Abstract
Background
Dietary nondigestible, short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharides (GFAs) lower the effector response in cow-milk-allergic (CMA) mice; and forkhead box P3 (Foxp3)–positive regulatory T cells (Tregs) were shown to contribute to this.
Objective
The aim of this study was to assess the contribution of interleukin 10 (IL-10) and transforming growth factor β (TGF-β) to the protective effect of the GFA diet in CMA mice.
Methods
Female C3H/HeOuJ mice, 3–4 wk old, were orally sensitized with cholera toxin (Sham) or whey and cholera toxin (Whey) 1 time/wk for 5 consecutive weeks and challenged with whey 1 wk later. The mice were fed a control or 1% GFA (9:2:1) (Whey+GFA) diet starting 2 wk before the first sensitization. In a second experiment, the mice were also injected with αIL-10 receptor (αIL-10r), αTGF-β, or isotype control antibodies 24 h before each sensitization. The acute allergic skin response, anaphylaxis score, whey-specific IgE, mucosal mast cell protease 1 (mMCP-1), and Treg frequency in the mesenteric lymph nodes (MLNs) and intestinal Foxp3, Il10, and Tgfb mRNA expression were determined.
Results
In Whey+GFA mice, intestinal Il10, Tgfb, or Foxp3 mRNA expression was 2–10 times higher (P < 0.05) and the MLN Treg frequency was 25% higher compared with Whey mice (P < 0.05). The acute allergic skin response was 50% lower in Whey+GFA mice compared with Whey mice (P < 0.01), and IL-10 receptor (IL-10r) or TGF-β neutralizing antibodies prevented this protective effect (P < 0.001). The Whey mice had higher serum mMCP-1 concentrations and whey–immunoglobulin E (-IgE) levels than Sham mice (P < 0.01), whereas these were not higher in Whey+GFA mice, and neutralizing antibodies partially interfered with these responses.
Conclusions
Dietary GFAs enhance the Treg frequency in the MLNs and mucosal IL-10 and TGF-β transcription while suppressing the allergic effector response. Neutralizing antibodies showed that the allergy-protective effect of the GFA diet was mediated by IL-10 and TGF-β in CMA mice.
Keywords: cow-milk allergy, nondigestible oligosaccharides, prevention, regulatory T cells, IL-10, TGF-β, mouse model
Introduction
Cow-milk allergy (CMA) is one of the most common food allergies among young children, affecting 2–3% of infants worldwide (1–3). Most of these children acquire tolerance against cow-milk proteins between the ages of 3 and 5 y (2, 4, 5). However, children who have or had CMA are predisposed to develop other food allergies or asthma later in life (6, 7). There are many different approaches to support tolerance induction, which include the use of specific dietary components to prevent the development of the allergic response to cow-milk protein. The effectiveness of a mixture of short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharides (GFAs) has been studied in mice sensitized with cow-milk-protein whey (1, 4). These oligosaccharides structurally and functionally mimic specific aspects of oligosaccharides present in human milk and can have a positive effect on the immune system and growth of beneficial bacteria in the intestine. They can partially prevent CMA symptoms in mice and were shown to reduce the risk of developing atopic dermatitis in infants (8–11).
Acquiring oral tolerance occurs through deletion, anergy, or active suppression (7, 12). Dendritic cells (DCs) take up a food particle and present it to naïve T cells in the Peyer's patches or mesenteric lymph nodes (MLNs), from where the activated T cells home back to the lamina propria (LP) in the small intestine. Under normal circumstances, anergy or deletion results in silencing or loss of T cells having recognition for epitopes from the food protein. Alternatively, various regulatory T cells (Tregs) develop, and these specific Tregs are important to acquire oral tolerance because they induce hyporesponsiveness via active suppression (12–15). If acquiring tolerance is disturbed, it can lead to an allergic reaction. Hadis et al. (16) showed that deleting forkhead box P3–positive (Foxp3+) Tregs breaks tolerance in an ovalbumin (OVA) mouse model. This break in tolerance was also observed when Tregs could not home toward the gut (16). In addition, transfer of splenic Tregs from CMA mice fed a specific oligosaccharide diet was shown to protect control diet–fed recipient mice from developing allergic symptoms when injected before sensitization with whey protein (17). This implicates an important role for this cell type in acquiring tolerance to food proteins and its involvement in allergy protection induced by dietary oligosaccharides.
When DCs skew naïve T cells toward a regulatory phenotype to induce active peripheral tolerance, the cytokines IL-10 and TGF-β play an important role (18). DCs produce TGF-β in the MLNs and, together with retinoic acid, induce Foxp3+ Tregs (19, 20). Furthermore, Tregs secrete TGF-β as well as IL-10 to inhibit proliferation and activation of other immune cells (20). Both IL-10 and TGF-β can inhibit the development and cytokine production of T-helper cells and cytokine production from mast cells (21). For a more complete overview of the functions of IL-10 and TGF-β, the reader is referred to Taylor et al. (22) and du Pre and Samsom (23).
To establish the role of IL-10 and TGF-β in tolerance compared with food allergy, various studies were performed. It was shown that cultured splenocytes of orally tolerized mice produce significantly higher concentrations of IL-10 than splenocytes of allergic mice (24). Furthermore, injecting IL-10 before applying a specific contact allergen inhibited the allergic response (25). For TGF-β it was shown that adding TGF-β to formula milk led to a beneficial T-helper 1 immune profile in a CMA rat model during suckling and even after weaning when the rats were rechallenged (26). In addition, it was shown that orally administrated TGF-β in an OVA-allergic mouse model is active in the intestine and enhances the induction of oral tolerance (27). In addition, orally administrated TGF-β after weaning could prolong the beneficial effects of breast milk in OVA-challenged mice (28). Hence, there are several indications that IL-10 and TGF-β are important in tolerance induction against food particles.
Currently, it is not known whether the preventive effect of nondigestible oligosaccharides in CMA occurs through indirect interactions with the intestinal microbiome, direct cell interactions with epithelial or immune cells, or interactions via soluble factors such as IL-10 or TGF-β locally in the intestine. From a previous experiment using GFAs in a similar setting, we have a strong indication that GFAs could act via either IL-10 or TGF-β (29). In the latter study, higher intestinal Il10 or Tgfb mRNA expression was observed in the GFA-fed allergic mice.
The aim of the current study was to assess the contribution of IL-10 and TGF-β to the protective effect of the GFA diet in CMA. Therefore, TGF-β or the receptor of IL-10 (IL-10r) was neutralized via specific antibody treatment before each oral sensitization to determine if this could abrogate the protective effect of the GFA diet.
Methods
Diet
A semisynthetic cow-milk-protein–free AIN-93G–based diet (milk proteins were replaced with soy proteins) was composed and mixed with an isocaloric supplementation of nondigestible oligosaccharides by Research Diet Services (Wijk bij Duurstede). One percent of a mixture of short-chain galacto-oligosaccharides, long-chain fructo-oligosaccharides, and pectin-derived acidic oligosaccharides (GFAs; 75.0%, 16.7%, and 8.3%, respectively) was added to the diet (17).
Animal model
Three- to four-week-old specific pathogen–free female C3H/HeOuJ mice, bred for ≥2 generations on a cow milk–free diet, were purchased from Charles River Laboratories (Saint Germain Nuelles, France). Mice were fed the control diet or the GFA diet starting directly at arrival for 2 wk before and during oral sensitization with the use of cholera toxin and whey protein (WPC60; Milei). Mice were orally sensitized via gavage 1 time/wk for 5 consecutive weeks, and at day 33, the mice were anesthetized and the acute allergic skin response and anaphylactic symptom scores were measured 30 min after intradermal whey challenge in the ear, as described previously (8). Mice were given an oral challenge at day 34 and were killed 18 h later via terminal bleeding under isoflurane/air anesthesia followed by cervical dislocation. Blood was collected and serum was stored at −20ºC until measurement of mouse mast cell protease 1 (mMCP-1; ELISA from Ebiosciences) and whey-specific IgE, as described previously (30). Animal procedures were approved by an independent ethics committee for animal experimentation (Animal Ethics Committee of Utrecht University, Utrecht, Netherlands) and complied with the principles of good laboratory animal care following the European Directive for the protection of animals used for scientific purposes. The group size in experiment 1 was calculated by using 2 × (Power[(Za + Zb)/2]) × Power(variation/2)/Power(difference/2), with Za = 1.96 and Zb = 1.28, using 17% for variation and 25% for the difference. The statistical power was calculated on the basis of the expected result for the acute skin response. In the experimental set-up for experiment 1 (Figure 1) no antibody treatment was used and power calculations, which would allow significant differences between the groups, were accepted at n = 6. The set-up for experiment 2 was according to the same protocol; however, in addition, the mice were injected intraperitoneally with either Rat IgG serum isotype control (Sigma-Aldrich), or an αIL-10 receptor (αIL-10r) antibody (Biolegend) or an αTGF-β antibody (200 μg/mouse), 24 h before each oral sensitization (Figure 1). The TGF-β hybridoma (clone 1D11) was cultured in IMDM Iscove's modified dulbecco's medium containing 1% FCS and gentamycin. Clarified supernatant was used to purify the antibody using affinity chromatography. The antibody was sterile preserved in PBS. For these studies, n = 10 mice/group (depending on the treatment) were used because the statistical power was calculated on the basis of the same expected difference in the acute skin response, but due to the use of antibodies a larger variation was accounted for. Due to the study size, this study was performed in 2 cohorts, which alternated 1 wk, and data were combined. For the acute skin response, an additional cohort of n = 4–6 mice was analyzed and added to the data set. All of the mice were housed under specific-pathogen-free conditions and fed ad libitum with unlimited access to water. Mice were housed with n = 6–10/cage, and cages were enriched with shelter and nesting material and kept at a reversed light-dark cycle.
FIGURE 1.
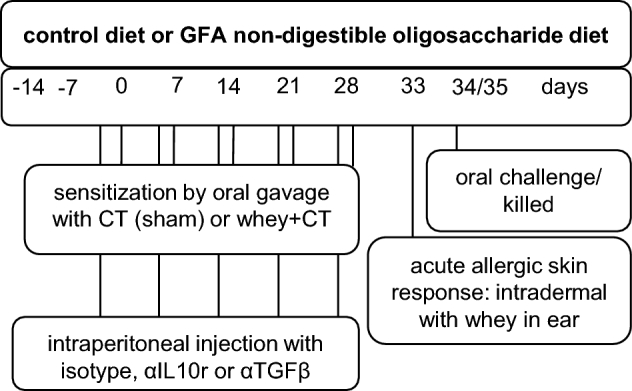
Schematic overview of the experimental designs. The first experimental set-up (data shown in experiment 1) was performed without injections of antibodies, whereas the second experimental set-up (data shown in experiment 2 and Supplemental Figure 1) included antibody injections. The intraperitoneal injection protocol is shown in the gray box written in black. CT, cholera toxin; GFA, short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharide; αIL10r, αIL-10 receptor.
Flow cytometry
MLNs were removed, placed on ice in Roswell Park Memorial Institute 1640/penicillin-streptomysin 1%, processed through a 70-nm filter to form single-cell suspension, and blocked for 30 min in PBS containing 1% BSA and 5% heat-inactivated FBS. 1x10E6 Cells were incubated at 4ºC for 30 min with CD8a-APC-Cy7; CD11c-PerCP-Cy5.5 and CD25-Pe-Cy7 were used from BD Biosciences and CD4-PerCP-Cy5.5, CD103-APC, and Foxp3-APC from eBiosciences or matching isotype controls. Extracellular stained cells were fixed with the use of 2% paraformaldehyde, and intracellular staining was performed according to the manufacturer's instructions (eBiosciences). The flow cytometry data are shown as a percentage of the control data (Sham).
Immunohistochemistry
Swiss rolls of the proximal small intestine were fixed in neutral 10% formalin for ≥24 h, and paraffin sections were embedded with the use of a LeicaTP1020, Leica biosystems, Amsterdam, The Netherlands. Tissue processing and Foxp3 staining were performed according to van den Elsen et al. (31). Foxp3+ cells were counted only in completely attached villi and crypts, and the number per villus/crypt or crypt unit was calculated.
qPCR
One centimeter of the proximal and distal small intestine and colon was collected shortly after killing and stored in RNAlater(Qiagen GmbH) at 4°C until further processing, which was described previously by Kerperien et al. (29). Validated primers for ribosomal protein S13 (Rps13), Foxp3, Tgfb1, and Il10 were purchased from SAbioscience (Qiagen). mRNA levels were calculated with CFX Manager software (version 1.6) and corrected for the expression of Rps13 with 100 × 2(Rps13 − gene of interest), as described previously (32).
Statistical analysis
For experiment 1, a multiple-comparison test of the whole data set was performed with the use of 1-factor ANOVA and Bonferroni post hoc test to correct for multiple comparisons (Graphpad Prism software, version 6). For experiment 2, all of the data except for the anaphylaxis score were analyzed with 1-factor ANOVA and Bonferroni post hoc test with preselected pairs (Graphpad Prism software, version 6). The preselected pairs were as follows: sham-sensitized mice fed the control diet (Sham) compared with all other groups, whey-sensitized mice fed the control diet (Whey) compared with whey-sensitized mice fed the GFA diet (Whey+GFA) or whey-sensitized mice fed the control diet treated with αIL-10r (Whey+αIL-10r) or whey-sensitized mice fed the control diet treated with αTGF-β; (Whey+αTGF-β;) Whey+GFA compared with whey-sensitized mice fed the GFA diet treated with αIL-10r (Whey+GFA+αIL-10r) or whey-sensitized mice fed the GFA diet treated with αTGF-β; (Whey+GFA+αTGF-β;) Whey+αIL-10r compared with Whey+GFA+αIL-10r or Whey+αTGF-β; Whey+GFA+αIL-10r compared with Whey+GFA+αTGF-β; and Whey+αTGF-β compared with Whey+GFA+αTGF-β. If required, log transformation was used to normalize data distribution. The anaphylaxis score (nonparametrical data, scores are defined stepwise from 0 to 4) was evaluated with the Kruskal-Wallis and Dunn's post hoc tests. P values <0.05 were considered significant, and data are shown as means ± SEMs.
Results
Acute allergic skin response and intestinal qPCR analysis (experiment 1)
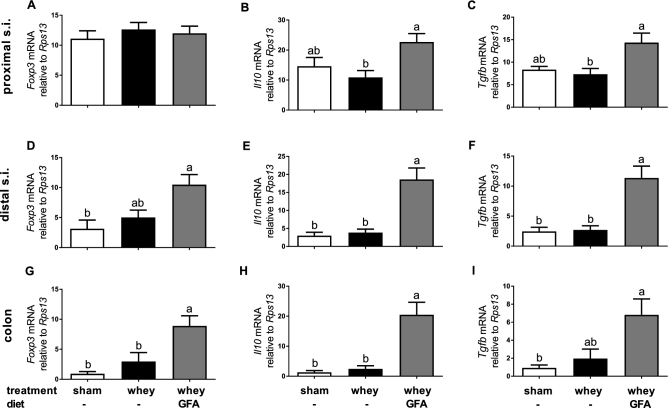
The acute allergic skin response was determined in experiment 1 in Sham, Whey, and Whey+GFA. The significantly higher acute skin response in the Whey compared with the Sham group (P < 0.0001) was reduced in the Whey+GFA group (P < 0.0001) (Figure 2). In the proximal part of the small intestine, only in Whey+GFA mice, the relative expression of Il10 and Tgfb was significantly higher compared with Sham mice (P < 0.05) (Figure 3A–C). In the distal ileum and colon, Foxp3, Il10, and Tgfb were significantly higher in the Whey+GFA group compared with the Whey and Sham groups (P < 0.05) (Figure 3D–I). One mouse died during the sensitization period, and some intestinal samples, did not yield enough RNA; therefore, the sample size became n = 4–6.
FIGURE 2.
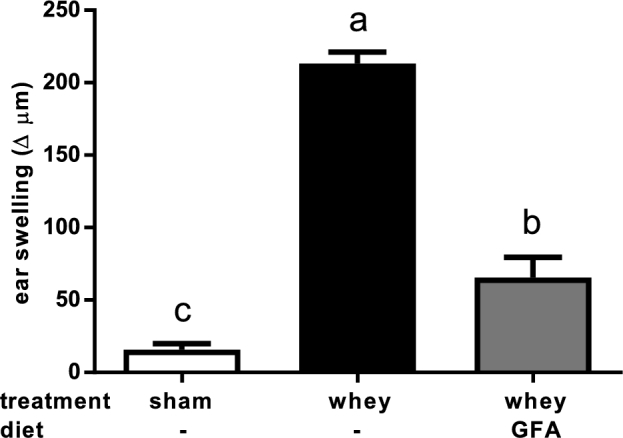
Acute allergic skin response in sham mice fed a control diet or in whey-sensitized mice fed a control or a GFA diet (experiment 1). The acute allergic skin response was measured 1 h after intradermal injection with whey in the ears. Values are means ± SEMs, n = 5–6. Labeled means without a common letter differ, P < 0.05. Δ indicates ear thickness before intradermal injection deducted from thickness after intradermal injection. GFA, short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharide.
FIGURE 3.
mRNA markers in the intestine in sham mice fed a control diet or whey-sensitized mice fed a control or a GFA diet (experiment 1). In the proximal small intestine (A–C), distal small intestine (D–F), and proximal colon (G–I), the relative expressions of consecutive transcription factor Foxp3, Il10, and Tgfb relative to Rps13 are shown. Values are means ± SEMs, n = 3–6. Labeled means without a common letter differ, P < 0.05. Foxp3, forkhead box P3; GFA, short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharide; Rps13, ribosomal protein S13; s.i., small intestine; Tgfb, transforming growth factor β.
Contribution of IL-10 and TGF-β to the allergy-protective effect of GFAs (experiment 2)
In the next experimental set-up (experiment 2), the effects of IL-10r or TGF-β neutralizing antibodies were studied and Sham mice were injected with an isotype antibody (Sham+isotype). Allergic control-diet-fed isotype-treated mice (Whey+isotype) showed a significantly higher acute allergic skin response compared with Sham+isotype mice (P < 0.001), which was suppressed by the GFA diet (P < 0.05) (Figure 4A). The significantly lower acute allergic skin response in mice fed the GFA diet was prevented when Whey+GFA mice were treated with either αIL-10r (Whey+GFA+αIL-10r) or αTGF-β (Whey+GFA+αTGF-β) (P < 0.001) (Figure 4A). For the anaphylactic symptom score, only Whey+isotype mice showed higher shock compared with Sham+isotype mice (P < 0.01) (Figure 4B). The anaphylactic symptom score did not differ between the whey-sensitized mice fed the GFA diet treated with isotype (Whey+GFA+isotype mice) and Whey+GFA+αIL-10r or Whey+GFA+αTGF-β mice. In 5 mice, no ear thickness measurement could be obtained (1 in Whey, 1 in Whey+GFA, 2 in Whey+αTGF-β, 1 in Whey+GFA+αTGF-β); these were removed from the analysis.
FIGURE 4.
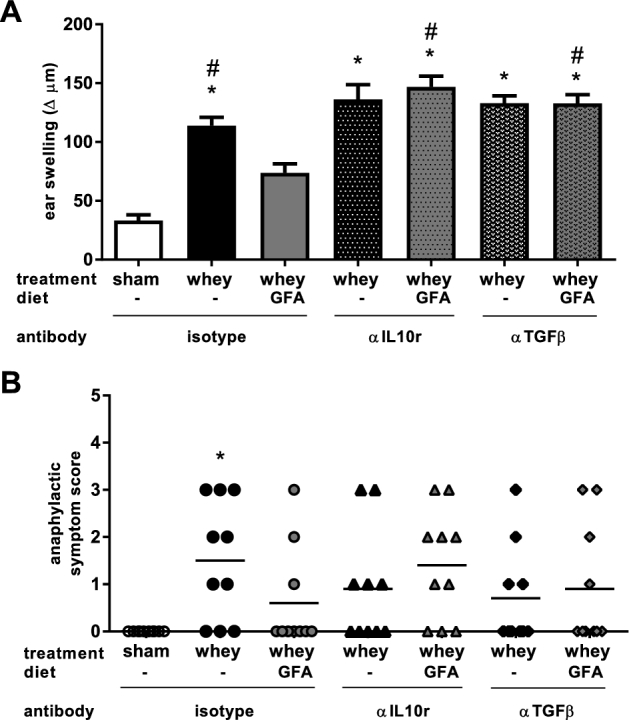
Clinical symptoms in sham mice fed a control diet and whey-sensitized mice fed a control or GFA diet treated with isotype control or αIL-10r or αTGF-β antibody (experiment 2). (A) The acute allergic skin response was measured 1 h after intradermal injection with whey in the ears. Δ indicates ear thickness before intradermal injection deducted from thickness after intradermal injection. Values are means ± SEMs, n = 10–16, compiled from 3 cohorts. Data were analyzed by using 1-factor ANOVA and Bonferroni post hoc test with preselected pairs. (B) The anaphylactic symptom scores of sham-sensitized mice fed a control diet and whey-sensitized mice fed a control or GFA diet treated with an isotype: αIL-10r or αTGF-β antibody (n = 10). Data were analyzed with the Kruskal-Wallis and Dunn's post hoc test. *Different from sham, P < 0.05; #different from whey+GFA, P < 0.05. GFA, short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharide; αIL10r, αIL-10 receptor.
mMCP-1 and whey-specific IgE (experiment 2)
In the Whey+isotype mice, serum mMCP-1 concentrations were higher than in the Sham+isotype group (P < 0.01) (Figure 5A). Serum mMCP-1 was not higher in the Whey+GFA mice, whereas it was higher in the Whey+GFA mice treated with αIL-10r or αTGF-β than in Sham+isotype mice (P < 0.01) (Figure 5A). Whey-specific IgE was higher in Whey+isotype mice (P < 0.01) but not in Whey+GFA+isotype mice (Figure 5B). However, in the Whey+GFA+αTGF-β mice, whey-specific IgE levels tended to be higher compared with Sham+isotype mice (P = 0.06) (Figure 5B). The results for whey-specific IgE remain inconclusive in Whey+GFA+αIL-10r mice. In 8 mMCP-1 serum samples (3 in Whey, 2 in Whey+GFA, 3 in Whey+αTGF-β) and 2 whey-IgE serum samples (1 in Whey, 1 in Whey+αTGF-β) of whey-sensitized mice the ELISA signal remained below the level of detection and therefore these were removed from the analysis.
FIGURE 5.
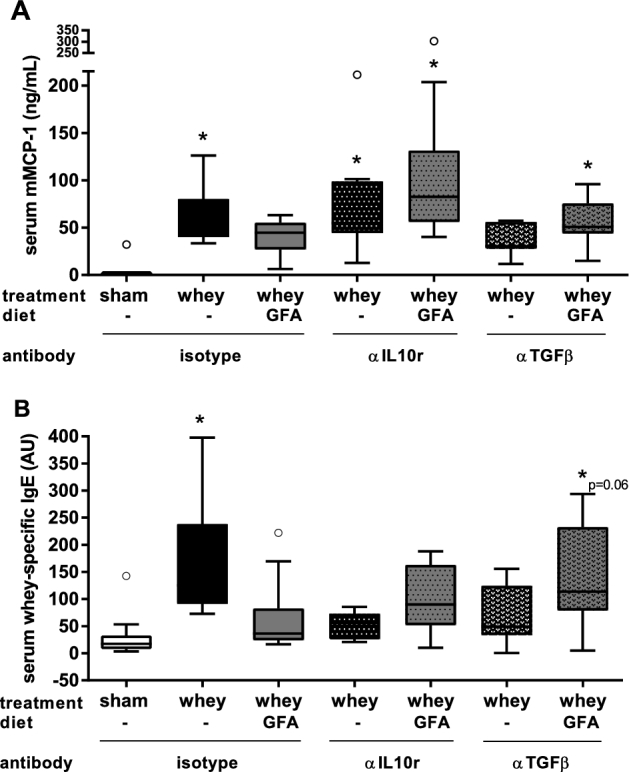
mMCP-1 and whey-specific IgE measured in serum of sham mice fed a control diet and whey-sensitized mice fed a control or GFA diet injected with an isotype control or an αIL-10r or αTGFβ antibody (experiment 2). (A) mMCP-1 (ng/mL) and (B) whey-specific-IgE were measured in serum. Values are means ± SEMs, n = 7–10. Data were analyzed by using 1-factor ANOVA and Bonferroni post hoc test with preselected pairs, if required. Log transformation was used to normalize data distribution. The circles represent outliers. *Different from sham (P < 0.05) or tended to differ, as indicated. AU, arbitrary units; GFA, short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharide; mMCP-1, mouse mast cell protease 1; αIL10r, αIL-10 receptor.
CD103+ DCs and Tregs in the MLNs (experiment 2)
The relative percentage of CD11c+ CD103+ DCs in Whey+αIL-10r or Whey+GFA+αIL-10r mice was higher compared with this DC subset in Whey+isotype mice in the MLNs (Figure 6A). The relative percentage of CD4+ CD25+ Foxp3+ Tregs was significantly higher in Whey+GFA+isotype compared with Sham+isotype mice (P < 0.05) (Figure 6B). The Treg population in Whey+αIL-10r or Whey+GFA+αIL-10r mice was significantly higher compared with Sham+isotype mice (P < 0.01). For both the CD103+ DCs and the Foxp3+ Tregs, flow cytometry dot plots are shown for the Whey+isotype and the Whey+αIL-10r groups (Supplemental Figure 1).
FIGURE 6.
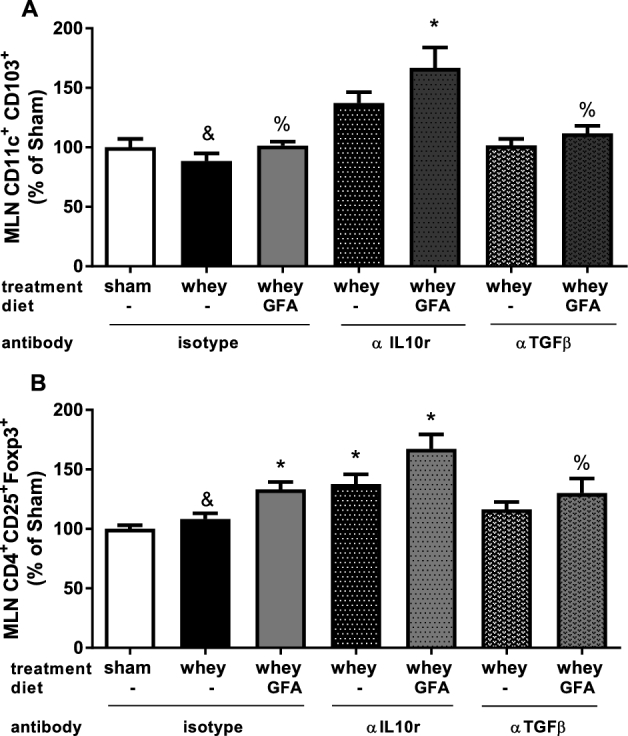
Percentage of CD103+ DCs and Foxp3+ CD25+ Tregs in the MLNs of sham mice fed a control diet and whey-sensitized mice fed a control or GFA diet treated with an isotype control or an αIL-10r or αTGF-β antibody relative to Sham (experiment 2). MLNs were stained for DC (CD11c-PERCP-Cy5.5+ and CD103-APC+) (A) and Tregs (CD4-PERCP-Cy5.5+ CD8a-APC-Cy7-, Foxp3-APC+ CD25-PE-Cy7+) (B). Results are shown in percentages relative to the mean of sham sensitized mice fed a control diet injected with the isotype antibody, which was set to 100%. Values are means ± SEMs, n = 9–10. Data were analyzed by using 1-factor ANOVA and Bonferroni post hoc test with preselected pairs. *Different from sham, P < 0.05; &different from whey+αIL-10r, P < 0.05; %different from whey+GFA+αIL-10r, P < 0.05. Representative flow cytometric plots are shown with actual percentages in the plots in Supplemental Figure 1. DC, dendritic cell; GFA, short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharide; Foxp3, forkhead box P3; MLN, mesenteric lymph node; Treg, regulatory T cell; αIL10r, αIL-10 receptor.
Foxp3+ cells in the LP of the small intestine (experiment 2)
After DCs have activated T cells in the MLNs, these T cells can be instructed to migrate toward the intestinal LP; therefore, Foxp3+ cells were stained in the intestinal LP (Figure 7). In the proximal part of the small intestine of the Whey+GFA+isotype mice, the Foxp3+ counts were not significantly higher than in Sham+isotype mice. However, significantly greater numbers of Foxp3+ cells were counted in both the Whey+αIL-10r as well as the Whey+GFA+αIL-10r group compared with Sham+isotype mice (P < 0.05) (Figure 7).
FIGURE 7.
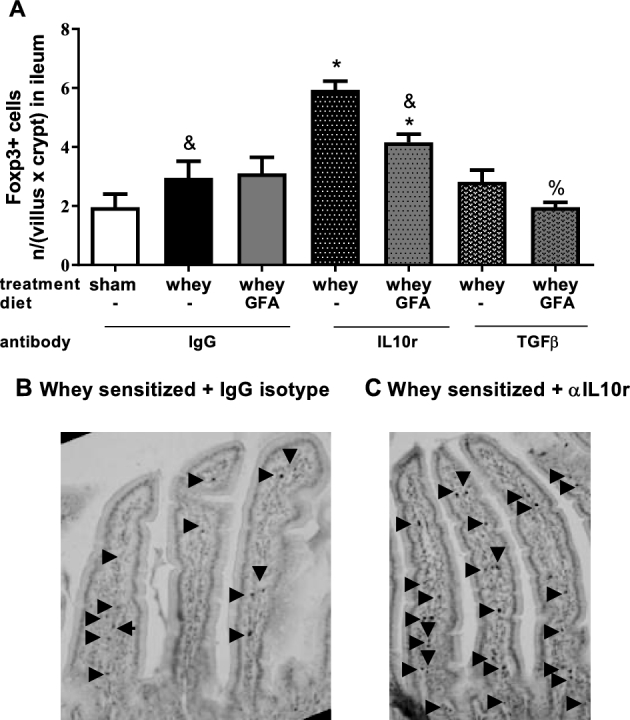
Foxp3+ cells in the proximal part of the small intestine (experiment 2). (A) IHC staining of Foxp3+ cells in the first part of the intestine of sham mice fed a control diet and whey-sensitized mice fed a control or GFA diet and treated with an isotype control or an αIL-10r or αTGF-β antibody in whey-sensitized mice fed a control diet treated with isotype (B) and in whey-sensitized mice fed a control diet treated with αIL-10r (C). Values are means ± SEMs, n = 3–4 (represented as Foxp3+ [n/(villus × crypt)]). *Different from sham, P < 0.05; &different from whey+αIL-10r, P < 0.05; %different from whey+GFA+αIL-10r, P < 0.05. → indicates positive intracellular staining for Foxp3. Foxp3, forkhead box P3; GFA, short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharide. IHC, immunohistochemical; IL10r, IL-10 receptor; αIL10r, αIL-10 receptor.
Discussion
In the current study, the contribution of IL-10 and TGF-β to the protective effect of a dietary intervention with GFAs in CMA prevention was investigated. We and others have shown that GFAs can effectively reduce allergic symptoms (8, 29, 33, 34). It was also shown that Tregs play a role in this CMA-protective effect, therefore we aimed to study the contribution of the soluble factors IL-10 and TGF-β. IL-10 and TGF-β support the development of Tregs, are produced by Tregs, and contribute to their suppressive effect (17, 35). In humans affected with CMA, lower amounts of IL-10 and TGF-β are produced by lymphocytes isolated from peripheral blood after in vitro stimulation with cow milk (36–38).
In our study, dietary intervention with GFAs reduced the acute allergic skin response while increasing Foxp3+ Tregs in the MLNs. By using qPCR it was observed that GFAs enhanced the expression IL-10 and TGF-β along the intestinal tract of the CMA mice. IL-10 and TGF-β are regulatory cytokines that are important for oral tolerance induction and proper immune function. The protective effect of GFAs on the acute allergic skin response in the CMA model was abolished upon injection with an αIL-10r or αTGF-β antibody (P < 0.001) and a similar pattern was shown for the anaphylactic symptom score, although significance was not attained. Anti–IL-10r or anti–TGF-β treatment did not negatively affect the acute allergic skin response, whey-specific IgE levels, or the anaphylactic symptom scores in control diet–fed CMA mice.
IL-10 and TGF-β are, among others, derived from Tregs, and the frequency of Foxp3+ Tregs in the MLNs of GFA-fed CMA mice was enhanced. However, the αIL-10r treatment itself resulted in a higher percentage of Foxp3+ Tregs in the MLNs and proximal part of the small intestine in both the control as well as the GFA-fed CMA mice (P < 0.05). This unexpected finding may have several explanations. In previous studies it was shown that Tregs derived from anti–IL-10 antibody exposed co-cultures of DCs, and neither Tregs nor Tregs derived from Il10−/− knockout mice could not suppress effector T cell activation (39). Hence, Tregs that develop in the absence of IL-10 were phenotypically normal but had lost their suppressive function. However, Tregs are also known to develop in the absence of IL-10, which is dependent on TGF-β (40). Indeed, in αIL-10r–treated mice the population of CD103+ DCs reached a higher percentage and TGF-β is known to contribute to the development of these cells that are involved in the generation of Foxp3+ Tregs (19).
In addition to the possibility that the Tregs lost their suppressive function upon αIL-10r treatment, it also may have blocked IL-10r on the target effector cells, making them nonresponsive to the effect of IL-10. Indeed, mucosal mast cell–derived mMCP-1 was higher in whey-sensitized mice fed the control diet with or without αIL-10r and in allergic mice fed the GFA diet and treated with the αIL-10r antibody (P < 0.05). However, mMCP-1 concentrations were not higher in mice fed GFAs. This suggests that IL-10 produced by cells generated in mice fed the GFA diet could be involved in the suppression of mast cell degranulation. IL-10 is known to suppress murine and human mast cell IgE receptor expression and signaling in vitro and in vivo in mice (41). Hence, IL-10 is suggested to be functionally involved in the protective effect of the GFA diet and may suppress effector cell degranulation.
In addition, the αTGF-β antibody abolished the protective effect of the GFA diet on the acute allergic skin response in CMA mice (P < 0.001). CMA mice fed the GFA diet had low whey-IgE and mMCP-1, whereas neutralization of TGF-β in these mice resulted in significantly higher mMCP-1 and the same tendency was shown for whey-IgE, comparable to that in control diet–fed CMA mice (P < 0.05). Thus, TGF-β may be involved in the protective effect of the GFA diet on whey sensitization and mast cell degranulation as well. However, this result remains inconclusive because αTGF-β treatment in control diet–fed whey-sensitized mice also resulted in low whey-IgE and mMCP-1 concentrations.
The acute allergic skin response is a reflection of effector cell degranulation, such as mast cells in the ear. The effect of TGF-β on mast cells depends on timing and other cytokines present. TGF-β can act as a chemoattractant for mast cells and enhance extracellular release of mMCP-1 together with IL-3 and IL-9, but TGF-β can also inhibit mast cell activation via attenuation of FcεR receptor expression (42–44). Recently, TGF-β isoforms 1 and 3 were shown to have suppressive effects on class switching and affinity maturation for IgG and IgA isoforms in human B cells (45). Hence, the anti-TGFβ treatment may have affected the production of IgE directly because TGF-β is known to significantly reduce STAT6 activation in the nucleus of B cells and STAT6 is critical for IgE class switching in B cells (46, 47). This may explain the dual outcome of the findings. The αTGF-β antibody effectively inhibited the protective effect of the GFA diet on acute allergic symptoms, indicating that TGF-β is possibly involved via inhibition of IgE production or mast cell degranulation. By contrast, TGF-β neutralization itself may have contributed to reduced mast cell recruitment and could have led to suppressed whey-IgE generation (48).
Neutralization of IL-10r may have implications for the capacity of TGF-β to instruct Tregs, and vice versa, neutralization of TGF-β may affect the generation of IL-10 secreting Tregs. According to Maynard et al. (40), in the small intestine, Foxp3− IL10+ Tregs were most prevalent and were most similar to type 1 Tregs (TR1). This regulatory subset is dependent on TGF-β for their development. They also show that all IL-10+ Treg subsets are dependent on TGF-β for their induction and maintenance (40). In addition to modifying mast cell function, TGF-β also controls Treg maintenance and is therefore, like IL-10, important in acquiring oral tolerance in CMA.
Mice fed the GFA diet had higher Il10 and Tgfb mRNA levels in intestinal tissue, which may be associated with the protective effect because neutralization of either one of these mediators results in total loss of the protective effect. Currently, it is not certain whether GFAs enhance intestinal Il10 and Tgfb mRNA expression directly or indirectly. Eiwegger et al. (49) showed that there is in vitro evidence of epithelial transport of oligosaccharides and that acidic oligosaccharides induce IL-10. According to Lehmann et al. (50), GFAs can directly influence DCs to produce IL-10 and to upregulate functional Tregs. These are indications that GFAs may also regulate DC function directly. However, GFAs may also modulate the intestinal microbiome, hereby contributing to allergy protection.
In summary, in allergic mice fed the GFA diet the percentage of Tregs in the MLNs and mucosal Il10 and Tgfb mRNA expression were higher compared with the control diet. Furthermore, the protective effect of the dietary intervention with GFAs on allergic symptoms in the murine CMA model can be prevented via αIL-10r or αTGF-β treatment. Considering that Tregs are involved in allergy protection (16, 17), this protective effect of the GFA diet may involve Treg-derived IL-10 and TGF-β.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—JK, LMJK, and LEMW: designed the research; JK, DV-G, TW, BCAMvE, GAH, and PC: conducted the research; LB: provided essential materials; JK, DV-G, PVJ, PC, and LEMW: analyzed data or performed statistical analysis; JK, DV-G, JG, LMJK, and LEMW: wrote or critically read the manuscript; JK and LEMW: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Author disclosures: JK, GAH, PC, and LEMW, no conflicts of interest. DV-G, TW, and PVJ are employed by Nutricia Research and report personal fees and nonfinancial support from Nutricia Research during the conduct of the study. BCAMvE is partly employed by Nutricia Research and reports personal fees and nonfinancial support from Nutricia Research. LB is employed by Bioceros but there is no conflict of interest. JG is partly employed by Nutricia Research and reports personal fees and nonfinancial support from Nutricia Research during the conduct of the study; in addition, he has a patent pending for nondigestible oligosaccharides for oral induction of tolerance against dietary proteins to Nutricia Research, Netherlands. LMJK is employed by Nutricia Research and report personal fees and nonfinancial support from Nutricia Research during the conduct of the study; in addition, he has a patent pending for nondigestible oligosaccharides for oral induction of tolerance against dietary proteins to Nutricia Research, Netherlands.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used:
- CMA
cow-milk allergy (allergic)
- DC
dendritic cell
- Foxp3
forkhead box P3
- GFA
short-chain galacto-, long-chain fructo-, and pectin-derived acidic oligosaccharide
- IL-10r
IL-10 receptor
- LP
lamina propria
- MLN
mesenteric lymph node
- mMCP-1
mouse mast cell protease 1
- OVA
ovalbumin
- RPS13
ribosomal protein S13
- Sham
sham-sensitized mice fed the control diet
- Sham+isotype
sham-sensitized mice injected with an isotype antibody
- Treg
regulatory T cell
- Whey
whey-sensitized mice fed the control diet
- Whey+GFA
whey-sensitized mice fed the GFA diet
- Whey+GFA+isotype
whey-sensitized mice fed the GFA diet treated with isotype
- Whey+GFA+αIL-10r
whey-sensitized mice fed the GFA diet treated with αIL-10r
- Whey+GFA+αTGFβ
whey-sensitized mice fed the GFA diet treated with αTGF-β
- Whey+isotype
whey-sensitized mice fed the control diet treated with isotype
- Whey+αIL-10r
whey-sensitized mice fed the control diet treated with αIL-10r
- Whey+αTGF-β
whey-sensitized mice fed the control diet treated with αTGF-β
- αIL-10r
αIL-10 receptor
References
- 1. Huang F, Kim JS. IgE-mediated cow's milk allergy in children. Curr Allergy Asthma Rep 2012;12:630–40. [DOI] [PubMed] [Google Scholar]
- 2. Sanchez-Garcia S, Cipriani F, Ricci G. Food allergy in childhood: phenotypes, prevention and treatment. Pediatr Allergy Immunol 2015;26:711–20. [DOI] [PubMed] [Google Scholar]
- 3. Du Toit G, Foong RX, Lack G. The role of dietary interventions in the prevention of IgE-mediated food allergy in children. Pediatr Allergy Immunol 2017;28:222–9. [DOI] [PubMed] [Google Scholar]
- 4. Brozek JL, Terracciano L, Hsu J, Kreis J, Compalati E, Santesso N, Fiocchi A, Schunemann HJ. Oral immunotherapy for IgE-mediated cow's milk allergy: a systematic review and meta-analysis. Clin Exp Allergy 2012;42:363–74. [DOI] [PubMed] [Google Scholar]
- 5. Fiocchi A, Schunemann HJ, Brozek J, Restani P, Beyer K, Troncone R, Martelli A, Terracciano L, Bahna SL, Rance F et al. , Diagnosis and Rationale for Acti on Against Cow's Milk Allergy (DRACMA): a summary report. J Allergy Clin Immunol 2010;126:1119–28, e12. [DOI] [PubMed] [Google Scholar]
- 6. Host A. Frequency of cow's milk allergy in childhood. Ann Allergy Asthma Immunol 2002;89:33–7. [DOI] [PubMed] [Google Scholar]
- 7. Brandtzaeg P. Food allergy: separating the science from the mythology. Nat Rev Gastroenterol Hepatol 2010;7:380–400. [DOI] [PubMed] [Google Scholar]
- 8. Schouten B, van Esch BC, Hofman GA, van Doorn SA, Knol J, Nauta AJ, Garssen J, Willemsen LE, Knippels LM. Cow milk allergy symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J Nutr 2009;139:1398–403. [DOI] [PubMed] [Google Scholar]
- 9. Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B et al. , Prebiotic effects: metabolic and health benefits. Br J Nutr 2010;104(Suppl 2):S1–63. [DOI] [PubMed] [Google Scholar]
- 10. Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, Klarczyk M, Schopfer H, Bockler HM, Wells J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr 2005;40:36–42. [DOI] [PubMed] [Google Scholar]
- 11. Nauta AJ, Garssen J. Evidence-based benefits of specific mixtures of non-digestible oligosaccharides on the immune system. Carbohydr Polym 2013;93:263–5. [DOI] [PubMed] [Google Scholar]
- 12. Lepski S, Brockmeyer J. Impact of dietary factors and food processing on food allergy. Mol Nutr Food Res 2013;57:145–52. [DOI] [PubMed] [Google Scholar]
- 13. Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebiq H et al. , Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med 2004;199:1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vickery BP, Scurlock AM, Jones SM, Burks AW. Mechanisms of immune tolerance relevant to food allergy. J Allergy Clin Immun 2011;127:576–84; quiz: 85–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol 2012;5:232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 2011;34:237–46. [DOI] [PubMed] [Google Scholar]
- 17. Schouten B, van Esch BC, Hofman GA, Boon L, Knippels LM, Willemsen LE, Garssen J. Oligosaccharide-induced whey-specific CD25(+) regulatory T-cells are involved in the suppression of cow milk allergy in mice. J Nutr 2010;140:835–41. [DOI] [PubMed] [Google Scholar]
- 18. Soyer OU, Akdis M, Ring J, Behrendt H, Crameri R, Lauener R, Akdis CA. Mechanisms of peripheral tolerance to allergens. Allergy 2013;68:161–70. [DOI] [PubMed] [Google Scholar]
- 19. Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 2007;204:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol 2013;23:R389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marshall JS, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Investig 1996;97:1122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology 2006;117:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. du Pre MF, Samsom JN. Adaptive T-cell responses regulating oral tolerance to protein antigen. Allergy 2011;66:478–90. [DOI] [PubMed] [Google Scholar]
- 24. Shandilya UK, Kapila R, Singh S, Dahiya D, Kapila S, Kansal VK. Induction of immune tolerance to caseins and whey proteins by oral intubation in mouse allergy model. J Anim Physiol Anim Nutr (Berl) 2014;98:467–75. [DOI] [PubMed] [Google Scholar]
- 25. Enk AH, Saloga J, Becker D, Mohamadzadeh M, Knop J. Induction of hapten-specific tolerance by interleukin 10 in vivo. J Exp Med 1994;179:1397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Penttila I. Effects of transforming growth factor-beta and formula feeding on systemic immune responses to dietary beta-lactoglobulin in allergy-prone rats. Pediatr Res 2006;59:650–5. [DOI] [PubMed] [Google Scholar]
- 27. Ando T, Hatsushika K, Wako M, Ohba T, Koyama K, Ohnuma Y, Katoh R, Ogawa H, Okumura K, Luo J et al. , Orally administered TGF-beta is biologically active in the intestinal mucosa and enhances oral tolerance. J Allergy Clin Immunol 2007;120:916–23. [DOI] [PubMed] [Google Scholar]
- 28. Rekima A, Macchiaverni P, Turfkruyer M, Holvoet S, Dupuis L, Baiz N, Annesi-Maesano I, Mercenier A, Nutten S, Verhasselt V. Long-term reduction in food allergy susceptibility in mice by combining breastfeeding-induced tolerance and TGF-beta-enriched formula after weaning. Clin Exp Allergy 2017;47:565–76. [DOI] [PubMed] [Google Scholar]
- 29. Kerperien J, Jeurink PV, Wehkamp T, van der Veer A, van de Kant HJ, Hofman GA, van Esch EC, Garssen J, Willemsen LE, Knippels LM. Non-digestible oligosaccharides modulate intestinal immune activation and suppress cow's milk allergic symptoms. Pediatr Allergy Immunol 2014;25:747–54. [DOI] [PubMed] [Google Scholar]
- 30. Schouten B, van Esch BC, Hofman GA, van den Elsen LW, Willemsen LE, Garssen J. Acute allergic skin reactions and intestinal contractility changes in mice orally sensitized against casein or whey. Int Arch Allergy Immunol 2008;147:125–34. [DOI] [PubMed] [Google Scholar]
- 31. van den Elsen LW, van Esch BC, Hofman GA, Kant J, van de Heijning BJ, Garssen J, Willemsen LE. Dietary long chain n-3 polyunsaturated fatty acids prevent allergic sensitization to cow's milk protein in mice. Clin Exp Allergy 2013;43:798–810. [DOI] [PubMed] [Google Scholar]
- 32. Garcia-Vallejo JJ, Van Het Hof B, Robben J, Van Wijk JA, Van Die I, Joziasse DH, Van Dijk W. Approach for defining endogenous reference genes in gene expression experiments. Anal Biochem 2004;329:293–9. [DOI] [PubMed] [Google Scholar]
- 33. de Kivit S, Saeland E, Kraneveld AD, van de Kant HJ, Schouten B, van Esch BC, Knol J, Sprikkelman AB, van der A LB, Knippels LM et al. , Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy 2012;67:343–52. [DOI] [PubMed] [Google Scholar]
- 34. Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child 2006;91:814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van den Elsen LW, Meulenbroek LA, van Esch BC, Hofman GA, Boon L, Garssen J, Willemsen LE. CD25+ regulatory T cells transfer n-3 long chain polyunsaturated fatty acids-induced tolerance in mice allergic to cow's milk protein. Allergy 2013;68:1562–70. [DOI] [PubMed] [Google Scholar]
- 36. Savilahti EM, Savilahti E. Development of natural tolerance and induced desensitization in cow's milk allergy. Pediatr Allergy Immunol 2013;24:114–21. [DOI] [PubMed] [Google Scholar]
- 37. Pérez-Machado MA, Ashwood P, Thomson MA, Latcham F, Sim R, Walker-Smith JA, Murch SH. Reduced transforming growth factor-β1-producing T cells in the duodenal mucosa of children with food allergy. Eur J Immunol 2003;33:2307–15. [DOI] [PubMed] [Google Scholar]
- 38. Beyer K, Castro R, Birnbaum A, Benkov K, Pittman N, Sampson HA. Human milk—specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol 2002;109:707–13. [DOI] [PubMed] [Google Scholar]
- 39. Chattopadhyay G, Shevach EM. Antigen-specific induced T regulatory cells impair dendritic cell function via an IL-10/MARCH1-dependent mechanism. J Immunol 2013;191:5875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol 2007;8:931–41. [DOI] [PubMed] [Google Scholar]
- 41. Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR et al. , IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol 2008;180:2848–54. [DOI] [PubMed] [Google Scholar]
- 42. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99–146. [DOI] [PubMed] [Google Scholar]
- 43. Yamazaki S, Nakano N, Honjo A, Hara M, Maeda K, Nishiyama C, Kitaura J, Ohtsuka Y, Okumura K, Oyawa H et al. , The transcription factor Ehf is involved in TGF-beta-Induced suppression of FcepsilonRI and c-Kit expression and FcepsilonRI-Mediated activation in mast cells. J Immunol 2015;195:3427–35. [DOI] [PubMed] [Google Scholar]
- 44. Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood 1999;93:3473–86. [PubMed] [Google Scholar]
- 45. Tsuchida Y, Sumitomo S, Ishigaki K, Suzuki A, Kochi Y, Tsuchiya H, Ota M, Komai T, Inoue M, Morita K et al. , TGF-beta3 inhibits antibody production by human B cells. PloS One 2017;12:e0169646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sugai M, Gonda H, Kusunoki T, Katakai T, Yokota Y, Shimizu A. Essential role of Id2 in negative regulation of IgE class switching. Nat Immun 2003;4:25–30. [DOI] [PubMed] [Google Scholar]
- 47. Okamura T, Sumitomo S, Morita K, Iwasaki Y, Inoue M, Nakachi S, Komai T, Shoda H, Miyazaki J, Fujio K et al. , TGF-beta3-expressing CD4+CD25(-)LAG3+ regulatory T cells control humoral immune responses. Nat Commun. 2015;6:6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halova I, Draberova L, Draber P. Mast cell chemotaxis—chemoattractants and signaling pathways. Front Immunol 2012;3:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, Urbanek R, Szepfalusi Z. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol 2010;21:1179–88. [DOI] [PubMed] [Google Scholar]
- 50. Lehmann S, Hiller J, van Bergenhenegouwen J, Knippels LM, Garssen J, Traidl-Hoffmann C. In vitro evidence for immune-modulatory properties of non-digestible oligosaccharides: direct effect on human monocyte derived dendritic cells. PloS One 2015;10:e0132304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.