Abstract
Background
Divalent metal-ion transporter 1 (DMT1) may transport copper, but studies to date on this topic have been equivocal. Previously, an ex vivo experiment showed that intestinal copper transport was impaired in Dmt1-mutant Belgrade rats.
Objective
In this study, we tested the hypothesis that intestinal DMT1 transports copper in vivo.
Methods
Intestine-specific Dmt1 knockout (Dmt1int/int) mice and normal (control) littermates (Dmt1fl/fl) were used. In study 1, intestinal copper absorption was assessed in 7-wk-old mice of both sexes and genotypes by oral-intragastric gavage of 64Cu under normal and iron-deficiency anemia (IDA) conditions. In study 2, both sexes and genotypes of 8-wk-old mice were fed diets with adequate iron concentrations [72 parts per million (ppm)] plus adequate (9 ppm) or excessive (183 ppm) copper concentrations for 4 wk. Iron- and copper-related physiologic variables were subsequently assessed.
Results
Study 1 showed that intestinal copper transport was enhanced in normal (∼11% increase in males, 35% in females) and anemic (∼42% increase in males, 35% in females) Dmt1int/int mice. Study 2 showed that, with adequate copper intakes, serum ceruloplasmin (Cp) activity was decreased (by ∼29% in males and 20% in females) and spleens were enlarged (by 3-fold in both sexes) in Dmt1int/int mice. Higher dietary copper increased hepatic copper concentrations (by ∼3.3-fold in males and 1.5-fold in females), restored serum Cp activity, and mitigated the noted splenomegaly in Dmt1int/int mice.
Conclusions
Copper homeostasis was disrupted in Dmt1int/int mice, particularly during IDA, despite the noted increases in intestinal copper transport. This was exemplified by the fact that extra dietary copper was required to restore serum Cp activity (a biomarker of copper status) and reduce the severity of the noted splenomegaly (which could reflect changes in erythropoietic demand) in Dmt1int/int mice. Collectively, these observations show that intestinal DMT1 is essential for the assimilation of sufficient quantities of dietary copper to maintain systemic copper homeostasis during IDA.
Keywords: intestine-specific Dmt1 knockout mice, copper absorption, Slc11a2, ceruloplasmin, iron-deficiency anemia, manganese, zinc
Introduction
Previous studies over the past several decades have shown that iron deficiency is associated with alterations in copper homeostasis (1–5). For example, during iron depletion, copper accumulates in the duodenal epithelium (3), and expression of an intestinal copper exporter, copper-transporting ATPase 1 (Atp7a), is strongly induced (3, 4, 6). Moreover, copper accumulates in the liver (5), and increases in serum copper are associated with enhanced ceruloplasmin (Cp) ferroxidase activity (7). Copper is thus likely redistributed during iron deficiency to tissues important for iron metabolism, exemplifying a physiologically-relevant relationship between iron and copper. What is unknown, however, is what protein (i.e., transporter) mediates increased copper uptake into duodenal enterocytes during low-iron conditions. Possible mechanisms of copper import would logically include copper transporter 1 (CTR1) (8, 9) and divalent metal-ion transporter 1 (DMT1), which may also transport dietary copper (10, 11). Given the strong induction of DMT1 expression during iron deficiency (3, 12, 13), it is a likely candidate for enhancing the assimilation of dietary copper. Conceivably, intestinal DMT1 could also transport copper during physiologic conditions, but one recent study suggests that this is not the case (14). Further experimentation is, however, warranted to establish definitively whether intestinal DMT1 transports copper or otherwise influences copper homeostasis.
DMT1 is a widely expressed ferrous iron/proton cotransporter that has emerged as a critical player in iron metabolism in humans and other mammals (15). In the intestine, DMT1 is the principal importer of diet-derived, nonheme iron (16, 17). In other body cells—for example, developing erythrocytes—DMT1 functions intracellularly in endosomes where it mediates ferrous iron transport into the cytosol after endocytosis of diferric transferrin (18). Although earlier studies showed that DMT1 could transport a variety of divalent cations (19), or even monovalent cations (e.g., Cu+) (11), more recent evidence suggests that iron is its main physiologic substrate, at least in mice (14). Despite this, however, there is also support for manganese transport by DMT1 in some tissues (20–22). Accumulating evidence also suggests that DMT1 may transport copper (10, 11, 23–26), but definitive in vivo studies are lacking. The current investigation was thus undertaken to test the hypothesis that lack of intestinal DMT1 will decrease intestinal copper transport and disrupt systemic copper homeostasis. The experimental approach used genetically-engineered mice lacking DMT1 only in the intestinal epithelium and control (normal) littermates. Notably, the lack of intestinal DMT1 activity led to compensatory increases in intestinal copper absorption, but systemic copper metabolism was nonetheless disturbed, showing that intestinal DMT1 is required for optimal assimilation of dietary copper.
Methods
Animal experiments
All animal studies were approved by the University of Florida Institutional Animal Care and Use Committee. Intestine-specific Dmt1 knockout (Dmt1int/int) mice and phenotypically normal (Dmt1fl/fl) control littermates on the 129S6 genetic background were used for this investigation. These mice have loxP sites flanking part of the Slc11a2 gene and express the CRE recombinase under the control of the intestinal epithelium-specific villin promoter (42). Breeders were obtained from Dr. Bryan Mackenzie, University of Cincinnati, and the breeding strategy was identical to what was previously reported (14). Genomic DNA was isolated from tail clips, and genotype was determined by PCR (14). Mice were killed by carbon dioxide exposure followed by thoracotomy. Blood was collected by cardiac puncture after carbon dioxide exposure (but before thoracotomy), and various organs were removed and weighed. Tissue samples and serum were preserved at −80°C for mineral analyses and other experiments.
Quantification of intestinal copper absorption (study 1)
Mice were housed in stainless steel, overhanging, wire-mesh-bottom cages with ad libitum access to food and water. Newly weaned mice were fed AIN-93G–based diets with adequate copper (AdCu) content and variable iron content (low, adequate, or high). In experiment 1 (“normal”), Dmt1fl/fl mice were fed an adequate-iron (AdFe) diet (50 ppm Fe; TD.130018; Envigo) for 4 wk, and Dmt1int/int mice were fed a high-iron diet [10,000 ppm (carbonyl) Fe; TD.130015; Envigo] for 1 wk followed by the AdFe diet for 3 wk (the experimental design is depicted in Figure 1A). This approach was designed to normalize hemoglobin concentrations in the Dmt1int/int mice to control concentrations. In experiment 2 [“iron-deficiency anemia” (IDA)], Dmt1fl/fl mice were fed a low-iron (LFe) diet (∼3 ppm Fe; TD.120105; Envigo) and Dmt1int/int mice were fed the AdFe diet for 4 wk. This experimental approach ensured that both groups of mice were iron deficient and anemic. The entire experiment was repeated twice. After the 4-wk dietary interventions, mice were feed-deprived overnight (but given free access to water) and then administered 20 µCi 64CuCl2 (394 mCi/µg; Washington University, St. Louis, Missouri) diluted into PBS containing 0.1 N HCl by oral, intragastric gavage (27). Immediately after gavage feeding, mice were fed the AdFe diet and water, and then killed 9 h later. This time point was selected because intestinal transit time is ∼11 h in mice (28) and excess copper can be excreted in the bile; thus, only minimal amounts of unabsorbed copper should have been lost in the feces. Moreover, biliary copper excretion should have been minimal because the mice were fed a diet with adequate copper content. In addition, the short half-life of 64Cu (12.7 h) necessitated a more brief experimental period. Whole-carcass, blood, and tissue radioactivity were measured using a WIZARD2 automatic gamma counter (Perkin Elmer). The 64Cu radioactive counts were normalized on the basis of the half-life of 64Cu. Intestinal copper absorption was calculated as follows: radioactivity in the carcass minus radioactivity in the entire gut (esophagus to anus) divided by the amount of radioactivity in the oral gavage solution (×100). Blood and tissue radioactive counts were normalized by volume or weight, respectively.
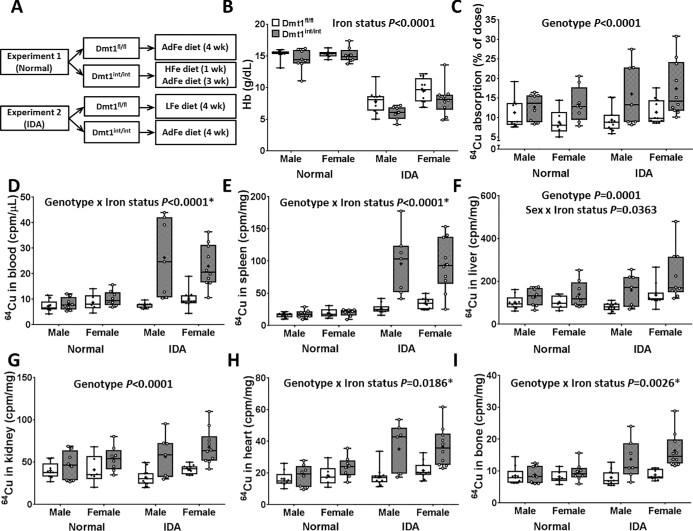
FIGURE 1.
Study design (A), blood hemoglobin concentrations (B), quantification of intestinal copper absorption (C), and radioactive counts in blood (D), spleen (E), liver (F), kidney (G), heart (H), and bone (I) of male and female 7-wk-old anemic and nonanemic Dmt1int/int and Dmt1fl/fl mice (study 1). Results are depicted as box plots and represent n = 9 (serum hemoglobin concentrations), n = 8 (64Cu absorption), n = 6 (64Cu radioactivity in blood and spleen), n = 11 (in liver and heart), n = 7 (in kidney), or n = 10 (in bone) mice/group. No significant 3-way interactions were noted. Significant 2-way interactions and some main effects are denoted in each panel. Genotype main effects: P < 0.0001 (panels D, E, H, and I).*Slice test results (panels D, E, H, and I): normal, genotype not significant; IDA, genotype P < 0.0001. AdFe, adequate iron; cpm, counts per minute; Dmt1, divalent metal-ion transporter 1; Dmt1fl/fl mice, phenotypically normal mice with the Slc11a2 gene “floxed”; Dmt1int/int mice, intestine-specific Dmt1 knockout mice; Hb, hemoglobin; HFe, high iron; IDA, iron-deficiency anemia; LFe, low iron; Normal, normal hematologic variables.
Copper dietary study (study 2)
Two-month-old mice of both sexes and genotypes, housed in standard shoebox cages, were fed either an AdCu diet (∼9 ppm Cu) or a high-copper (HCu) diet (∼183 ppm Cu) [both with AdFe content (∼72 ppm)] for 4 wk (the experimental design is depicted in Figure 1A). Diets were fabricated on the basis of the AIN-93G formulation (Dyets, Inc.; the diet compositions are shown in Supplemental Table 1) (29–31). Mice had ad libitum access to food and water. The entire experiment was repeated 3 times. Mice were weighed weekly, and after being killed, blood and tissues were harvested. Serum hemoglobin and hematocrit concentrations were measured by standard methods (6). Serum Cp activity was assessed by a para-phenylenediamine assay, which quantifies the amine oxidase activity of Cp, as previously described (7). All experiments outlined below were performed with the mice described here in this section (i.e., as part of study 2).
Spleen histology
Spleen tissue samples were rinsed in PBS, fixed with 4% (wt:vol) paraformaldehyde, and embedded in paraffin. Sections were cut and then stained with hematoxylin and eosin and analyzed by light microscopy. The proportion of red compared with white pulp was assessed (by a blinded observer) by quantifying the area of periarterial lymphatic sheaths, representing white pulp, and comparing it to total area of whole-organ cross-sections in three 200× fields of view per mouse (32). Images were captured and areas calculated using cellSens Standard 1.11 software.
Quantification of serum and tissue mineral concentrations
Serum and liver, spleen, heart and kidney tissue samples were digested with HNO3 at 90°C for 3 h. Digested tissues were diluted in MilliQ water before measurement by inductively coupled plasma–MS (NexION 300X; Perkin Elmer) (27). Serum and tissue iron, copper, manganese, and zinc concentrations were normalized by volume or mass, respectively.
Renal erythropoietin mRNA expression analysis
Total RNA was isolated from mouse kidney with RNAzol RT reagent (Molecular Research Center, Inc.) following the manufacturer's protocol. RNA concentration was measured with a Nanodrop spectrophotometer, and RNA integrity was assessed by agarose gel electrophoresis. SYBR-Green qRT-PCR was performed according to a well-established protocol (10, 27). The 2−∆∆Ct analysis method was used to calculate fold changes in erythropoietin (Epo) mRNA expression, which was normalized to expression of cyclophilin (which did not vary significantly between samples). Primer sequences are listed in Supplemental Table 2.
Statistical analyses
Results are depicted as box plots displaying the minimum, the lower (25th percentile), the median (50th percentile), the upper (75th percentile), and the maximum ranked sample. The mean value is indicated by a “+” sign. Data were analyzed by 2- or 3-factor ANOVA using JMP (version 12.2) software. Levene's test was used to test for equal variance. Some data sets (Figure 1C–I; Figure 2B, C, E; Figure 3A, C; Figure 4A, B, D, E) were transformed as a log10 scale to adjust for unequal variance. Data were then tested again to ensure equal variance before analysis by 3-factor ANOVA. Similarly, for 2-factor ANOVA, some data sets, including kidney/body weight, heart/body weight, and liver, spleen, heart, and renal iron concentrations, were log10 transformed to adjust for unequal variance. Again, data were retested to ensure equal variance before analysis by 2-factor ANOVA. Hemoglobin and hematocrit data, which were not equally distributed even after log10 transformation, were analyzed by a mixed-model test, for which hemoglobin concentrations and hematocrit levels (percentage) were allowed to vary by genotype. Even if data sets were log10 transformed before conducting statistical analyses, the nontransformed data are shown in the figures for ease of interpretation. If significant 2-factor (if 2-factor ANOVA was used) or 3-factor (if 3-factor ANOVA was used) interactions were noted, multiple pairwise comparisons were made by Tukey's post hoc analysis, and differences between individual groups are denoted in the figures and tables. In addition, simple effects analyses were made by slice tests (i.e., multiple-comparison F tests), which compare the levels of one factor for each level of the other factor. In this case, this approach allowed us to independently compare the influence of genotype in the normal and IDA groups in the copper-absorption experiments, and the influence of genotype and sex on serum copper concentrations. The use of slice tests is indicated by “*” in Figure 1 (panels D, E, H, and I) and Figure 2A, and individual effect P values are presented in the figure legends. Differences between groups were considered significant at P < 0.05. Significant 2-way interactions (when 3-factor ANOVA was used) and main effects (for both 2- and 3-factor ANOVA) are also indicated where appropriate.
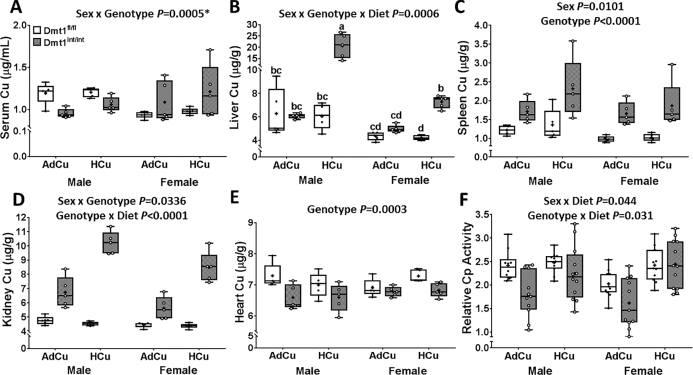
FIGURE 2.
Copper content in serum (A), liver (B), spleen (C), kidney (D), and heart (E) and relative serum Cp activity (F) of 2-mo-old mice of both genotypes and sexes fed diets with AdFe and either AdCu or HCu for 4 wk (study 2). Results are depicted as box plots and represent n = 5 (A–E) or n = 10–12 (F) mice/group. A significant 3-way interaction was noted for liver copper concentrations (B); different letters above whiskers indicate differences between groups. Significant 2-way interactions and/or main effects are also denoted in each panel. Genotype main effects: P < 0.0001 (D), P = 0.0005 (F). *Slice test results (panel A): female, genotype P = 0.0095; male, genotype P = 0.0093; wild-type, sex P = 0.0015; knockout, sex P = 0.0489. AdCu, adequate copper; AdFe, adequate iron; Cp, ceruloplasmin; Dmt1, divalent metal-ion transporter 1; Dmt1fl/fl mice, phenotypically normal mice with the Slc11a2 gene “floxed”; Dmt1int/int mice, intestine-specific Dmt1 knockout mice; HCu, high copper.
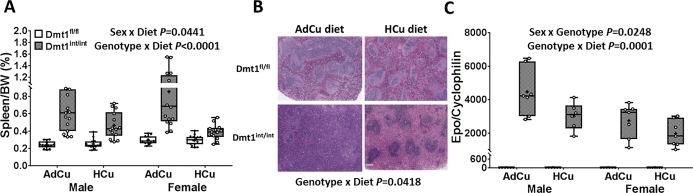
FIGURE 3.
Relative spleen weights (A), example images of spleen histology (B), and renal Epo mRNA expression (C) in 2-mo-old mice of both genotypes and sexes fed diets with AdFe and either AdCu or HCu for 4 wk (study 2). Relative proportions of red and white pulp in the spleen were calculated by mean periarterial lymphatic sheath area/total area (B) (n = 3 mice/group). The significant 2-way interaction shown (panel B) refers to the relative proportions of white compared with red pulp. Results are depicted as box plots for n = 12–16 (A) or n = 5–8 (C) mice/group. Genotype main effects: P < 0.0001 (panels A and C). AdCu, adequate copper; AdFe, adequate iron; BW, body weight; Dmt1, divalent metal-ion transporter 1; Dmt1fl/fl mice, phenotypically normal mice with the Slc11a2 gene “floxed”; Dmt1int/int mice, intestine-specific Dmt1 knockout mice; Epo, erythropoietin; HCu, high copper.
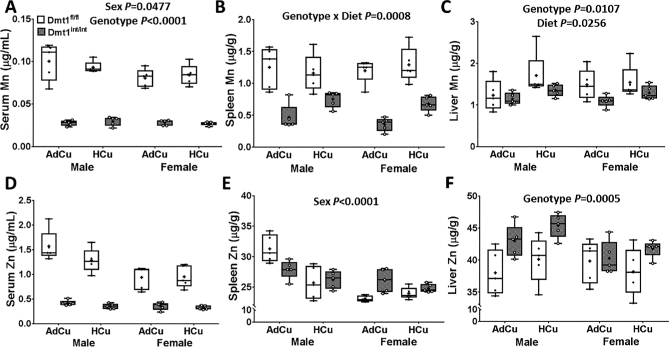
FIGURE 4.
Manganese concentrations in serum (A), spleen (B), and liver (C), and zinc concentrations in serum (D), spleen (E), and liver (F) of 2-mo-old mice of both genotypes and sexes fed diets with AdFe and either AdCu or HCu for 4 wk (study 2). Results are depicted as box plots representing n = 5 mice/group. No significant 3-way interactions were noted. Significant 2-way interactions and/or main effects are denoted in each panel. Genotype main effects: P < 0.0001 (panels A, B, and D). AdCu, adequate copper; AdFe, adequate iron; Dmt1, divalent metal-ion transporter 1; Dmt1fl/fl mice, phenotypically normal mice with the Slc11a2 gene “floxed”; Dmt1int/int mice, intestine-specific Dmt1 knockout mice; HCu, high copper.
Results
Phenotypical characterization of experimental mice
The Dmt1int/int mice were challenging to breed because litter sizes were small (2–4 pups), and only 50% of pups were the correct genotype for use here, making it difficult to obtain enough mice of both sexes for various experiments. The number of mice used for individual experiments thus varied. It was previously reported that male adult Dmt1int/int mice had severe IDA and other pathologic perturbations associated with systemic iron depletion (14). Here (as part of study 2), we fed mice of both genotypes and sexes defined diets with AdFe contents in combination with adequate or HCu content for 4 wk, and then assessed various iron-related variables. With adequate iron and copper intake, Dmt1int/int mice of both sexes were smaller, severely anemic, and had smaller livers and larger kidneys and hearts than their control littermates (Table 1). Serum iron was also lower in Dmt1int/int mice of both sexes, and liver, spleen, heart, and kidney iron concentrations were also significantly decreased (Table 2). These observations confirm the findings reported earlier (14), and show that intestinal DMT1 is important for adequate assimilation of dietary iron in mice. Furthermore, none of these physiologic variables changed when mice were fed an AdFe diet with HCu content (data not shown).
TABLE 1.
Hematologic variables, BWs, and relative tissue weights of 3-mo-old male and female mice fed an adequate-iron diet with adequate copper content for 4 wk (study 2)1
| Sex, genotype | Hemoglobin, g/dL | Hematocrit, % | BW, g | Liver/BW, % | Kidney/BW, % | Heart/BW, % |
|---|---|---|---|---|---|---|
| Male, Dmt1fl/fl | 15.3 ± 1.0 | 59.5 ± 6.6 | 29.2 ± 3.7a | 4.07 ± 0.39 | 1.26 ± 0.14 | 0.47 ± 0.08c |
| Male, Dmt1int/int | 1.5 ± 0.2 | 7.4 ± 1.7 | 19.4 ± 1.7c | 3.41 ± 0.26 | 1.58 ± 0.12 | 1.82 ± 0.28a |
| Female, Dmt1fl/fl | 15.4 ± 0.8 | 60.4 ± 3.7 | 22.1 ± 2.3b | 3.71 ± 0.38 | 1.04 ± 0.06 | 0.45 ± 0.03c |
| Female, Dmt1int/int | 1.8 ± 0.4 | 10.4 ± 2.9 | 21.1 ± 1.9b,c | 3.16 ± 0.27 | 1.31 ± 0.07 | 1.16 ± 0.33b |
| Two-way interactions and main effects, P | ||||||
| Sex | 0.30 | 0.10 | 0.0001 | 0.0009 | <0.0001 | <0.0001 |
| Genotype | <0.0001 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| Sex × genotype | 0.39 | 0.36 | <0.0001 | 0.52 | 0.82 | <0.0001 |
Values are means ± SDs, n = 13–16/group. Data were analyzed by 2-factor ANOVA. Labeled means without a common superscript letter differ, P < 0.05. None of the physiological variables assessed here changed when mice were fed an adequate-iron diet with high copper content (data not shown). BW, body weight; Dmt1, divalent metal-ion transporter 1; Dmt1fl/fl mice, phenotypically normal mice with the Slc11a2 gene “floxed”; Dmt1int/int mice, intestine-specific Dmt1 knockout mice.
TABLE 2.
Serum and tissue iron concentrations of 3-mo-old male and female mice fed an adequate-iron diet with adequate copper content for 4 wk (study 2)1
| Iron | |||||
|---|---|---|---|---|---|
| Sex, genotype | Serum, µg/mL | Liver, µg/g | Spleen, mg/g | Heart, µg/g | Kidney, µg/g |
| Male, Dmt1fl/fl | 9.70 ± 2.83 | 157 ± 24.8b | 1.44 ± 0.217 | 110 ± 11.2 | 100 ± 8.79a |
| Male, Dmt1int/int | 3.80 ± 2.68 | 22.9 ± 1.59c | 0.036 ± 0.007 | 44.8 ± 3.36 | 28.9 ± 2.83b |
| Female, Dmt1fl/fl | 5.07 ± 1.37 | 269 ± 57.7a | 1.70 ± 0.258 | 117 ± 11.7 | 117 ± 10.1a |
| Female, Dmt1int/int | 2.31 ± 2.51 | 21.3 ± 2.46c | 0.036 ± 0.008 | 63.5 ± 35.7 | 25.9 ± 2.63b |
| Two-way interactions and main effects, P | |||||
| Sex | 0.0120 | 0.0023 | 0.51 | 0.15 | 0.58 |
| Genotype | 0.0010 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sex × genotype | 0.17 | 0.0002 | 0.31 | 0.37 | 0.0057 |
Values are means ± SDs, n = 5/group. Data were analyzed by 2-factor ANOVA. Labeled means without a common superscript letter differ, P < 0.05. None of the physiological variables assessed here changed when mice were fed an adequate-iron diet with high copper content (data not shown). Dmt1, divalent metal-ion transporter 1; Dmt1fl/fl mice, phenotypically normal mice with the Slc11a2 gene “floxed”; Dmt1int/int mice, intestine-specific Dmt1 knockout mice.
Copper-absorption studies
The dietary feeding regimens, intended to normalize hematologic status between genotypes or to induce IDA in control mice, are shown in Figure 1A. In experiment 1 (“normal”), blood hemoglobin concentrations were similar in all mice and close to normal values, whereas in experiment 2 (“IDA”), all mice were iron deficient and anemic (Figure 1B). Copper absorption and tissue 64Cu accumulation were higher in Dmt1int/int mice in both experiments (Figure 1C–I). The increases in copper absorption and tissue accumulation, however, tended to be more robust in mice with IDA (as indicated by significant genotype × iron status interactions; Figure 1D, E, H, I). In fact, slice tests showed that genotype effects were only significant in the IDA groups (individual P values are presented in Figure 1 legend).
Serum and tissue copper concentrations and serum Cp activity
Our next objective was to assess possible perturbations of copper homeostasis in Dmt1int/int mice fed diets with AdFe and AdCu contents. Serum and tissue copper concentrations were thus assessed, and serum Cp activity was quantified (because Cp is a well-established biomarker of copper status) (33–35). Under these (normal) dietary conditions, serum and hepatic copper concentrations did not vary significantly by genotype (Figure 2A, B). Splenic and renal copper concentrations were, however, higher in Dmt1int/int mice of both sexes (Figure 2C, D), perhaps reflecting increased intestinal copper absorption. Conversely, copper concentrations in the heart were lower in the knockout mice of both sexes (Figure 2E), as was serum Cp activity (Figure 2F). Given the noted cardiac copper depletion and decrements in serum Cp activity, we postulated that increasing copper intake would correct these abnormalities. Mice of both genotypes and sexes were thus fed a HCu diet (with adequate iron) for 4 wk and all of these physiological variables were assessed again. HCu feeding increased serum copper in Dmt1int/int mice of both sexes, with the increase being more robust in females (Figure 2A). In control mice, serum copper concentrations were, however, not affected by HCu intake. Moreover, no diet effect was noted for spleen or heart copper concentrations. Renal and hepatic copper concentrations were further increased by higher copper intake (Figure 2B, D), but no effect was noted in Dmt1fl/fl mice. Last, serum Cp activity, which was reduced in Dmt1int/int mice, was restored close to control values by HCu feeding in males and females. Overall, these data show that the lack of intestinal DMT1 disrupts copper metabolism in mice when dietary copper intake is at normal levels. When dietary copper was elevated, copper homeostasis was partially restored in Dmt1int/int mice, as reflected by increases in serum and liver copper concentrations and serum Cp activity.
Spleen weight and morphology, and renal Epo mRNA expression
With AdCu intakes, Dmt1int/int mice of both sexes had enlarged spleens and increased proportions of red pulp (likely reflecting extramedullary, splenic erythropoiesis) (32) (Figure 3A, B). Renal Epo expression was also strongly induced, reflecting enhanced erythroid demand (Figure 3C). These notable physiologic alterations could relate to iron depletion; however, because copper deficiency also causes splenomegaly (36–38) and an iron-deficiency–like anemia (1, 5, 33, 35), we postulated that copper depletion exacerbated the anemia in Dmt1int/int mice. If true, correcting the copper imbalance could reduce erythropoietic demand. Indeed, increasing dietary copper intake partially corrected the noted splenomegaly and re-established the balance between red and white pulp in both sexes of Dmt1int/int mice (Figure 3A, B). In control mice, HCu intake did not, however, influence spleen size or red-to-white pulp ratios. We also noted that copper supplementation blunted the induction of renal Epo mRNA expression (Figure 3C). Quantification of Epo mRNA levels is a useful proxy for circulating protein concentrations, because the Epo gene is transcriptionally upregulated when erythroid demand increases (21). These observations suggested that copper depletion (or impaired copper utilization) contributed to the enhanced erythropoietic demand in the Dmt1int/int mice.
Quantification of serum and tissue manganese and zinc concentrations
Interactions among dietary, essential minerals are common, given their similar physiochemical properties. Moreover, some published reports suggested that DMT1 can transport manganese (20–22) and one early report suggested that DMT1 could also transport zinc (19). Serum and tissue manganese and zinc concentrations were thus assessed in experimental mice. With AdCu intake, serum, spleen, and liver manganese concentrations were lower in Dmt1int/int mice (Figure 4A–C). The manganese concentration in the heart was not, however, different between genotypes (data not shown). Serum zinc concentrations were also dramatically reduced in Dmt1int/int mice (Figure 4D), whereas the splenic zinc concentration was not influenced by genotype (Figure 4E). Hepatic zinc concentrations, conversely, increased in the knockout mice (Figure 4F). Surprisingly, higher dietary copper intake also influenced manganese metabolism. For example, splenic manganese concentrations increased with HCu consumption in Dmt1int/int mice, but not in controls (Figure 4B). Liver manganese concentrations were lower in both sexes of Dmt1int/int mice, and surprisingly, higher copper intake increased hepatic manganese concentrations (Figure 4C). Manganese concentrations in the heart, however, did not change with higher copper intake (data not shown). Collectively, these observations show that lack of intestinal DMT1 impairs manganese and zinc absorption and/or utilization in mice.
Discussion
Copper transport by DMT1 remains enigmatic, because most studies on DMT1 function have been performed in various in vitro model systems, with more physiologically relevant in vivo experimentation lacking. In the original DMT1 cloning study, copper transport was noted in Xenopus oocytes expressing rat DMT1 (19); however, this observation was later refuted (39). Several in vitro studies exemplify copper transport by DMT1 (10, 11, 23, 24, 26, 40, 41). One current study with relevance to this topic utilized Belgrade rats, which harbor an inactivating point mutation in the Slc11a2 gene (encoding DMT1) (10). Copper transport experiments in isolated duodenal loops showed that copper absorption was significantly higher in iron-deprived control rats, as compared with (naturally) iron-deficient Belgrade rats. The potential physiologic significance of this observation was, however, limited by the ex vivo experimental approach. A more recent investigation addressed this issue using the same mice that were used in the current investigation (14). Before experimentation, the severe anemia in the Dmt1int/int mice was corrected by iron injections, and then copper absorption was assessed by in vivo gavage of 64Cu. It was noted that 64Cu accumulated in blood, duodenal enterocytes, and liver to the same extent in Dmt1int/int and Dmt1fl/fl mice at all time points tested, so these authors concluded that DMT1 is not required for intestinal absorption of copper under physiologic (i.e., normal) conditions.
Here, we sought to extend this previous investigation to include mice of both sexes studied under physiologic conditions and during IDA. This approach was important because iron metabolism is notably different in males and females (43, 44), and moreover, because DMT1 could have different functional properties during iron depletion. Under both circumstances [normal (experiment 1) and IDA (experiment 2)] and in both sexes, intestinal copper (64Cu) absorption was enhanced and copper accumulation in all tissues tested increased. Unexpectedly then, the lack of intestinal DMT1 increases intestinal copper absorption. Because previous studies in laboratory rodents (27, 34) and in humans (45, 46) have clearly established that dietary copper absorption responds to changes in body copper requirements, the most logical interpretation of these findings is that copper absorption was enhanced in response to disruption of copper homeostasis. Supporting this possibility, iron-deficient Dmt1int/int mice fed a diet with AdCu content had reduced serum Cp activity and enlarged spleens (compared with controls). Interestingly, there is ample evidence linking splenomegaly and reductions in serum Cp activity to copper depletion (33–38). Low cardiac copper concentrations were also documented in both sexes of Dmt1int/int mice when copper intake was adequate. Importantly, it was previously reported that depletion of cardiac copper induced production and secretion of a heart-specific, regulatory factor that enhanced intestinal copper absorption (47). This hormonal factor has never been identified, but nonetheless, this could be one trigger for increased intestinal copper transport in Dmt1int/int mice.
Given that Dmt1int/int mice with IDA showed signs of copper depletion with AdCu intakes (despite increased intestinal copper absorption), we hypothesized that increasing systemic copper concentrations would prevent (or reverse) some of the noted physiologic abnormalities. Supporting this postulate, HCu intake restored serum Cp activity close to control values in Dmt1int/int mice. This likely reflects re-establishment of copper homeostasis, because reductions in serum Cp activity typify moderate to severe copper deficiency (1, 5, 48–50). Restoration of serum Cp activity occurred concurrently with significant increases in serum and hepatic copper concentrations in the knockout mice fed the HCu diet. Higher liver copper content was thus necessary to allow normal Cp production (and activity), showing that lack of intestinal DMT1 in the setting of IDA impairs hepatic copper utilization. Moreover, higher dietary copper intake increased splenic copper concentrations and reduced spleen size and decreased the relative proportion of red pulp in Dmt1int/int mice. Increasing dietary copper amounts did not, however, restore cardiac copper concentrations to normal levels in Dmt1int/int mice. This investigation thus did not clarify whether cardiac copper depletion contributed to the noted cardiac hypertrophy, which is important because deficiencies of both iron and copper are associated with this notable physiologic perturbation (5, 33–35). Overall, these experimental observations suggested that knockout of intestinal DMT1 increased copper requirements during IDA, because copper supplementation corrected some of the noted copper-related pathologies. Specificity for copper was established since HCu feeding did not alter serum, hepatic, splenic, or cardiac iron concentrations (data not shown). Moreover, increases in copper absorption in Dmt1int/int mice were insufficient to prevent the development of notable copper-related abnormalities at normal (i.e., adequate) dietary copper intakes. DMT1 must thus be required for optimal intestinal copper transport during IDA.
Also notable were significant decreases in serum manganese and zinc concentrations in Dmt1int/int mice. Previous studies, mentioned above, support possible manganese transport by DMT1, but little evidence of zinc transport by DMT1 has been published to date. Decreases in serum manganese were associated with lower splenic and hepatic manganese concentrations, but manganese concentrations in other tissues were not remarkably different when comparing genotypes. Interestingly, increasing dietary copper intake was associated with increases in hepatic manganese concentrations. To our knowledge, such an interaction between copper and manganese has not been previously reported. Furthermore, hepatic zinc concentrations were elevated in Dmt1int/int mice. Lack of intestinal DMT1, or the severe IDA associated with loss of DMT1, thus disrupts manganese and zinc homeostasis in mice. Because the focus of this investigation is on alterations of copper metabolism in Dmt1int/int mice, assessment of the functional consequences of altered manganese and zinc metabolism must await future experimentation. Importantly, however, manganese and zinc are not known to directly relate to any of the copper- (or iron-) related physiologic variables assessed in the current study.
In summary, many of the pathologies noted in DMT1int/int mice, including decrements in serum and tissue iron concentrations, blood hemoglobin and hematocrit concentrations, body weight, and liver mass, as well as increases in heart mass, typify murine iron deficiency. Lack of intestinal DMT1 thus does not appear to have any particular iron-related influence that differs from iron deficiency more generally. What is, however, notable are disruptions of copper homeostasis in iron-deficient Dmt1int/int mice when dietary copper intake was at a typically adequate amount. Increasing dietary copper consumption corrected the noted copper deficiency, without altering serum or tissue iron concentrations, proving that copper depletion was the underlying cause of these physiologic perturbations. Ablation of intestinal DMT1 thus increases copper requirements during IDA. Collectively, these observations show that intestinal DMT1 is essential for optimal assimilation of dietary copper and normal systemic copper homeostasis.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—XW, SV, and JFC: conceived of the investigation and designed the experimental approach; XW: directed and performed the experiments with the technical assistance of SRLF, J-HH, CD, RRW, PX, and AG; XW: analyzed data and prepared figures; XW, AG, SV, and JFC: interpreted data and drafted the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by grant R01 DK074867 from the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK) and grant R01 DK109717 from NIDDK and the Office of Dietary Supplements (ODS) (to JFC). XW is a recipient of a doctoral scholarship from the China Scholarship Council.
Author disclosures: XW, SRLF, J-HH, CD, RRW, PX, AG, SV, and JFC, no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used:
- AdCu
adequate copper
- AdFe
adequate iron
- Cp
ceruloplasmin
- DMT1
divalent metal-ion transporter 1
- Dmt1fl/fl mice
phenotypically normal mice with the Slc11a2 gene “floxed”
- Dmt1int/int mice
intestine-specific Dmt1 knockout mice
- Epo
erythropoietin
- HCu
high copper
- IDA
iron-deficiency anemia
References
- 1. Gulec S, Collins JF. Molecular mediators governing iron-copper interactions. Annu Rev Nutr 2014;34:95–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins JF, Prohaska JR, Knutson MD. Metabolic crossroads of iron and copper. Nutr Rev 2010;68:133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem 2005;280:36221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins JF, Franck CA, Kowdley KV, Ghishan FK. Identification of differentially expressed genes in response to dietary iron deprivation in rat duodenum. Am J Physiol Gastrointest Liver Physiol 2005;288:G964–71. [DOI] [PubMed] [Google Scholar]
- 5. Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals 2003;16:9–40. [DOI] [PubMed] [Google Scholar]
- 6. Gulec S, Collins JF. Investigation of iron metabolism in mice expressing a mutant Menke's copper transporting ATPase (Atp7a) protein with diminished activity (Brindled; Mo (Br) (/y)). PLoS One 2013;8:e66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ranganathan PN, Lu Y, Jiang L, Kim C, Collins JF. Serum ceruloplasmin protein expression and activity increases in iron-deficient rats and is further enhanced by higher dietary copper intake. Blood 2011;118:3146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 2006;4:235–44. [DOI] [PubMed] [Google Scholar]
- 9. Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, Spears JW, Thiele DJ. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem 2010;285:32385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang L, Garrick MD, Garrick LM, Zhao L, Collins JF. Divalent metal transporter 1 (Dmt1) mediates copper transport in the duodenum of iron-deficient rats and when overexpressed in iron-deprived HEK-293 cells. J Nutr 2013;143:1927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arredondo M, Munoz P, Mura CV, Nunez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol 2003;284:C1525–30. [DOI] [PubMed] [Google Scholar]
- 12. Trinder D, Oates PS, Thomas C, Sadleir J, Morgan EH. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut 2000;46:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology 2001;120:1412–9. [DOI] [PubMed] [Google Scholar]
- 14. Shawki A, Anthony SR, Nose Y, Engevik MA, Niespodzany EJ, Barrientos T, Ohrvik H, Worrell RT, Thiele DJ, Mackenzie B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am J Physiol Gastrointest Liver Physiol 2015;309:G635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shawki A, Knight PB, Maliken BD, Niespodzany EJ, Mackenzie B. H(+)-coupled divalent metal-ion transporter-1: functional properties, physiological roles and therapeutics. Curr Top Membr 2012;70:169–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuqua BK, Vulpe CD, Anderson GJ. Intestinal iron absorption. J Trace Elem Med Biol 2012;26:115–9. [DOI] [PubMed] [Google Scholar]
- 17. Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol 2014;307:G397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garrick MD. Human iron transporters. Genes Nutr 2011;6:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997;388:482–8. [DOI] [PubMed] [Google Scholar]
- 20. Kim J, Buckett PD, Wessling-Resnick M. Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS One 2013;8:e64944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo YA, Li Y, Wessling-Resnick M. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 2013;38:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hansen SL, Trakooljul N, Liu HC, Moeser AJ, Spears JW. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J Nutr 2009;139:1474–9. [DOI] [PubMed] [Google Scholar]
- 23. Arredondo M, Mendiburo MJ, Flores S, Singleton ST, Garrick MD. Mouse divalent metal transporter 1 is a copper transporter in HEK293 cells. Biometals 2014;27:115–23. [DOI] [PubMed] [Google Scholar]
- 24. Espinoza A, Le Blanc S, Olivares M, Pizarro F, Ruz M, Arredondo M. Iron, copper, and zinc transport: inhibition of divalent metal transporter 1 (DMT1) and human copper transporter 1 (hCTR1) by shRNA. Biol Trace Elem Res 2012;146:281–6. [DOI] [PubMed] [Google Scholar]
- 25. Han M, Chang J, Kim J. Loss of divalent metal transporter 1 function promotes brain copper accumulation and increases impulsivity. J Neurochem 2016;138:918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin C, Zhang Z, Wang T, Chen C, James Kang Y. Copper uptake by DMT1: a compensatory mechanism for CTR1 deficiency in human umbilical vein endothelial cells. Metallomics 2015;7:1285–9. [DOI] [PubMed] [Google Scholar]
- 27. Ha JH, Doguer C, Collins JF. Consumption of a high-iron diet disrupts homeostatic regulation of intestinal copper absorption in adolescent mice. Am J Physiol Gastrointest Liver Physiol 2017;313:G353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bellier S, Da Silva NR, Aubin-Houzelstein G, Elbaz C, Vanderwinden JM, Panthier JJ. Accelerated intestinal transit in inbred mice with an increased number of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol 2005;288:G151–8. [DOI] [PubMed] [Google Scholar]
- 29. Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr 1997;127:838S–41S. [DOI] [PubMed] [Google Scholar]
- 30. Reeves PG, Rossow KL, Lindlauf J. Development and testing of the AIN-93 purified diets for rodents: results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr 1993;123:1923–31. [DOI] [PubMed] [Google Scholar]
- 31. Reeves PG, Nielsen FH, Fahey GC Jr.. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 32. Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol 2006;34:455–65. [DOI] [PubMed] [Google Scholar]
- 33. Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr 1998;67:952S–9S. [DOI] [PubMed] [Google Scholar]
- 34. Linder MC. Biochemistry of copper. New York: Plenum; 1991. [Google Scholar]
- 35. Williams DM. Copper deficiency in humans. Semin Hematol 1983;20:118–28. [PubMed] [Google Scholar]
- 36. Prohaska JR, Downing SW, Lukasewycz OA. Chronic dietary copper deficiency alters biochemical and morphological properties of mouse lymphoid tissues. J Nutr 1983;113:1583–90. [DOI] [PubMed] [Google Scholar]
- 37. Prohaska JR, Lukasewycz OA. Copper deficiency during perinatal development: effects on the immune response of mice. J Nutr 1989;119:922–31. [DOI] [PubMed] [Google Scholar]
- 38. Mulhern SA, Koller LD. Severe or marginal copper deficiency results in a graded reduction in immune status in mice. J Nutr 1988;118:1041–7. [DOI] [PubMed] [Google Scholar]
- 39. Illing AC, Shawki A, Cunningham CL, Mackenzie B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J Biol Chem 2012;287:30485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tennant J, Stansfield M, Yamaji S, Srai SK, Sharp P. Effects of copper on the expression of metal transporters in human intestinal Caco-2 cells. FEBS Lett 2002;527:239–44. [DOI] [PubMed] [Google Scholar]
- 41. Han O, Wessling-Resnick M. Copper repletion enhances apical iron uptake and transepithelial iron transport by Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 2002;282:G527–33. [DOI] [PubMed] [Google Scholar]
- 42. Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest 2005;115:1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jacobs A. Sex differences in iron absorption. Proc Nutr Soc 1976;35:159–62. [DOI] [PubMed] [Google Scholar]
- 44. Harrison-Findik DD. Gender-related variations in iron metabolism and liver diseases. World J Hepatol 2010;2:302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Turnlund JR, Keyes WR, Peiffer GL, Scott KC. Copper absorption, excretion, and retention by young men consuming low dietary copper determined by using the stable isotope 65Cu. Am J Clin Nutr 1998;67:1219–25. [DOI] [PubMed] [Google Scholar]
- 46. Turnlund JR, Keyes WR, Anderson HL, Acord LL. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am J Clin Nutr 1989;49:870–8. [DOI] [PubMed] [Google Scholar]
- 47. Kim BE, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab 2010;11:353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Legleiter LR, Spears JW. Plasma diamine oxidase: a biomarker of copper deficiency in the bovine. J Anim Sci 2007;85:2198–204. [DOI] [PubMed] [Google Scholar]
- 49. Broderius M, Mostad E, Wendroth K, Prohaska JR. Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents. Comp Biochem Physiol C Toxicol Pharmacol 2010;151:473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Olivares M, Mendez MA, Astudillo PA, Pizarro F. Present situation of biomarkers for copper status. Am J Clin Nutr 2008;88:859S–62S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.