Abstract
Developmental dyslexia (DD) is a heritable condition characterized by persistent difficulties in learning to read. White matter alterations in left-lateralized language areas, particularly in the arcuate fasciculus (AF), have been observed in DD, and diffusion properties within the AF correlate with (pre-)reading skills as early as kindergarten. However, it is unclear how early these alterations can be observed. We investigated white matter structure in 14 infants with (FHD+; ages 6.6–17.6 months) and 18 without (FHD−; ages 5.1–17.6 months) familial risk for DD. Diffusion scans were acquired during natural sleep, and early language skills were assessed. Tractography for bilateral AF was reconstructed using manual and automated methods, allowing for independent validation of results. Fractional anisotropy (FA) was calculated at multiple nodes along the tracts for more precise localization of group differences. The analyses revealed significantly lower FA in the left AF for FHD+ compared with FHD− infants, particularly in the central portion of the tract. Moreover, expressive language positively correlated with FA across groups. Our results demonstrate that atypical brain development associated with DD is already present within the first 18 months of life, suggesting that the deficits associated with DD may result from altered structural connectivity in left-hemispheric regions.
Keywords: arcuate fasciculus, developmental dyslexia, diffusion tensor imaging, infants, reading disabilities
Introduction
Developmental dyslexia (DD) is a brain-based learning disability characterized by difficulties with reading and reading-related skills, such as fluent word recognition, spelling or written word comprehension that cannot be attributed to low general cognitive skills (Peterson and Pennington 2012).
Substantial evidence indicates that structural and functional brain abnormalities underlie DD. Functional MRI studies have revealed that school-age children and adults with DD exhibit reduced or absent functional activation in perisylvian, occipito-temporal, and parieto-temporal cortical regions, as well as increased activation in left inferior frontal regions, during reading and reading-related tasks relative to controls (for a summary, see Schlaggar and McCandliss 2007; Gabrieli 2009; Richlan et al. 2009, 2011). Individuals with DD also show decreased gray matter volume and altered sulcal patterns in left occipito-temporal and temporo-parietal brain regions compared with typical readers (e.g., Hoeft et al. 2007; Pernet et al. 2009; Richlan et al. 2013; Im et al. 2015), and gray matter volume indices in these areas correlate positively with reading and reading-related skills (e.g., Kronbichler et al. 2008; He et al. 2013).
These cortical brain regions are connected through axonal bundles, and diffusion metrics in the region of these axonal bundles have also been shown to be atypical in individuals with DD, below-average readers, and young children at risk for DD (e.g., Wandell and Yeatman 2013; Vandermosten et al. 2015). In particular, individuals diagnosed with DD and below-average readers exhibit reduced fractional anisotropy (FA), a measure which reflects fiber density, axonal diameter, and myelination (Ben-Shachar et al. 2007), in the arcuate fasciculus (AF), an axonal bundle connecting the posterior region of the temporo-parietal junction with the frontal cortex that plays a critical role in reading and language processing (Catani et al. 2005; Catani and Mesulam 2008). Patients with lesions in the left AF exhibit profound deficits in various skills important for successful reading, such as phonological processing, reading fluency, or language comprehension (Breier et al. 2008; Rauschecker et al. 2009; Shinoura et al. 2013). In school-age children, FA of the left AF correlates with phonological awareness (Yeatman et al. 2011), a key predictor of the later reading outcome (Snowling et al. 2000). Moreover, changes in volume of the AF between ages 5 and 9 predict reading outcome during the developmental period when children become fluent readers (Myers et al. 2014). Several other white matter pathways, such as the left superior longitudinal fasciculus, the left inferior longitudinal fasciculus (Steinbrink et al. 2008; Yeatman, Dougherty and Ben-Shachar et al. 2012), and the left inferior fronto-occipital fasciculus (Vandermosten et al. 2015) have been strongly linked to successful reading, but the results are less consistent than for the AF.
DD is highly heritable and several candidate susceptibility genes for DD (e.g., ROBO1, DCDC2, DYX1C1, and KIAA0319) have been reported, the majority of which are involved in brain development (Galaburda et al. 2006; Grigorenko 2009). Experimental interference of these genes in rodents causes atypical neuronal migration, which in turn results in localized gray matter malformations that affect cortical circuitry (Paracchini et al. 2006; Wang et al. 2006), and postmortem studies of individuals with DD have suggested similar cortical abnormalities (Galaburda et al. 1985). Recent studies suggest that FA of temporo-parietal white matter is particularly highly heritable (Darki et al. 2012; Jahanshad et al. 2013).
The genetic basis for DD is further supported by evidence that structural and functional brain alterations can already be detected in a group of kindergarteners at risk for DD, which indicates that DD-related brain abnormalities are not a result of reading failure, but rather seem to predate reading instruction (Raschle et al. 2011; Raschle et al. 2012a; Saygin et al. 2013; Simon et al. 2013; Raschle et al. 2014; Vandermosten et al. 2015). However, although most kindergarten beginners have not yet received formal reading instruction, reading subskills including phonological awareness have already begun to develop by this age, as most preschool-age children receive some instruction at home or in a preschool setting (Anthony et al. 2007). Therefore, it remains unclear to what extent the association between white matter structure and phonological awareness is a result of linguistic experience-dependent brain changes in early childhood or infancy, or genetic predisposition.
To date, EEG studies have demonstrated alterations in neurophysiological activity in infants with a familial risk of reading and language disorders in response to various prelinguistic and linguistic stimuli, including rapid auditory processing, categorical syllable perception, and phoneme perception (e.g., Guttorm et al. 2001; Benasich and Tallal 2002; Richardson et al. 2003). Moreover, these functional differences are predictive of later language and reading outcomes (Molfese 2000; Guttorm et al. 2010; Leppänen et al. 2012) and are observed in individuals diagnosed with DD (e.g., Gaab et al. 2007). However, no study to date has characterized structural brain alterations in infants with a familial risk for DD, of which 34–77% will most likely develop DD (Pennington and Lefly 2001; Snowling et al. 2003). Investigating white matter organization in at-risk infants can provide crucial information on how genetically driven alterations in structural connectivity develop and give rise to previously reported functional alterations in infants. Knowledge about alterations in structural connectivity may in turn inform new theoretical models for future research in children with DD, prereading children and infants at risk, and especially animal models.
In the present study, we employed manual and automated tractography methods of diffusion tensor imaging (DTI) to compare diffusion properties of the left and right AF between FHD+ and FHD− infants. Given the evidence of functional and structural brain alterations in kindergarten children at risk for DD, as well as functional brain alterations in infants at risk for DD, we hypothesized that FHD+ infants would already demonstrate altered white matter diffusion metrics, namely decreased FA, in the left AF. We further hypothesized a positive correlation between standardized language scores and FA of the left AF.
Materials and Methods
Subjects
After extensive data quality review, 14 FHD+ (7 female; mean age = 333 ± 118 days) and 18 FHD− (10 female; mean age = 298 ± 99 days) infants were included in the analyses. Participating families completed a socioeconomic status (SES) questionnaire adapted from the MacArthur Research Network (http://macses.ucsf.edu/default.php; see Supplementary Table 1), and a home literacy questionnaire (see Supplementary Table 2). Based on parental reports, infants were classified as FHD+ if they had at least 1 first-degree (n = 6) or second-degree relative (n = 5) diagnosed with DD, or if they had at least 1 first- or second-degree relative with a lifelong reading difficulty as determined via a questionnaire (n = 3). Infants were classified as FHD− if they had no relatives with a DD diagnosis or a history of reading difficulties. All infants were born after 37 weeks of gestation, had no reported history of neurological disease or trauma, and had no reported hearing or vision problems. The study was approved by the Institutional Review Board of the Boston Children's Hospital, and written informed consent was provided by the legal guardians.
Mullen Scales of Early Learning
Subjects completed the Mullen Scales of Early Learning (MSEL) (Mullen 1995), a standardized assessment of development from birth to 5 years 9 months. The MSEL consists of 5 subtests: 1) Expressive Language; 2) Receptive Language; 3) Fine Motor Skills; 4) Gross Motor Skills; 5) Visual Reception. Two-sample t-tests were used to compare standard scores on the 5 subtests between FHD+ and FHD− subjects. Complete scores for all subtests were not acquired for all subjects due to time restrictions or lack of cooperation (for an overview, see Supplementary Table 3). Analyses for the Receptive Language and Visual Reception subtests were not performed because less than half of the scores (4 and 6, respectively) were available for FHD+ infants. In addition, available measures were used for correlation analysis with the diffusion-based data.
Image Acquisition
The MRI scans were completed while the infants were naturally sleeping. Diffusion scans and structural T1-weighted images were acquired on a 3.0 Tesla Siemens TrioTim MRI scanner with a 32-channel radio frequency head coil (see Supplementary Material and Raschle et al. 2012b for details of the scanning procedure). Diffusion-weighted echo planar images were acquired using 64 slices with a thickness of 2.0 mm from 30 gradient directions and 10 unweighted images without skip. Additional imaging parameters were as follows: flip angle = 90°; TE = 88 ms; TR = 8320 ms; TA = 5:59 min; field of view = 256 × 256 mm; b = 1000 s/mm2. Structural T1-weighted whole-brain multi-echo magnetization-prepared rapid gradient-echo sequences with prospective motion correction (mocoMEMPRAGE) were acquired with a spatial resolution of 1.1 × 1.1 × 1.0 mm (176 slices). Further imaging parameters are as follows: flip angle = 7°; TE = 1450 ms; TR = 2270 ms; TA = 4:51 min; field of view = 220 × 220 mm; in-plane acceleration (GRAPPA) factor of 2.
Data Preprocessing
Diffusion-weighted imaging is particularly sensitive to the effects of motion, and removing motion outliers or regressing out motion diminishes these effects (Yendiki et al. 2013). Therefore, scans were evaluated for motion and scanner-induced outliers using DTIprep software (Liu et al. 2010). Motion parameters were found by rigidly registering the interleaved subvolumes. Translation threshold was set to 2.0 mm and rotation threshold to 0.5°. All detected motion outliers were flagged and subsequently removed using an in-house script. Subjects (n = 6) with 5 or more motion outliers were excluded from the analysis. All data were visually inspected, and 3 additional subjects (2 FHD−, 1 FHD+) were excluded because of poor quality data after motion outliers were removed. To exclude the possibility that the results were biased by differences in motion between the 2 groups, composite movement scores (mean of linear motion parameters [X, Y, Z] in mm) were calculated. An independent t-test was used to compare the composite motion scores between FHD+ and FHD− infants. The analysis of the motion revealed no significant differences between the groups (all P > 0.65).
Further preprocessing was done with the FMRIB Software Library (FSL), version 4.1.617 (www.fmrib.ox.ac.uk/fsl). This included the following steps: 1) creation of a brain mask of the reference volume (no diffusion) by segregating brain tissue from nonbrain tissue using Brain Extraction Tool 18 (Smith 2002); 2) eddy current and head movement correction using EDDYCORRECT from FMRIB's Diffusion Toolbox; 3) rotation of the gradients by taking the corrected parameters from the eddy current and head movement into account; and 4) local fitting of diffusion tensors and constructing of individual FA maps using DTIFIT from FMRIB's Diffusion Toolbox.
Manual Tractography
Manual Tractography Procedure
The present analyses were performed on the left and right arcuate fasciculi because of evidence of alterations in the AF in children and adults diagnosed with DD (e.g., Odegard et al. 2009). In order to determine whether any observed differences between groups are specific to the AF, the left and right corticospinal tracts (CST) were also reconstructed and used as control tracts. A standardized protocol was used to compute fiber tractography using the Diffusion Toolkit 0.5 and TrackVis 0.5.1 (TrackVis.org n.d.) (Supplementary Material and Supplementary Fig. 4). Two investigators conducted tractography blinded to the subjects' condition. Interrater reliability of mean FA values for the bilateral arcuate fasciculi for all subjects was calculated using an interclass correlation coefficient. A mean of the FA values obtained by the 2 investigators was used for statistical analysis.
Statistical Analysis for Manual Tractography
Independent two-sample t-tests with an alpha level of P < 0.05 were used to compare FA of the left and right arcuate fasciculi and left and right CST between FHD+ and FHD− infants with age in days as a covariate. The relationship between age and FA are displayed for each group separately in the Supplementary Figure 1. Results were corrected for multiple comparisons using a Bonferroni correction (adjusted alpha level P = 0.0125) (Benjamini et al. 2001). Because the right AF was detected in less than half of the subjects, an χ2 test was used to determine differences between FHD+ and FHD− infants in the number of subjects with a trackable right AF. The significance level was set to P < 0.05. Additionally, a Pearson's correlation was used to compute the relationship between age-corrected mean FA of the left AF, left CST, and right CST with expressive language MSEL standard scores, composite SES, and home literacy environment scores.
Automated Tractography
Automated Tractography Procedure
Automated Fiber Quantification (AFQ; https://github.com/jyeatman/AFQ) was used as an independent validation method in order to (a) confirm the mean FA of the manual tractography obtained across the entire tract and (b) characterize the nature of the group differences. AFQ is an automated tractography method that reconstructs 18 major white matter tracts and calculates diffusion properties at multiple equidistant nodes along the trajectory of each tract, instead of calculating mean diffusion properties across entire tracts, allowing for a more precise localization of group differences. Diffusion measures differ along the trajectory of the tract, partially due to passing fibers and axons that do not span the whole length of the tract. Therefore, obtaining mean diffusion measures across an entire tract may not adequately express precise group differences. AFQ, which has been used to examine white matter alterations in individuals with cerebral palsy and ventricular dilation, as well as premature and typically developing children, is described extensively in Yeatman, Dougherty and Myall et al. (2012). A description of the procedure with parameters used is as follows: first, whole-brain tractography was computed using a deterministic streamline tracking algorithm seeded with a white matter mask defined as all voxels with an FA of >0.1. Fiber tracking was terminated if the minimum angle between the last path segment and the next step direction was >40°. Next, individual fibers were assigned to a particular tract if they cross through the 2 waypoint ROIs that characterize the central portion of that tract. These ROIs were anatomically defined using the subject's T1-image based on the procedure described in Wakana et al. (2004) and were created in MNI space on a group-average diffusion dataset. These ROIs were converted into each subject's native space based on an estimated nonlinear transformation into MNI template space. Then, fiber tract probability maps developed by Hua et al. (2008) were transformed into each subject's native space and used to determine the probability of individual fibers belonging to their assigned fiber group based on the voxels that they pass through. Fibers that passed through low-probability voxels were excluded from the fiber group. The fiber groups were further cleaned by removing all fibers that were >4 standard deviations above the mean fiber length or >5 standard deviations away from the core of the fiber group. Fiber groups were then clipped so that they only spanned the region between the 2 waypoint ROIs, and the resulting clipped tract was segmented into 100 equidistant nodes. Spline interpolation was used to calculate FA, radial diffusivity (RD), and axial diffusivity (AD) across each tract and at each of the 100 nodes of each tract for each subject. These diffusion properties were calculated by taking a weighted average of the measures of each fiber at that node. The weight of each fiber was determined based on the probability that the fiber belonged to the fiber group.
Similar to previous studies that used AFQ (Yeatman, Dougherty and Myall et al. 2012), we found that the lowest FA along the trajectory of the AF was in the region of the principal arc of the tract. Because FA decreases in areas of high curvature and in regions containing fibers with different orientations (Ben-Shachar et al. 2007), individual differences in FA may be due to differences in the position or size of the AF or diffusion properties of crossing tracts, and not necessarily to the diffusion properties of the AF itself. In order to address this issue, a tract alignment procedure described in Yeatman, Dougherty and Myall et al. (2012) was employed to coregister anatomically analogous nodes of the left AF across subjects and ensure that equivalent regions were compared. An automated procedure was used to locate the node with the lowest FA value in the left AF for each subject, and the left AF of each subject was coregistered based on the location of this node (Supplementary Fig. 2). Because we only included the segments that overlapped across all subjects, 50 out of the original 100 segments were used in the analyses. For a more detailed description of these coregistration methods, see Yeatman, Dougherty and Myall et al. (2012).
Because AFQ uses strict criteria for tract identification, the tracts of interest could not be located in all subjects. The number of infants in which automated tractography was successfully obtained for each tract are as follows: 28 infants (14 FHD+ and 14 FHD−) for the left AF, 10 infants (2 FHD+ and 8 FHD−) for the right AF, 29 infants (13 FHD+ and 16 FHD−) for the left CST, and 30 infants (13 FHD+ and 17 FHD−) for the right CST. There are a large number of DTI studies demonstrating asymmetry of the AF in children and adults (Catani et al. 2007; Nucifora et al. 2005; Parker et al. 2005; Powell et al. 2006; Vernooij et al. 2007). The absence of a trackable right AF may be due to this left-lateralization of the AF and is in line with previous studies that were unable to identify a right AF even in adult subjects (Catani and Mesulam 2008). For example, Dubois et al. (2009) reported leftward asymmetry in the AF in fetal and neonate brains. This asymmetry, which is mainly characterized by larger volume of the left AF, is probably related to asymmetries of the superior temporal sulcus and the planum temporale. In the present manuscript, the automated tractography method most likely failed to identify the right AF in 8/10 FHD+ infants because (a) the fibers were not continuous and fully developed yet and/or (b) the fibers did not pass through the chosen ROIs.
Statistical Analysis for Automated Tractography
In order to validate the results of the manual tractography, two-sample t-tests with age in days as a covariate were used to compare mean FA values of the left AF between FHD+ and FHD− subjects by averaging the FA values of all of the nodes with an alpha level of P < 0.05, Bonferroni corrected for multiple comparisons with an adjusted alpha level of 0.017 (0.05/3 tests for left AF, left and right CST). Because only a total of 10 subjects (2 FHD+ and 8 FHD−) had a good-quality right AF based on visual inspection (the approximate correct location and the characteristic shape of the AF were used as the 2 criteria), analyses on the right hemisphere were not carried out. In order to investigate whether group differences in FA are driven by differences in (RD) or AD, two-sample t-tests were used to compare RD and AD of the left AF between FHD+ and FHD− subjects.
In order to statistically test differences in diffusion properties in each segment of the left AF and correct for multiple comparisons at each node along the tract, Monte-Carlo permutations were used by allocating the subjects randomly to 1 of 2 groups and creating 5000 randomly assigned pairs of groups (P < 0.05, controlled for multiple comparisons). For each random group pair, the 2 randomly generated groups were compared and the number of consecutive significant segments was calculated, which is the appropriate approach for Monte-Carlo permutations for consecutive datasets (Nichols and Holmes 2002; Langer et al. 2013) that simultaneously corrects for multiple comparisons by using the multiple comparisons nonparametric permutation approach of Holmes et al. (1996). Group differences were also computed for the left and right CST in order to determine whether observed group differences were specific to the AF.
Multivariate Pattern Classification
In order to investigate whether FA values in the segments along the left AF could predict whether an infant belonged to the FHD+ or FHD− group, a multivariate pattern analysis (MVPA) was employed. All FA measures of the equidistant segments were entered as individual features. A linear support vector machine classifier was trained and used a leave-one-out cross-validation combined with a univariate rank searching method to evaluate discriminative power. Significance level was set to P < 0.05 corrected for multiple comparisons. See Supplementary Material for a detailed description of the MVPA.
Results
Group Characteristics
There were no significant differences in age, gender (Supplementary Table 3), SES (Supplementary Table 1), or home literacy environment (Supplementary Table 2) between FHD+ and FHD− infants (all P > 0.121). No significant differences between FHD+ and FHD− infants were observed in performance on the Fine Motor, Gross Motor, or Expressive Language subtests of the MSEL (all P > 0.163) (Supplementary Table 1).
Manual Tractography
Group Differences
A high inter-rater reliability (r = 0.85) of mean FA values for the bilateral AF supported the reliability of the manual tractography. A χ2 test revealed that the right AF was less likely to be tracked in the FHD+ infants than the FHD− infants (Z = −4.70, P = 0.030). Because mean FA values of the left AF were significantly correlated with age (r = 0.51, P = 0.004), age was regressed out for the subsequent analyses. FHD+ infants demonstrated a significantly lower age-corrected mean FA in the left AF compared with FHD− infants (t = 3.06, P = 0.012, Bonferroni corrected). There were no significant group differences in age-corrected mean FA in the right AF (t = 0.88, P = 0.386), left CST (t = 0.30, P = 0.770), or right CST (t = −0.25, P = 0.806).
Correlation with MSEL, SES, and Home Literacy Variables
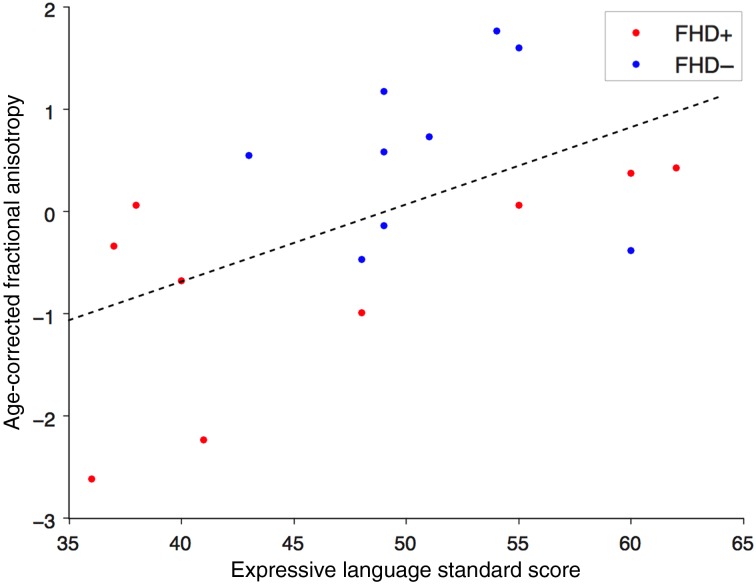
The correlation analyses between MSEL scores and FA values revealed a significant positive correlation between standard scores on the Expressive Language subtest and age-corrected mean FA of the left AF (Pearson r = 0.53, P = 0.022) (Fig. 1). There was no significant correlation between age-corrected FA of the left AF and expressive language scores in the FHD+ (Pearson r = 0.62, P = 0.073) nor FHD− (Pearson r = −0.13, P = 0.715) groups separately. Moreover, no significant relationship was found between the standardized Expressive Language and age (r = −0.29, P = 0.19). No significant relationship was observed between Receptive Language standard scores and mean FA of the left AF, home literacy environment or SES composite scores (all P > 0.155). Additionally, there was no significant correlation between FA of the left or right CST and Expressive Language, Receptive Language, home literacy and SES composite scores (all P > 0.371).
Figure 1.
Relationship between FA and expressive language. Scatterplot depicts the correlation between the age-corrected FA values of the left AF and the standard scores of the Expressive Language subtest of the MSEL. The red dots represent infants with a familial risk for dyslexia and the blue dots represent infants with no familial risk.
Automated Tractography
Validation of Tractography Results
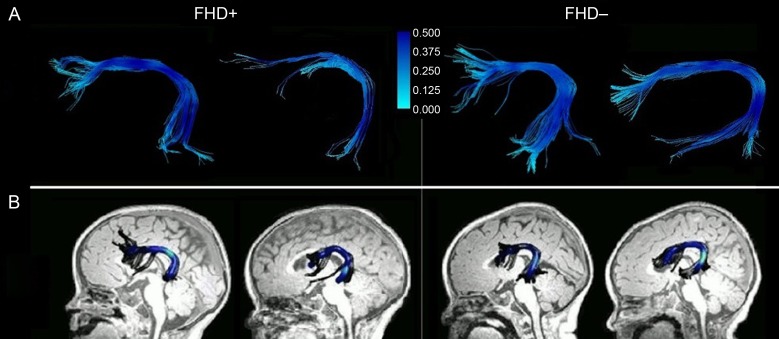
First, the correlation between mean FA of the left AF from the manual and automated tractography was calculated. There was a significant correlation between the 2 measures (r = 0.77, P = 0.002). See Figure 2 for examples of left AF tractography for both methods.
Figure 2.
Exemplary left arcuate fasciculi. AF tractography in infants with (FHD+) and without (FHD−) a familial risk for DD using (A) manual and (B) automated methods. The intensity of the color represents the magnitude of the FA.
Group Differences
An χ2 test revealed that the right AF was less likely to be tracked in the FHD+ infants than the FHD− infants (Z = 5.72, P = 0.017). Because the right AF could only be located in 2 FHD+ infants, further analyses on the right AF were not performed.
FHD+ infants demonstrated a significantly lower mean age-corrected FA in the left AF compared with FHD− infants, supporting the results of the manual tractography analyses (t = 2.73, P = 0.011, Bonferroni corrected). There were no group differences in age-corrected FA of the left CST (t = 1.53, P = 0.139) or right CST (t = −0.36, P = 0.720). In order to determine whether the mean FA group differences were driven by differences AD or RD, mean AD and RD of the left AF were compared between FHD+ and FHD− infants. There was a significantly higher mean AD in FHD+ infants compared with FHD− infants (t = 2.85, P = 0.024, Bonferroni corrected), but no difference in mean RD (t = −1.37, P = 0.182).
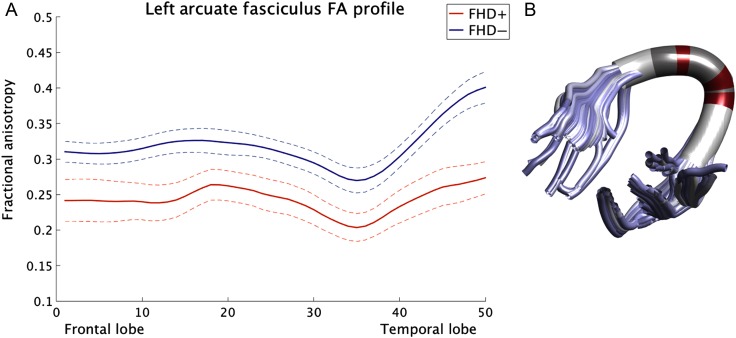
Next, the left AF was segmented into 50 equidistant nodes, and diffusion properties at each node were compared between FHD+ and FHD− infants. FHD+ infants exhibited significantly lower age-corrected FA in the compact region anterior and posterior to the principal arc of the left AF compared with FHD− infants (Fig. 3). FHD+ infants also exhibited significantly lower age-corrected AD in the left AF (Supplementary Fig. 3), but there were no group differences in age-corrected RD.
Figure 3.
Fractional anisotropy differences between infants with (FHD+) and without (FHD−) a familial risk for DD. (A) Tract profile of the left AF depicting FA values in FHD+ and FHD− infants at each of the 50 nodes. To illustrate the commonly reported dip in FA in the arc of the AF, we displayed the age-uncorrected FA values along the tract. However, the results for the age-corrected FA group differences look equivalent. The solid line represents the mean FA and the dashed line denotes one standard deviation. (B) Regions in which FHD+ infants exhibit significantly lower FA than FHD− infants are marked in red, and regions in which there are no significant differences between groups are marked in dark gray. Regions colored in white were not included in this specific analysis due to insignificant overlap between the infants (see also Supplementary Fig. 1).
MVPA Results
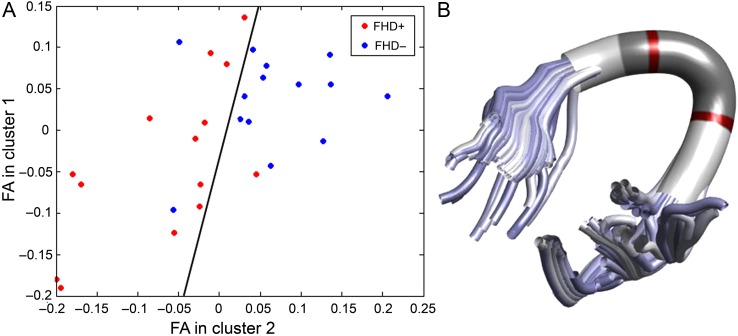
The MVPA yielded a prediction accuracy of 82% (P = 0.001, corrected for multiple comparisons), with a sensitivity of 85% and specificity of 79% (Fig. 4). The most discriminative features were found in the segments posterior to the principal arc in the left AF.
Figure 4.
Multivariate pattern classification of infants with (FHD+) and without (FHD−) a familial risk for DD using a linear support vector machine. (A) Classification is based on the FA values along the left AF and identifies a decision boundary (shown as a black line) that maximizes the margin to the nearest training cases of either class between 2 predictive FA clusters. The FA values are age-corrected and z-transformed. The red dots represent the FHD+ infants and the blue dots represent the FHD− infants. In (B), the 2 predictive segment clusters depicted in (A) are plotted in red.
Discussion
The present study is the first to demonstrate structural brain alterations in infants with a familial risk of DD. In accordance with previous neuroimaging evidence of altered white matter structure in left temporo-parietal brain regions in individuals with DD, FHD+ infants show reduced FA and AD in the left AF compared with FHD− infants. No significant differences between groups were observed in the left or right CST, suggesting that detectable white matter alterations in FHD+ infants are specific to the white matter tracts connecting language-related areas in the left hemisphere. These results were validated with an independent method, which, in addition to measuring mean FA of the entire tract, also investigates local differences along the trajectory of the tract. An MVPA that utilized FA values of the left AF to classify infants as FHD+ or FHD− yielded a high prediction accuracy, suggesting that our measures are highly sensitive in detecting early developmental brain differences. Additionally, mean FA in the left AF correlated positively with expressive language skills, which strongly predict future reading skills (Duff et al. 2015), across both groups, suggesting a general relationship between language measures and diffusion metrics of language-related regions in infancy.
These results are consistent with previous evidence of white matter alterations in individuals with DD. Evidence of reduced FA in left temporo-parietal and fronto-temporal white matter regions, particularly the left AF, is well-described in individuals with DD and below-average readers (Klingberg et al. 2000; Deutsch et al. 2005; Niogi and McCandliss 2006; Steinbrink et al. 2008; Carter et al. 2009; Odegard et al. 2009; Rollins et al. 2009; Rimrodt et al. 2010; Vandermosten et al. 2012). However, it remained unclear whether these white matter alterations predate the onset of formal reading instruction or are a result of it, or whether they may be caused by atypical language development. Here, we were able to show that infants with a familial risk for DD, of which 34–77% will most likely develop DD (Hallgren 1950; DeFries et al. 1987; Gilger et al. 1992; Gallagher et al. 2000; Pennington and Lefly 2001), already show the characteristic white matter alterations observed in individuals with a diagnosis of DD.
To date, only 2 DTI studies have shown alterations in the AF in prereaders and beginning readers at risk for DD (Saygin et al. 2013; Vandermosten et al. 2015), and it is not clear from these studies whether the observed alterations are present at birth, or emerge in parallel to or are influenced by (certain stages of) language development. However, here we show that the same atypicalities shown in at-risk prereaders are already present in at-risk infants with no detectable expressive or receptive language deficits, suggesting that the white matter alterations associated with DD risk are already present within the first 18 months of life of life. Based on the EEG literature (Molfese 2000) and on molecular genetic work on DD susceptibility genes (Ramos et al. 2008; Tammimies et al. 2013; Centanni et al. 2014) combined with the evidence that the embryo begins to perceive speech sounds in the 16th week of gestation at the latest (Isaacson 1988), it is plausible that such white matter alterations in the phonological system are present earlier, possibly even in utero, but to date no study has examined this.
Several DD susceptibility genes (e.g., KIAA0319, DYX1C1, DCDC2, and ROBO1) that participate in brain development and neuronal migration have been reported. Because single-nucleotide polymorphisms acting on these genes have only been directly linked to gray matter, and not to white matter (see Kere 2014), one could hypothesize that atypical gene expression in individuals with a genetic predisposition for DD may cause neuronal migration abnormalities that lead to cortical gray matter malformations, which in turn lead to alterations in cortical connectivity in these areas that underlie the characteristic perceptual and cognitive deficits (e.g., auditory perception, receptive/expressive language, verbal working memory) associated with DD. The altered structure observed in the left AF in FHD+ infants may form the basis for the functional disconnection between temporo-parietal and frontal brain regions observed in individuals with DD (Richlan et al. 2011).
Previous studies have shown that the AF can be either segmented into 1 long direct segment connecting Wernicke's and Broca's areas and 2 indirect segments (an anterior part between Broca's area and the inferior parietal lobe and a posterior part between Wernicke's area and the inferior parietal lobule) (Catani and Mesulam 2008), or it can be segmented into 2 parts, one projecting from Wernicke's to Broca's area and one from Wernicke's to the premotor cortex (Perani et al. 2011). However, the part of the AF projecting from the temporal cortex to Broca's area could not be detected in 3-day-old newborns (Perani et al. 2011). Interestingly, our study revealed direct connections between the temporal cortex (Wernicke's area) and inferior frontal cortex (Broca's area) in an infant sample. This might be explained by (a) the fact that the infants in the present study were much older (infants versus newborns) and (b) differences in the tractography, which relies primarily on ROIs to ensure that the main portions of the AF are identified objectively and reliably in all subjects. Moreover, the tractography method employed here enabled us to divide the AF into multiple segments, allowing for examination of local differences. This is especially important in the arc, which is the transition zone between the anterior indirect segment and the posterior indirect segment, as described in Catani and Mesulam (2008), and the present study showed significant differences between the 2 groups in precisely this region as well as neighboring segments.
Our results also contribute to the abundance of evidence of atypical brain functioning in infants at familial risk for DD. Infants with a familial risk of reading disorders exhibit alterations in cortical brain responses to language-related and auditory stimuli compared with infants with no familial risk (Leppänen et al. 2002; Guttorm et al. 2003; Richardson et al. 2003; Friedrich et al. 2004; van Leeuwen et al. 2006, 2007; van Herten et al. 2008). Our findings propose that these previously reported functional neural alterations in infants with a familial risk for DD might be driven in part by underlying differences in left-lateralized language-related white matter regions.
Research on DD suggests that the white matter alterations that underlie reading impairments are mainly rooted in atypical RD rather than in AD in school-age children (Yeatman et al. 2011) and adults (Vandermosten et al. 2012). RD may be related to the degree of myelination, whereas AD may reflect axonal maturation (Burzynska et al. 2010), although it should be noted that the precise neurobiological properties that AD and RD reflect are highly debated (Wheeler-Kingshott and Cercignani 2009). However, here we observed reduced AD but preserved RD in infants at familial risk for DD, suggesting that reduced white matter FA in infants at risk for DD may be caused by other pathophysiological processes, such as altered axonal development. However, it is also possible that the reduced FA and AD observed in FHD+ infants may alternatively reflect more crossing fibers in left temporo-parietal areas and therefore delayed or altered axonal pruning in those infants. New methods need to be developed in order to disentangle precisely the effects of myelination vs. pruning on FA.
Limitations
The MSEL is a broad measure of language, and language assessment in infancy is in general difficult to perform (Aylward and Aylward 2011). Therefore, the obtained language scores must be interpreted with caution. Moreover, this study would ideally be carried out in neonates, because by 12 months, infants have acquired phonological, prosodic, and lexical-semantic processing skills with neural representations that are remarkably similar to those of the adult brain (Kuhl 2010; Männel and Friederici 2013; Gómez et al. 2014). The phonological system appears to emerge early in development, as there is evidence that basic phoneme discrimination as measured by mismatch negativity is already evident in utero (Mahmoudzadeh et al. 2013), and phonological processing skills improve rapidly within the first months of life (Dehaene-Lambertz et al. 2002). Despite this, infants have not yet undergone the majority of language development. Furthermore, increasing the number of subjects could both enable more robust correlational analysis and improve the quality of the MVPA analysis because, for both objectives, our sample is rather small.
It is also important to note that because risk status for infants included in the present study was determined based on family history, and not on genetic data, inferences about the genetic nature of the observed white matter alterations should be made cautiously. Furthermore, because the present data are cross-sectional, it remains to be determined which FHD+ infants will later exhibit reading difficulties and receive a DD diagnosis, and whether diffusion metrics of the left arcuate in infancy can predict later reading outcome. Future longitudinal research has to quantify the specificity of the observed effects in the AF and other structural brain regions. Nevertheless, our MVPA analysis accurately predicted the group allocation of the infants, which highlights the possibility of implementing such measures as objective biomarkers in future prospective studies.
Conclusion
In the present study, we demonstrated for the first time that previously reported left-hemispheric white matter alterations in individuals with DD could already be detected in a group of infants with familial risk for DD. Our results provide further support for early atypical brain development in DD and suggest that DD may originate from alterations in structural connectivity in left hemispheric brain regions that are evident in infancy. This study further supports the hypothesis that brain alterations in DD are innate (Galaburda et al. 2006) and that they are causal to atypical language and reading development in DD and not a result of it. However, future studies are needed to determine whether alterations of the AF are predictive of later reading performance and whether these measures may be utilized, if sufficient sensitivity and specificity is demonstrated, as an early general premarker for DD or language-based learning disabilities. Identification of children at risk for DD earlier in the developmental time course could offer the possibility for individualized instruction, early implementation of effective interventions, and amelioration of skills that are essential for reading.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the Harvard Catalyst/NIH (5UL1RR025758 to N.G. and P.E.G.), the William Hearst Fund (to N.G and P.E.G) and a training grant from the National Institute of Health (NIH T32 DC000038-22 to J.Z.).
Supplementary Material
References
- Anthony JL, Williams JM, McDonald R, Francis DJ. 2007. Phonological processing and emergent literacy in younger and older preschool children. Ann Dyslexia. 57:113–137. [DOI] [PubMed] [Google Scholar]
- Aylward GP, Aylward BS. 2011. The changing yardstick in measurement of cognitive abilities in infancy. J Dev Behav Pediatr JDBP. 32:465–468. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. 2002. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 136(1):31–49. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. 2001. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 125:279–284. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Wandell BA. 2007. White matter pathways in reading. Curr Opin Neurobiol. 17:258–270. [DOI] [PubMed] [Google Scholar]
- Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. 2008. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol. 29:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li S-C, Lindenberger U, Heekeren HR. 2010. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. NeuroImage. 49:2104–2112. [DOI] [PubMed] [Google Scholar]
- Carter JC, Lanham DC, Cutting LE, Clements-Stephens AM, Chen X, Hadzipasic M, Kim J, Denckla MB, Kaufmann WE. 2009. A dual DTI approach to analyzing white matter in children with dyslexia. Psychiatry Res. 172:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Allin MPG, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. 2007. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. 104:17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. 2005. Perisylvian language networks of the human brain. Ann Neurol. 57:8–16. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. 2008. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex J Devoted Study Nerv Syst Behav. 44:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Booker AB, Sloan AM, Chen F, Maher BJ, Carraway RS, Khodaparast N, Rennaker R, LoTurco JJ, Kilgard MP. 2014. Knockdown of the dyslexia-associated gene Kiaa0319 impairs temporal responses to speech stimuli in rat primary auditory cortex. Cereb Cortex N Y N 1991. 24:1753–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darki F, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T. 2012. Three dyslexia susceptibility genes, DYX1C1, DCDC2, and KIAA0319, affect temporo-parietal white matter structure. Biol Psychiatry. 72:671–676. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Fulker DW, LaBuda MC. 1987. Evidence for a genetic aetiology in reading disability of twins. Nature. 329:537–539. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. 2002. Functional neuroimaging of speech perception in infants. Science. 298:2013–2015. [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, Wandell B. 2005. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex J Devoted Study Nerv Syst Behav. 41:354–363. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. 2009. Structural asymmetries in the infant language and sensori-motor networks. Cereb Cortex. 19(2):414–423. [DOI] [PubMed] [Google Scholar]
- Duff FJ, Reen G, Plunkett K, Nation K. 2015. Do infant vocabulary skills predict school-age language and literacy outcomes? J Child Psychol Psychiatry. 568:848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M, Weber C, Friederici AD. 2004. Electrophysiological evidence for delayed mismatch response in infants at-risk for specific language impairment. Psychophysiology. 41:772–782. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gabrieli JDE, Deutsch GK, Tallal P, Temple E. 2007. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: an fMRI study. Restor Neurol Neurosci. 25:295–310. [PubMed] [Google Scholar]
- Gabrieli JDE. 2009. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 325:280–283. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. 2006. From genes to behavior in developmental dyslexia. Nat Neurosci. 9:1213–1217. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. 1985. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 18:222–233. [DOI] [PubMed] [Google Scholar]
- Gallagher A, Frith U, Snowling MJ. 2000. Precursors of literacy delay among children at genetic risk of dyslexia. J Child Psychol Psychiatry. 41:203–213. [PubMed] [Google Scholar]
- Gilger JW, Pennington BF, DeFries JC. 1992. A twin study of the etiology of comorbidity: attention-deficit hyperactivity disorder and dyslexia. J Am Acad Child Adolesc Psychiatry. 31(2):343–348. [DOI] [PubMed] [Google Scholar]
- Gómez DM, Berent I, Benavides-Varela S, Bion RAH, Cattarossi L, Nespor M, Mehler J. 2014. Language universals at birth. Proc Natl Acad Sci USA. 111:5837–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko EL. 2009. Speaking genes or genes for speaking? Deciphering the genetics of speech and language. J Child Psychol Psychiatry. 50:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PHT, Hämäläinen JA, Eklund KM, Lyytinen HJ. 2010. Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. J Learn Disabil. 43:391–401. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PHT, Tolvanen A, Lyytinen H. 2003. Event-related potentials in newborns with and without familial risk for dyslexia: principal component analysis reveals differences between the groups. J Neural Transm Vienna Austria 1996. 110:1059–1074. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppanen PH, Richardson U, Lyytinen H. 2001. Event-related potentials and consonant differentiation in newborns with familial risk for dyslexia. J Learn Disabil. 34(6):534–544. [DOI] [PubMed] [Google Scholar]
- Hallgren B. 1950. Specific dyslexia (congenital word-blindness); a clinical and genetic study. Acta Psychiatr Neurol Suppl. 65:1–287. [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, Chen C, Lu Z-L, Dong Q. 2013. Decoding the neuroanatomical basis of reading ability: a multivoxel morphometric study. J Neurosci Off J Soc Neurosci. 33:12835–12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A et al. 2007. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 104:4234–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. 1996. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 16:7–22. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PCM, Mori S. 2008. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Ellen Grant P, Gaab N. 2015. Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners. Cereb Cortex. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson G. 1988. Antenatal diagnosis of congenital deafness. Ann Otol Rhinol Laryngol. 97:124–127. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI et al. 2013. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. NeuroImage. 81:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kere J. 2014. The molecular genetics and neurobiology of developmental dyslexia as model of a complex phenotype. Biochem Biophys Res Commun. 452:236–243. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. 2000. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 25:493–500. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G. 2008. Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum Brain Mapp. 29:613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. 2010. Brain mechanisms in early language acquisition. Neuron. 67:713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer N, Pedroni A, Jäncke L. 2013. The problem of thresholding in small-world network analysis. PloS One. 8:e53199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen PHT, Hämäläinen JA, Guttorm TK, Eklund KM, Salminen H, Tanskanen A, Torppa M, Puolakanaho A, Richardson U, Pennala R et al. 2012. Infant brain responses associated with reading-related skills before school and at school age. Neurophysiol Clin Clin Neurophysiol. 42:35–41. [DOI] [PubMed] [Google Scholar]
- Leppänen PHT, Richardson U, Pihko E, Eklund KM, Guttorm TK, Aro M, Lyytinen H. 2002. Brain responses to changes in speech sound durations differ between infants with and without familial risk for dyslexia. Dev Neuropsychol. 22:407–422. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, Styner M. 2010. Quality Control of Diffusion Weighted Images. Proc SPIE Int Soc Opt Eng. 7628:76280J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudzadeh M, Dehaene-Lambertz G, Fournier M, Kongolo G, Goudjil S, Dubois J, Grebe R, Wallois F. 2013. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc Natl Acad Sci USA. 110:4846–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel C, Friederici AD. 2013. Accentuate or repeat? Brain signatures of developmental periods in infant word recognition. Cortex J Devoted Study Nerv Syst Behav. 49:2788–2798. [DOI] [PubMed] [Google Scholar]
- Molfese DL. 2000. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain Lang. 72:238–245. [DOI] [PubMed] [Google Scholar]
- Mullen EM. 1995. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services, Inc. [Google Scholar]
- Myers CA, Vandermosten M, Farris EA, Hancock R, Gimenez P, Black JM, Casto B, Drahos M, Tumber M, Hendren RL et al. 2014. White matter morphometric changes uniquely predict children's reading acquisition. Psychol Sci. 25:1870–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. 2006. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 44:2178–2188. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC. 2005. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport. 16(8):791–794. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, Black J. 2009. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 47:1972–1977. [DOI] [PubMed] [Google Scholar]
- Paracchini S, Thomas A, Castro S, Lai C, Paramasivam M, Wang Y, Keating BJ, Taylor JM, Hacking DF, Scerri T et al. 2006. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet. 15:1659–1666. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, Lambon Ralph MA. 2005. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 24(3):656–666. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Lefly DL. 2001. Early reading development in children at family risk for dyslexia. Child Dev. 72:816–833. [DOI] [PubMed] [Google Scholar]
- Perani D, Saccuman MC, Scifo P, Anwander A, Awander A, Spada D, Baldoli C, Poloniato A, Lohmann G, Friederici AD. 2011. Neural language networks at birth. Proc Natl Acad Sci USA. 108:16056–16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet C, Andersson J, Paulesu E, Demonet JF. 2009. When all hypotheses are right: a multifocal account of dyslexia. Hum Brain Mapp. 30:2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. 2012. Developmental dyslexia. Lancet. 379:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS. 2006. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 32(1):388–399. [DOI] [PubMed] [Google Scholar]
- Ramos RL, Smith PT, DeCola C, Tam D, Corzo O, Brumberg JC. 2008. Cytoarchitecture and transcriptional profiles of neocortical malformations in inbred mice. Cereb Cortex N Y N 1991. 18:2614–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Chang M, Gaab N. 2011. Structural brain alterations associated with dyslexia predate reading onset. NeuroImage. 57:742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Stering PL, Meissner SN, Gaab N. 2014. Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia. Cereb Cortex N Y N 1991. 24:2489–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. 2012. a. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci USA. 109:2156–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Benasich AA, Gaab N. 2012. b. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann N Y Acad Sci. 1252:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker AM, Deutsch GK, Ben-Shachar M, Schwartzman A, Perry LM, Dougherty RF. 2009. Reading impairment in a patient with missing arcuate fasciculus. Neuropsychologia. 47:180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson U, Leppänen PHT, Leiwo M, Lyytinen H. 2003. Speech perception of infants with high familial risk for dyslexia differ at the age of 6 months. Dev Neuropsychol. 23:385–397. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. 2009. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 30:3299–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. 2011. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 56:1735–1742. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. 2013. Structural abnormalities in the dyslexic brain: a meta-analysis of voxel-based morphometry studies. Hum Brain Mapp. 34:3055–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Peterson DJ, Denckla MB, Kaufmann WE, Cutting LE. 2010. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex J Devoted Study Nerv Syst Behav. 46:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins NK, Vachha B, Srinivasan P, Chia J, Pickering J, Hughes CW, Gimi B. 2009. Simple developmental dyslexia in children: alterations in diffusion-tensor metrics of white matter tracts at 3T. Radiology. 251:882–891. [DOI] [PubMed] [Google Scholar]
- Saygin ZM, Norton ES, Osher DE, Beach SD, Cyr AB, Ozernov-Palchik O, Yendiki A, Fischl B, Gaab N, Gabrieli JDE. 2013. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J Neurosci Off J Soc Neurosci. 33:13251–13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. 2007. Development of neural systems for reading. Annu Rev Neurosci. 30:475–503. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Midorikawa A, Onodera T, Tsukada M, Yamada R, Tabei Y, Itoi C, Saito S, Yagi K. 2013. Damage to the left ventral, arcuate fasciculus and superior longitudinal fasciculus-related pathways induces deficits in object naming, phonological language function and writing, respectively. Int J Neurosci. 123:494–502. [DOI] [PubMed] [Google Scholar]
- Simon G, Lanoë C, Poirel N, Rossi S, Lubin A, Pineau A, Houdé O. 2013. Dynamics of the anatomical changes that occur in the brains of schoolchildren as they learn to read. PloS One. 8:e81789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp. 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling M, Bishop DV, Stothard SE. 2000. Is preschool language impairment a risk factor for dyslexia in adolescence? J Child Psychol Psychiatry. 41:587–600. [DOI] [PubMed] [Google Scholar]
- Snowling MJ, Gallagher A, Frith U. 2003. Family risk of dyslexia is continuous: individual differences in the precursors of reading skill. Child Dev. 74:358–373. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Müller H-P, Juengling FD, Kassubek J, Riecker A. 2008. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0T. Neuropsychologia. 46:3170–3178. [DOI] [PubMed] [Google Scholar]
- Tammimies K, Vitezic M, Matsson H, Le Guyader S, Bürglin TR, Ohman T, Strömblad S, Daub CO, Nyman TA, Kere J et al. 2013. Molecular networks of DYX1C1 gene show connection to neuronal migration genes and cytoskeletal proteins. Biol Psychiatry. 73:583–590. [DOI] [PubMed] [Google Scholar]
- TrackVis.org. n.d. Martinos Center for Biomedical Imaging, Massachussetts General Hospital. [Google Scholar]
- Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P. 2012. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain J Neurol. 135:935–948. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Vanderauwera J, Theys C, De Vos A, Vanvooren S, Sunaert S, Wouters J, Ghesquière P. 2015. A DTI tractography study in pre-readers at risk for dyslexia. Dev Cogn Neurosci. 14:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herten M, Pasman J, van Leeuwen TH, Been PH, van der Leij A, Zwarts F, Maassen B. 2008. Differences in AERP responses and atypical hemispheric specialization in 17-month-old children at risk of dyslexia. Brain Res. 1201:100–105. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen T, Been P, Kuijpers C, Zwarts F, Maassen B, van der Leij A. 2006. Mismatch response is absent in 2-month-old infants at risk for dyslexia. Neuroreport. 17:351–355. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen T, Been P, van Herten M, Zwarts F, Maassen B, van der Leij A. 2007. Cortical categorization failure in 2-month-old infants at risk for dyslexia. Neuroreport. 18:857–861. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. 2007. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage. 35(3):1064–1076. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. 2004. Fiber tract-based atlas of human white matter anatomy. Radiology. 230:77–87. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Yeatman JD. 2013. Biological development of reading circuits. Curr Opin Neurobiol. 23:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Paramasivam M, Thomas A, Bai J, Kaminen-Ahola N, Kere J, Voskuil J, Rosen GD, Galaburda AM, Loturco JJ. 2006. DYX1C1 functions in neuronal migration in developing neocortex. Neuroscience. 143:515–522. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M. 2009. About “axial” and “radial” diffusivities. Magn Reson Med. 61:1255–1260. [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. 2012. Development of white matter and reading skills. Proc Natl Acad Sci USA. 109:E3045–E3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. 2012. Tract profiles of white matter properties: automating fiber-tract quantification. PloS One. 7:e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, Ben-Shachar M. 2011. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J Cogn Neurosci. 23:3304–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. 2013. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 88C:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.