. Mol. Oncol. 2016 Apr;10(4):566–574. doi: 10.1016/j.molonc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J.A., Page K., Blighe K., Hava N., Guttery D., Ward B., Brown J., Ruangpratheep C., Stebbing J., Payne R., Palmieri C., Cleator S., Walker R.A., Coombes R.C. Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res. 2012;22(2):220–231. doi: 10.1101/gr.123497.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood J.L., Corcoran C., Brown H., Sharpe A.D., Musilova M., Kohlmann A. Optimised pre-analytical methods improve KRAS mutation detection in circulating tumour DNA (ctDNA) from patients with non-small cell lung cancer (NSCLC) PLoS One. 2016 Feb 26;11(2) doi: 10.1371/journal.pone.0150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroun M., Lyautey J., Lederrey C., Olson-Sand A., Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta. 2001;313(1–2):139–142. doi: 10.1016/s0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- Swinkels D.W., Wiegerinck E., Steegers E.A., de Kok J.B. Effects of blood-processing protocols on cell-free DNA quantification in plasma. Clin. Chem. 2003 Mar;49(3):525–526. doi: 10.1373/49.3.525. [DOI] [PubMed] [Google Scholar]
- Szpechcinski A., Struniawska R., Zaleska J., Chabowski M., Orlowski T., Roszkowski K., Chorostowska-Wynimko J. Evaluation of fluorescence-based methods for total vs. amplifiable DNA quantification in plasma of lung cancer patients. J. Physiol. Pharmacol. 2008 Dec;59(Suppl. 6):675–681. [PubMed] [Google Scholar]
- Taback B., O'Day S.J., Hoon D.S. Quantification of circulating DNA in the plasma and serum of cancer patients. Ann. N. Y. Acad. Sci. 2004 Jun;1022:17–24. doi: 10.1196/annals.1318.004. [DOI] [PubMed] [Google Scholar]
- Thijssen M.A., Swinkels D.W., Ruers T.J., de Kok J.B. Difference between free circulating plasma and serum DNA in patients with colorectal liver metastases. Anticancer Res. 2002 Jan-Feb;22(1A):421–425. [PubMed] [Google Scholar]
- Toro P.V., Erlanger B., Beaver J.A., Cochran R.L., VanDenBerg D.A., Yakim E., Cravero K., Chu D., Zabransky D.J., Wong H.Y., Croessmann S., Parsons H., Hurley P.J., Lauring J., Park B.H. Comparison of cell stabilizing blood collection tubes for circulating plasma tumor DNA. Clin. Biochem. 2015 Oct;48(15):993–998. doi: 10.1016/j.clinbiochem.2015.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetani N., Hiramatsu S., Hoon D.S. Higher amount of free circulating DNA in serum than in plasma is not mainly caused by contaminated extraneous DNA during separation. Ann. N. Y. Acad. Sci. 2006 Sep;1075:299–307. doi: 10.1196/annals.1368.040. [DOI] [PubMed] [Google Scholar]
- van Dessel L.F., Beije N., Helmijr J.C., Vitale S.R., Kraan J., Look M.P., de Wit R., Sleijfer S., Jansen M.P., Martens J.W., Lolkema M.P. Application of circulating tumor DNA in prospective clinical oncology trials - standardization of preanalytical conditions. Mol. Oncol. 2017 Mar;11(3):295–304. doi: 10.1002/1878-0261.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginkel J.H., van den Broek D.A., van Kuik J., Linders D., de Weger R., Willems S.M., Huibers M.M.H. Preanalytical blood sample workup for cell-free DNA analysis using Droplet Digital PCR for future molecular cancer diagnostics. Cancer Med. 2017 Oct;6(10):2297–2307. doi: 10.1002/cam4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.