Abstract
Doxorubicin (DOX), one of the most commonly used anticancer medications, has been reported to affect fertility by damaging ovarian follicles; however, the dose-dependent toxicity of DOX on the dynamic follicle development and oocyte maturation has not been well-defined. Our objective is to determine the effects of human-relevant exposure levels of DOX on follicular functions across developmental time. In vitro cultured multilayered secondary mouse follicles were treated with DOX at 0, 2, 20, 100, and 200 nM for 24 h, and follicle development, hormone secretion, and oocyte maturation were analyzed. DOX caused dose-dependent toxicity on follicle growth, survival, and secretion of 17β-estradiol (E2). At 200 nM, DOX induced DNA damage and apoptosis in follicle somatic cells first and then in oocytes, which was correlated with the uptake of DOX first to the somatic cells followed by germ cells. Follicles treated with DOX at 0, 2, and 20 nM showed similar oocyte metaphase II (MII) percentages after in vitro oocyte maturation; however, 20 nM DOX significantly increased the number of MII oocytes with abnormal spindle morphology and chromosome misalignment. In an effort to harmonize the in vitro study to in vivo treatment, dose-dependent toxicity on oocyte meiotic maturation was found in 16-day-old CD-1 mice treated with DOX at 0, 0.4, 2, and 10 mg/kg, consistent with the in vitro oocyte maturation outcomes. Our study demonstrates that DOX has dose-dependent toxicity on ovarian follicle development, hormone secretion, and oocyte maturation, which are three key factors to support the female reproductive and endocrine functions.
Keywords: doxorubicin, follicle development, ovarian toxicity, oocyte maturation
With the advances of early disease detection and therapeutic treatments, anticancer outcomes have significantly improved. In the last 30 years, the 5-year cancer survival rates increased from 49% to 68% (National Cancer Institute, 2015; Siegel et al., 2016). Some cancer diseases such as breast cancer and childhood leukemia have survival rates >80–90% (Gansler et al., 2010; Siegel et al., 2016). With this increased number of cancer survivors, there is now more concern regarding the side effects during and following anticancer treatments (Di Maio et al., 2016). Ovarian toxicity resulting from chemotherapy is one of the major concerns for both women in reproductive age (15–44 years of age) and childhood cancer survivors (0–15 years of age) (Morgan et al., 2012). To date, multiple chemotherapeutic chemicals have been shown to damage ovarian follicles as well as increase the risk of premature ovarian failure (POF), early menopause, and even infertility (Bines et al., 1996; Morgan et al., 2012).
Doxorubicin (DOX), an anthracycline antitumor antibiotic, is one of the most commonly used anticancer medications for many malignant tumors such as lymphomas, leukemia, and breast cancer (Blum and Carter, 1974). The mechanism of DOX induced anticancer actions is not conclusively identified, but it has been reported to inhibit DNA resealing by forming the DOX–DNA–topoisomerase II (TOP2A) complex and activate the p53 mediated cancer cell apoptosis (Minotti et al., 2004). However, the clinical usage of DOX is limited because of its toxicity in multiple organ systems such as cardiotoxicity, hepatotoxicity, myelosuppression, and reproductive toxicity (Damodar et al., 2014; Hofland et al., 2005; Zhang et al., 2012).
Follicle is the basic functional unit of the ovary and has primary functions to support oocyte maturation and hormone secretion to maintain the human 28-day menstrual cycle and support pregnancy. DOX has been reported to exhibit ovarian toxicity and affect fertility in multiple models which are shown below (Morgan et al., 2012). In female cancer patients, there was a dramatically increased amenorrhea incidence after receiving chemotherapy including DOX, suggesting the loss of growing follicles and decreased estrogen levels (Ben-Aharon et al., 2010; Petrek et al., 2006). In in vitro models, DOX induced both mouse germinal vesicle (GV) and metaphase II (MII) oocyte apoptosis at concentrations of 0.2–10 μM, whereas fertilized oocytes were resistant to the DOX toxicity (Bar-Joseph et al., 2010; Perez et al., 1997). In animal models, DOX at concentration of 10 mg/kg increased mouse follicle apoptosis and reduced ovarian size and ovulation rate (Ben-Aharon et al., 2010). Roti Roti et al. (2012) revealed that mouse growing follicles exhibited apoptosis following 8 h after DOX injection (20 mg/kg via intraperitoneal injection, i.p.), and primordial follicles started to show apoptosis at 48 h. Kropp et al. (2015) demonstrated that 20 mg/kg of DOX significantly resulted in follicle apoptosis and decreased the litter size, and the TOP2 inhibitor dexrazoxane protected the DOX induced ovarian toxicity and infertility. These data indicate that mitotically active growing follicles are more sensitive than the quiescent primordial follicles toward DOX’s ovarian toxicity and DOX has long-term effects of permanent ovarian insufficiency and infertility.
Despite the known ovarian toxicity, DOX has not been studied in the encapsulated in vitro follicle growth (eIVFG) model, which maintains follicle three-dimensional architecture and recapitulates key events of follicle development and oocyte maturation (Xiao et al., 2015a,b; Xu et al., 2006a). Further, previous studies have relied on the denuded oocytes without surrounding granulosa cells, which does not represent DOX exposure of intact follicles during anticancer treatment (Bar-Joseph et al., 2010; Perez et al., 1997). Therefore, by using both eIVFG and in vivo animal models, the present study aimed to determine the human-relevant exposure levels of DOX on follicle development, hormone secretion, and oocyte maturation, which are particularly critical for understanding the reproductive toxicity of DOX during chemotherapy in young female cancer patients.
MATERIALS AND METHODS
Animals
Immature follicles were isolated from ovaries harvested from 16-day-old CD-1 female mice. All mice were housed in polypropylene cages and provided food and water ad libitum. Animals were kept on a 12-h light/dark cycle (7:00 am–7:00 pm) at 23 ± 1 °C with 30–50% relative humidity. Animals were fed Teklad Global irradiated 2919 or 2916 chow (Madison, WI), which does not contain soybean or alfalfa meal to minimize the exposure to phytoestrogens. All methods used in this study were approved by Northwestern University Institutional Animal Care and Use Committee (IACUC) and correspond to National Institutes of Health guidelines and public law.
Follicle isolation, encapsulation, and culture
Multilayered secondary follicles (150–180 μm, type 5b) were isolated from 16-day-old CD-1 female mice as previously described (Xiao et al., 2015a; Xu et al., 2006a). Only follicles that displayed intact morphology were selected for encapsulation and culture. Follicles were placed in maintenance media containing 50% minimal essential medium (αMEM Glutamax) and 50% Nutrient Mixture (F-12 with Glutamax) with 1% fetal bovine serum (FBS, Life Technology, Grand Island, NY) for 2 h before encapsulation. Selected follicles were then encapsulated individually in 0.5% alginate (NovaMatrix, Sandvika, Norway) as previously described (Xu et al., 2006a; Xu et al., 2006b). Alginate beads were placed in 96-well plates, with each well containing 100 μl growth media (50% αMEM Glutamax and 50% F-12 Glutamax supplemented with 3 mg/ml bovine serum albumin [BSA] [Sigma-Aldrich, St. Louis, MO], 10 mIU/ml recombinant follicle-stimulating hormone [rFSH; from A. F. Parlow, National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD], 1 mg/ml bovine fetuin [Sigma-Aldrich, St. Louis, MO], 5 μg/ml insulin, 5 μg/ml transferrin, and 5 μg/ml selenium [Sigma-Aldrich, St. Louis, MO]). For all experiments, follicles were maintained at 37 °C. Half of the growth media (50 μl) was replaced and stored for further hormone analysis every other day. Follicles were imaged at each media change using an inverted Leica DM IRB microscope with 4× and 20× objectives (Leica Microsystems, Buffalo Grove, IL). Follicle growth curves were obtained by plotting the average follicle diameter, which was calculated by averaging two perpendicular measurements from basement membrane to basement membrane of each follicle in ImageJ software (National Institutes of Health, Bethesda, MD).
In Vitro Exposure of DOX to Follicles
In cancer patients, the DOX plasma concentration peaks at 200 nM for 20–30 h and then decreases because of the metabolic biotransformation and excretion (Barpe et al., 2010; Lee et al., 1980; Sturgill et al., 2000). Compared to its metabolites, DOX is more cytotoxic and remains high in both tumor and normal tissues (Speth et al., 1988). Thus, in vitro cultured follicles were exposed to DOX (Sigma-Aldrich, St. Louis, MO) at concentrations of 0, 2, 20, 100, and 200 nM on day 1 of eIVFG for 24 h, and follicles were washed 3 times with growth medium to remove the remaining DOX and were continued in culture for up to 8 days. Follicles were considered dead if they had unhealthy appearing oocytes and/or granulosa cells, or if the integrity of the oocyte and somatic cell interface was visibly compromised. Follicle survival rate was calculated by dividing the number of survived follicles on days 0, 2, 4, 6, and 8 to the number of cultured follicles on day 0. The follicle survival rate in the control group was >90% and <10% of follicles showed unhealthy follicle morphology and degenerated oocyte during the culture. The follicle growth curve and survival rates on days 0, 2, 4, 6, 8 were plotted and the lethal concentration 50 (LC50) with 90% confidence interval (CI) of DOX on in vitro cultured follicles was calculated.
Hormone Measurements
17β-estradiol (E2) concentrations in the conditioned follicle culture media on days 2, 4, and 8 were measured using ELISA kits (ES180S, Calbiotech, Spring Valley, CA) according to the manufacturer’s instructions. All assays were run in duplicates and medium collected from wells without follicles was used as negative control.
DOX Fluorescence Distribution in Ovarian Follicles
DOX distribution was examined based on its autofluorescence with excitation at 488 nm and emission at 590 nm using confocal microscopy (Leica, Buffalo Grove, IL). When follicles were incubated with DOX <200 nM, the fluorescence was under the detectable limit, therefore, follicles were incubated with DOX at 200 nM to determine its uptake and accumulation, and follicles incubated with the same media but without DOX were used as negative control. To determine whether the DOX fluorescence distribution pattern is consistent to the DOX induced toxicity, the same concentration of DOX at 200 nM was selected for the following studies of γ-H2AX staining, TUNEL, and RT-qPCR.
γ-H2AX Staining
DNA double strands breaks (DSBs) were determined by γ-H2AX immunofluorescent staining. Follicles exposed to 200 nM DOX for different time periods (0, 4 and 24 h) were fixed in 4% paraformaldehyde for 1 h at room temperature (RT), washed in 1× PBS for 3 times, and immersed in permeabilization solution with 1× PBS containing 10% goat serum (Jackson ImmunoResearch, West Grove, PA), 3% BSA and 1% Triton X-100 for 1 h at RT. Follicles were then incubated in a 1:1000 dilution of rabbit polyclonal to γ-H2AX (AB2893, Abcam, Cambridge, MA) in blocking solution (1x PBS containing 10% goat serum, 3% BSA and 1% Triton X-100) at 4 °C overnight, and the blocking solution without rabbit polyclonal to γ-H2AX was used as negative control. On the second day, follicles were washed for 3 times in washing solution of 1× PBS with 1% goat serum, 3% BSA and 0.1% Triton X-100, blocked in blocking solution for 1 h at RT, and then incubated in a 1:100 dilution of Alexa Fluor 488 Goat Anti-Rabbit IgG (Life Technology, Grand Island, NY) in blocking solution at 4 °C overnight. Follicles were washed in washing buffer 3 times and mounted in Vectashield containing DAPI. Fluorescent signals were visualized using confocal microscopy and the position with follicles containing oocyte nucleus were imaged to include both follicle somatic cells and oocyte nucleus chromosomes.
Apoptotic Gene Analysis
It has been demonstrated that the pro-apoptotic genes Caspase 3 (Casp3), Forkhead Box O1 (Foxo1) and Forkhead Box O3 (Foxo3) mRNA expression levels are well correlated with the protein levels during the ovarian follicle apoptosis (Mikaeili et al., 2016; Shen et al., 2014; Slot et al., 2006). To investigate the correlation between the DOX distribution and DOX induced follicular cell apoptosis, follicles were collected 24 h after DOX exposure and were snap frozen on dry ice for quantitative reverse transcription PCR (RT-qPCR). Total RNA of follicles was extracted using Trizol (Invitrogen, Carlsbad, CA). cDNA was reverse-transcribed from 1 μg of total RNA using Superscript III reverse transcriptase with random primers (Invitrogen, Carlsbad, CA). RT-qPCR was performed in 384-well plates using SYBR-Green I intercalating dye on ABI 7900 (Applied Biosystems, Carlsbad, CA). RT-qPCR thermos cycle was programmed for 30 s at 95 °C, followed by 35 cycles of 30 s at 95 °C and 30 s at 56 °C, and ended with 30 s at 72 °C and 5 min at 72 °C. The mRNA expressions levels of apoptotic genes were normalized by the expression of Gapdh (glyceraldehyde-3-phosphate dehydrogenase). Primer sequences were: Casp3 forward: CCTCAGAGAGACATTCATGG, Casp3 reversed: AGGAATAGTAACCAGGTGCTG; Foxo1 forward: TCTACGAGTGGATGGTGAAG, Foxo1 reversed: GGTTCGAGGACGAAATGTA; Foxo3 forward: AACCAGACACTCCAAGACCT; Foxo3 reversed: TACAGTGAGGAGCCTGAGAG; Gapdh forward: GCCGAGAATGGGAAGCTTGTCAT, Gapdh reversed: GTGGTTCACACCCATCACAAACAT.
TUNEL Assay
Follicle apoptosis was determined by terminal deoxynucleotidyl transferase 2′-Deoxyuridine, 5′-Triphosphate nick end labeling (TUNEL) assay using the DeadEnd Fluorometric TUNEL System (G3250, Promega, Madison, MI) following manufacturer’s instructions, and the incubation buffer without rTdT enzyme was used as the negative control.
Oocyte Maturation
Oocyte maturation was performed on day 8 of eIVFG. Follicles were removed from alginate beads and incubated for 16 h in maturation media (αMEM with 10% fetal bovine serum, 1.5 IU/ml human chorionic gonadotropin (hCG), 10 ng/ml epidermal growth factor [EGF] [BD Biosciences, Franklin Lakes, NJ], and 10 mIU/ml rFSH) at 37 °C in 5% CO2 in air. Oocytes were then denuded from surrounding cumulus cells using 0.3% hyaluronidase (Sigma-Aldrich, St. Louis, MO). Oocytes were considered to be arrested at prophase I in the germinal vesicle (GV) stage if the nucleus was intact, and were considered to have undergone germinal vesicle breakdown (GVBD) if the nucleus was not visible. If a polar body was present in the perivitelline space, the oocytes were classified as metaphase II (MII). Fragmented or shrunken oocytes were classified as degenerated (D).
Oocyte Spindle and Chromosome Analysis
Oocytes obtained following hCG stimulation were fixed in 3.8% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) containing 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO) for 1 h at 37 °C. Oocytes were washed 3 times in blocking solution with 1× PBS containing 0.3% BSA and 0.01% Tween-20, incubated overnight in a 1:100 dilution of mouse anti-α-tubulin (Cell Signaling Technology, Danvers, MA) in blocking solution, and the blocking solution without mouse anti-α-tubulin was used as negative control. Oocytes were washed 3 times with blocking solution, mounted using Vectashield containing DAPI (Vector Laboratories, Burlingame, CA), and analyzed using an EVOS FL AUTO microscope (Life Technology, Grand Island, NY) in a blinded fashion. Oocytes with barrel-shaped bipolar spindles and well-organized microtubule fibers, along with tightly aligned chromosomes on the metaphase plate, were scored as normal. All other configurations were considered abnormal.
DOX Exposure and Oocyte Maturation In Vivo
According to the Food and Drug Administration regulations (UCM078932), the conversion from human relevant doses to mouse was calculated based on the human weight and body surface area at 60 kg and 1.62 m2, respectively. In humans, the DOX treatment dosage ranges from 8 to 400 mg/m2, which is equivalent to 0.1–10 mg/kg. In order to confirm whether the in vivo DOX exposure affects the oocyte maturation and spindle morphology and chromosome alignment as in vitro, 21-day-old CD-1 female mice were intraperitoneally injected once with either 0.4, 2, and 10 mg/kg of DOX dissolved in 100 μl dimethyl sulfoxide (DMSO) or with 100 μl DMSO only as control. Superovulation was performed 6 days after DOX treatment by intraperitoneal injection of 5 IU of pregnant mare’s serum gonadotropin (PMSG) (Sigma-Aldrich, St. Louis, MO) into the adult female mice, which was followed by a 5-IU hCG injection 48 h after the PMSG injection. MII oocytes were collected from the ampulla of the oviduct 14 h after the hCG injection. Oocytes were denuded from surrounding cumulus cells using 0.3% hyaluronidase, and the oocyte spindle morphology and chromosome alignment were analyzed as described earlier.
Statistical Analyses
Follicle growth, survival, hormone secretion, and oocyte reproductive outcomes were analyzed from three to five independent cultures in which 10–15 follicles were included for each experimental group. Follicle growth, survival, and hormone secretion in different DOX-treated groups were analyzed using Repeated Measures ANOVA. Categorized data including cell apoptotic gene expression, MII oocyte percentage, and percentage with spindle abnormality and chromosome misalignment were analyzed by Kruskal–Wallis H test, and the post hoc test was performed to compare the difference between two groups if the significant difference was observed. The significance level was set at P < 0.05.
RESULTS
DOX Exhibited Dose-Dependent Toxicity on InVitro Cultured Follicles
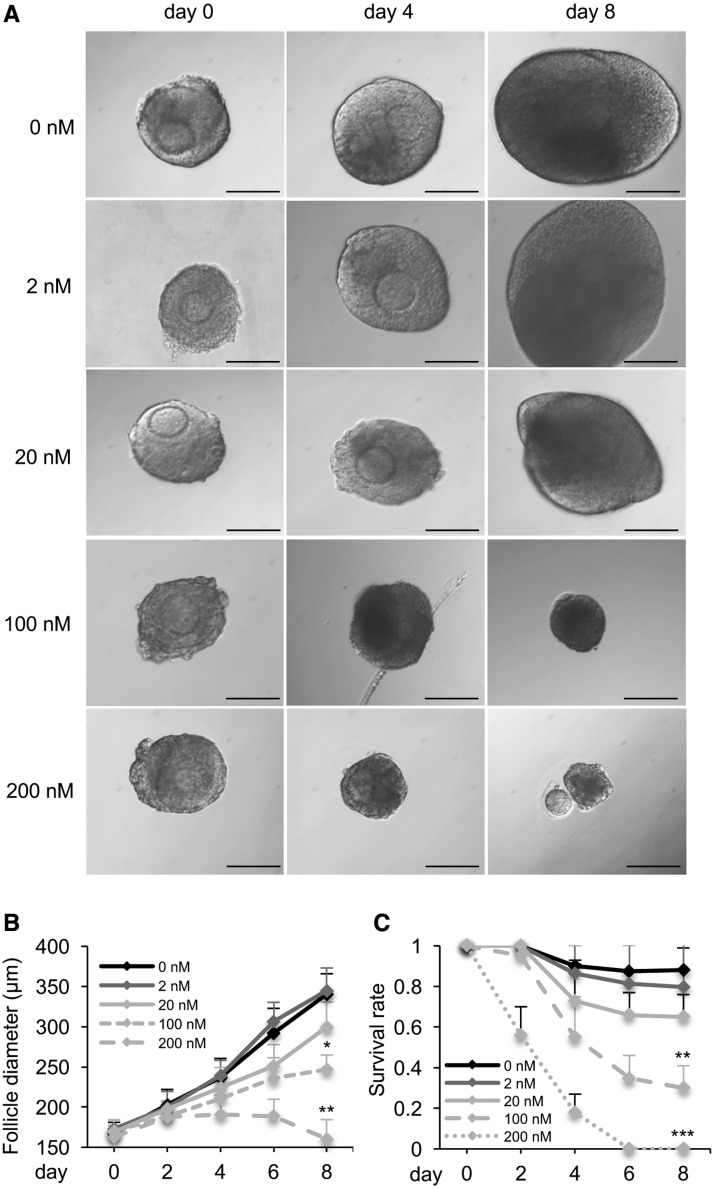
In the control group, the alginate encapsulation maintained follicles’ three-dimensional architecture and supported follicle growth from multilayered secondary stage to antral stage (Figure 1A), with the follicle diameter increased from 167.3 ± 13.8 μm on day 0 to 340.5 ± 25.3 μm on day 8 (Figure 1B), and the follicle survival rate was 87.5% ± 11.1% on day 8 (Figure 1C). The follicle growth and survival were comparable between control and 2 nM DOX-treated groups, and were slightly decreased when follicles were treated with 20 nM DOX (Figs. 1B and C). When follicles were exposed to DOX at 100 and 200 nM, follicle growth was significantly inhibited, and none developed to the antral stage by day 8 (Figs. 1A and B). Moreover, follicle survival rates were significantly decreased with over 80–90% follicles showing dark granulosa cells layers and shrunk and/or extruded oocytes (Figs. 1A and C). The LC50 of DOX on in vitro cultured ovarian follicles was 75.5 nM (95% CI: 68.7–82.3 nM). These results demonstrate the dose-dependent toxicity of DOX on ovarian follicle growth and survival.
FIG. 1.
Effect of doxorubicin (DOX) on follicle growth and survival during encapsulated in vitro follicle growth (eIVFG). A, Representative images of follicles treated with different doses of DOX on days 0, 4, and 8. B, Follicle diameters and C, follicle survival rates from days 0 to 8 after DOX exposure at 0–200 nM. Scale bar: 100 μm; error bar: standard deviation; *P < 0.05, **P < 0.01, and ***P < 0.001 compared to control group. N = 20–40 follicles for three replicates.
DOX Altered Ovarian Hormone Secretion on InVitro Cultured Follicles
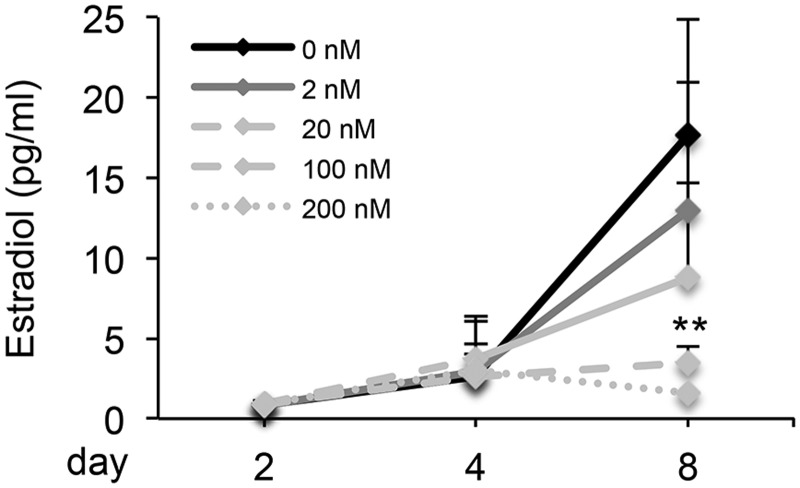
We next investigated the follicle secretion of E2, which is essential for supporting follicle development and oocyte maturation, as well as maintaining ovarian cyclicity and pregnancy. In the control group, the E2 concentration slightly rose from 0.9 ± 0.1 ng/ml on day 2 to 2.6 ± 1.5 ng/ml on day 4, and then significantly rose to 17.6 ± 7.3 ng/ml on day 8, suggesting the transition of in vitro cultured follicles from immature preantral stage to mature antral stage. Follicles treated with DOX from 2 to 200 nM had similar E2 secretion levels on days 2 and 4, however, the E2 secretion showed a decreasing trend in 2 and 20 nM DOX-treated groups, and was significantly decreased in 100 and 200 nM DOX-treated groups on day 8 (Figure 2).
FIG. 2.
Effect of DOX on 17β-estradiol (E2) secretion of in vitro cultured follicles on days 2, 4, and 8. **P < 0.01 compared to control group; error bar: standard deviation. N = 20–40 follicles for three replicates.
DOX Distributed in Follicles in a Time and Cell Type-Dependent Pattern
We next examined the DOX fluorescence distribution over a 24-h period of exposure at 200 nM. At 1 h, DOX started to show positive fluorescence in some theca cells and the outer layers of granulosa cells, and then distributed to most of the granulosa cells at 4 h; however, there was no DOX accumulation within the oocyte nucleus (Figure 3). At 24 h, the oocyte nucleus showed positive DOX fluorescence and dispersed oocyte chromatins (Figure 3). These results suggest that DOX has a time and cell type-dependent distribution patterns on ovarian follicles.
FIG. 3.
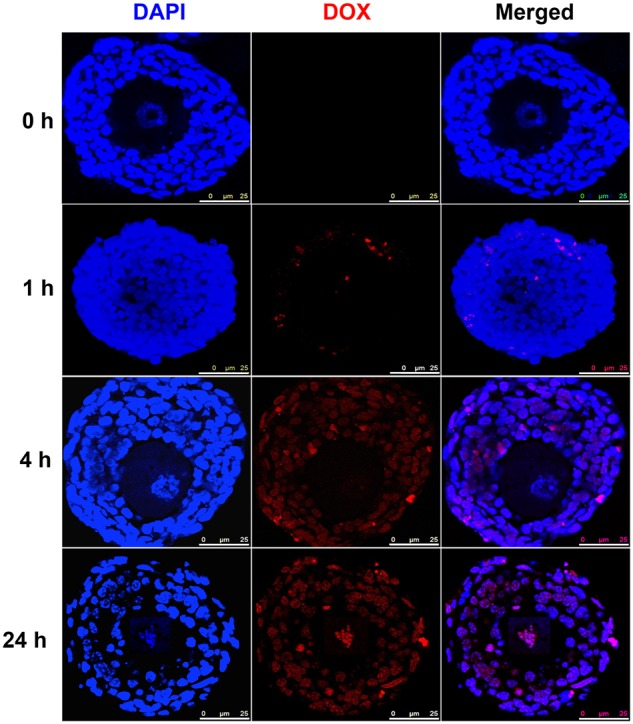
Representative images of doxorubicin (DOX) fluorescence distribution in follicles after DOX exposure at 200 nM for 0, 1, 4, and 24 h. Blue: DAPI; red: DOX fluorescence. Scale bar: 25 μm. N = 5–10 follicles for each time points and replicate, three replicates were performed, and follicles at each time points showed similar DOX fluorescence distribution patterns.
DOX Induced Follicle DNA Damage and Apoptosis
γ-H2AX is a well-defined biomarker for DNA double-strand breaks (Kuo and Yang, 2008; Kurz et al., 2004). As the major mechanism of DOX’s anticancer effect is the induction of DNA double-strands breaks and DNA damage in tumor cells, γ-H2AX was examined in follicles treated with 200 nM DOX at different time periods. Follicles treated with DOX for 4 h started to exhibit positive γ-H2AX staining, however, the signals were limited in outer layers of somatic cells including both theca and granulosa cells, but not within oocytes (Figure 4). At 24 h, the inner layers of granulosa cells exhibited DNA damage, and the oocytes showed positive γ-H2AX staining with dispersed oocyte chromatin (Figure 4).
FIG. 4.
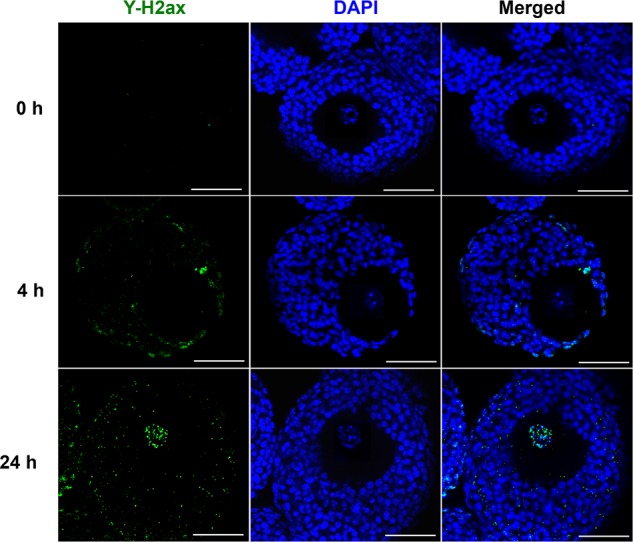
Representative images of follicular cell DNA damage after doxorubicin (DOX) exposure at 200 nM for 0, 4, and 24 h. Blue: DAPI; green: γ-H2ax. Scale bar: 50 μm. N = 5–10 follicles for each time points and replicate, three replicates were performed, and follicles at each time point showed similar DNA damage patterns.
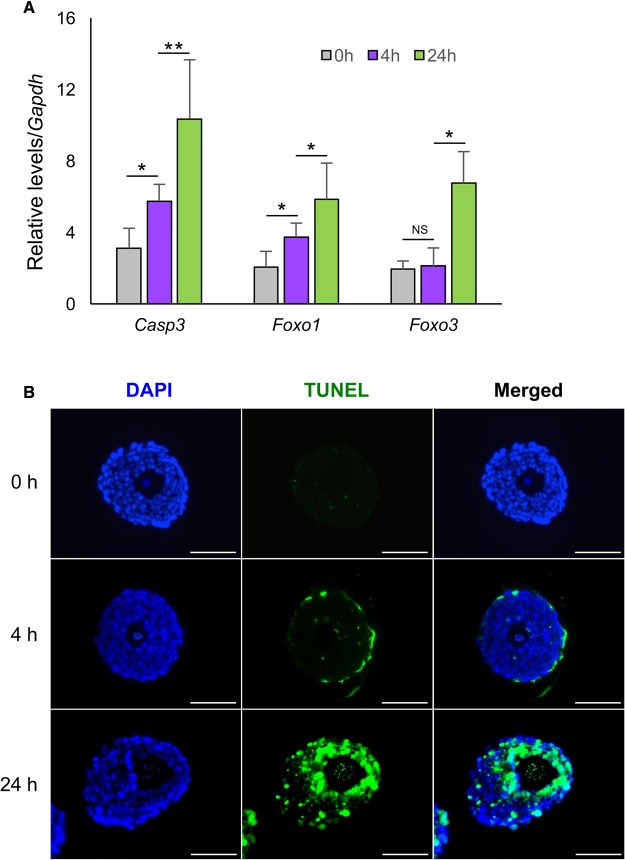
RT-qPCR and TUNEL staining were performed to determine whether DOX induced DNA damage triggers follicle apoptosis. At the transcriptional level, the mRNA expression levels of pro-apoptotic genes Casp3 was significantly increased at both 4 and 24 h (Figure 5B). Foxo1, the transcription factor contributing to follicle granulosa cell apoptosis, had significantly increased expression levels in both 4 and 24 h DOX-treated follicles; however, the oocyte apoptotic marker, Foxo3, had no significant increase until 24 h (Figure 5B). TUNEL staining demonstrated that the follicle theca cells underwent apoptosis 4 h after DOX exposure, but the positive TUNEL staining was barely detectable in granulosa cells and oocytes until 24 h (Figure 5A). These results demonstrate a dynamic pattern of DOX induced DNA damage and apoptosis in follicle somatic cells (theca and granulosa cells) and germ cells (oocyte).
FIG. 5.
Follicle apoptosis after DOX exposure at 200 nM for 0, 4, and 24 h. A, Expression levels of follicle apoptotic genes after DOX exposure at 200 nM. *P < 0.05, **P < 0.01; error bar: standard deviation. B, Representative images of follicles with TUNEL staining. Blue: DAPI; green: DNA fragmentation. Scale bar: 100 μm. N = 40–50 follicles for RNA extraction and three replicates in total in (A) and N = 5–10 follicle for TUNEL staining in (B).
DOX Disrupted Oocyte Meiotic Maturation on InVitro Cultured Follicles
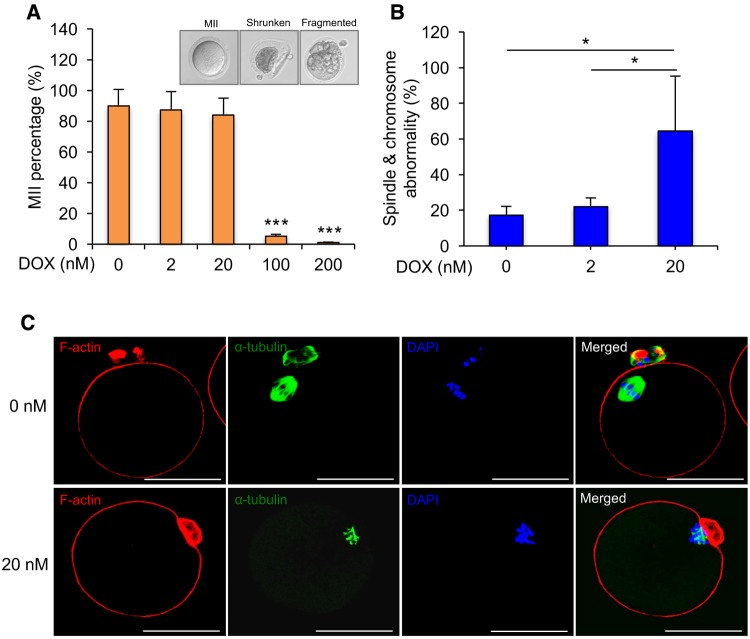
Oocyte maturation was performed on day 8 in follicles treated with different concentrations of DOX. In 0, 2, and 20 nM DOX-treated groups, there was no significant difference in the MII percentages after hormone stimulation (89.8% ± 10.8%, 87.3% ± 11.4%, and 84.0% ± 10.9%, respectively) (Figure 6A). However, only 5.2% ± 1.2% and 1.3% ± 0.2% of oocytes had polar body extruded in 100 and 200 nM DOX-treated groups, respectively, and most of oocytes were shrunken or fragmented (Figure 6A), which were expected as the follicle growth and development were severely inhibited, thus subsequently disrupting the oocyte maturation.
FIG. 6.
Effect of DOX exposure on oocyte maturation in vitro. A, MII percentages after oocyte maturation for in vitro cultured follicles treated with DOX at 0, 2, 20, 100, and 200 nM on day 8, and the representative images of MII, shrunken, and fragmented oocytes. B, Incidence of spindle abnormality and chromosome misalignment in MII oocytes from follicles treated with DOX at 0, 2, and 20 nM. C, Representative images of meiotic spindles organization (green) and chromosome distribution (blue) of MII oocytes treated with DOX at 0 or 2 and 20 nM. *P < 0.05 and ***P < 0.001; error bar: standard deviation. Blue: DAPI; green: α-tubulin; red: F-actin; scale bar in (C): 50 μm. N = 30–40 follicles in each DOX exposure group and three replicates in total.
To further investigate the quality of oocytes after low doses (0–20 nM) of DOX exposure, oocytes that had progressed to MII stage were analyzed for spindle integrity and chromosome alignment, two critical markers of oocyte meiotic and developmental competence (Li et al., 2014; Moon et al., 2003). 82.7% ± 14.5% and 79.1% ± 13.6% of MII oocytes from follicles treated with 0 and 2 nM DOX had barrel-shaped bipolar spindles and tightly aligned chromosomes on the metaphase plate, respectively (Figs. 6B and C). However, a significant higher percentage (64.58% ± 30.68%) of MII oocytes from follicles treated with 20 nM DOX exhibited misshaped spindles and/or dispersed chromosomes on the metaphase plate (Figs. 6B and C), suggesting the human-relevant low exposure level of DOX does not disrupt follicle growth, but does adversely affect oocyte meiotic maturation.
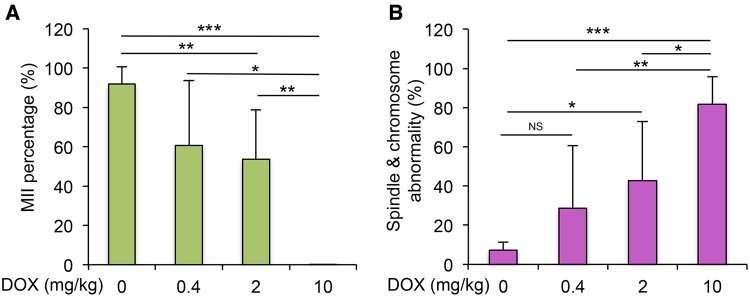
DOX Exhibited Dose-Dependent Toxicity on Oocyte Meiotic Maturation InVivo
To further confirm the effect of DOX on oocyte maturation, an in vivo experiment was performed by exposing DOX to 21-day-old mice at 0, 0.4, 2, and 10 mg/kg, and then retrieving oocytes for analysis as done in vitro. More than 90% of oocytes reached MII stage and had polar body extrusion in the control group, however, the MII percentages of oocyte from DOX-treated mice dose-dependently decreased (60.8% ± 32.8%, 53.1% ± 25.07%, and 0.2% ± 0.05% in 0.4, 2, and 10 mg/kg DOX-treated mice, respectively, Figure 7A). Particularly in the 10-mg/kg DOX-treated mice, most of the oocytes underwent degeneration. The percentage of MII oocytes with spindle or chromosome abnormality in 0.4 mg/kg DOX-treated mice was increased compared to the control group (28.6 ± 32.0% vs 7.3% ± 4.0%), but the difference was not significant (Figure 7B). However, in the 2- and 10-mg/kg DOX-treated groups, 42.8% ± 30.2% and 81.8% ± 13.9% of MII oocytes showed abnormal spindle morphology and/or chromosome misalignment, which were significantly higher than that in the control group (Figure 7B). Taken together of both in vivo and in vitro data, we conclude that the DOX has dose-dependent toxicity on oocyte meiotic maturation.
FIG. 7.
Effect of DOX exposure on oocyte maturation in vivo. A, MII percentage of oocytes from mice treated with DOX at 0, 0.4, 2, and 10 mg/kg. B, Incidence of spindle abnormality and chromosome misalignment of MII oocytes from mice treated with DOX at 0, 0.4, 2, and 10 mg/kg. *P < 0.05, **P < 0.01, and ***P < 0.001; NS: nonsignificant; error bar: standard deviation. N = 4–6 mice and 20–30 oocytes per mice in each DOX-treated dosage.
DISCUSSION
The primary functions of the follicle are to synthesize and secrete steroid hormones to maintain the ovarian cyclicity and to support oocyte maturation for fertilization and pregnancy. In vitro follicle cultures provide a dynamic method for evaluating the ovarian toxicity of both pharmaceutical drugs and environmental chemicals and efforts in harmonizing patients dosing with in vivo animal data and in vitro models are critically important (Ahn et al., 2013; Mahalingam et al., 2016; Patel et al., 2016; Xu et al., 2015).
DOX treatment dosage in cancer patients ranges from 8 to 400 mg/m2 (equivalent to 0.1–10 mg/kg), which depends on the body size, cancer type, and disease condition (Ferguson et al., 1993; Scheithauer et al., 1985). After entering systemic circulation, DOX is rapidly and extensively distributed into most of tissues, including the ovary (Roti Roti et al., 2012; Speth et al., 1988), and the plasma concentration is maintained at 2–200 nM for 20–30 h (Barpe et al., 2010; Lee et al., 1980). Doxorubicinol is the major metabolite of DOX through phase I reduction, and then doxorubicinol is converted to glucuronide or sulfate conjugates for excretion through phase II biotransformation (Sturgill et al., 2000). Compared to doxorubicinol and its conjugates, DOX is more cytotoxic, and remains high in both tumor and other tissues because the metabolic biotransformation and excretion are slow (Speth et al., 1988). Therefore, in the in vitro study, DOX, but not its metabolites, was used to treat in vitro cultured follicles at human relevant exposure levels (2–200 nM) and time periods (0–24 h). It has been demonstrated that 12 mg/kg of DOX treatment resulted in the plasma DOX concentration at 0.02–0.1 ng/μl in mouse, which is equivalent to 3.6–184 nM (Johansen, 1981). Thus, 0–10 mg/kg of DOX was selected in the in vivo experiment, which resulted in the similar DOX plasma concentrations as the follicle culture media DOX levels of in vitro study.
It has been reported that DOX’s ovarian distribution is consistent with the intimate interactions between tissues and blood supply, as DOX accumulates more in the medulla area than the outwards cortex area, suggesting the larger growing follicles (secondary, early antral, and antral follicles) experience more DOX exposure than smaller primordial and primary follicles (Roti Roti et al., 2012). Thus, multilayered secondary follicles were tested in our study, which represent growing follicles in the ovary, and our follicle survival results with the significantly increased follicle apoptosis and death are consistent with the previous studies (Ben-Aharon et al., 2010; Roti Roti et al., 2012). The mechanism of DOX’s anticancer actions is related to intercalation with cancer cell DNA and topoisomerase II and activate cancer cell apoptosis (Gewirtz, 1999; Minotti et al., 2004). During follicle growth from preantral to antral stage, the proliferation of granulosa cells are mitotically active and therefore are potential target of DOX’s cytotoxicity (Robker and Richards, 1998). We demonstrate that DOX has a dose-dependent toxicity on follicle growth and survival, and has LC50 at 75 nM on in vitro cultured follicles. When DOX exposure level is >100 nM, none of the follicles develop to the antral stage (Figure 1) and all follicles undergo atresia through apoptosis. Previous study has shown that primordial follicles exhibited granulosa cell apoptosis 48 h later than primary, secondary, and antral follicles, suggesting the cell and follicle type-dependent toxicity of DOX (Roti Roti et al., 2012). Recently, another commonly used chemotherapeutic cyclophosphamide has been shown to damage growing follicles, which subsequently induced primordial follicle activation and diminished the ovarian reserve (Kalich-Philosoph et al., 2013). Here, we only tested the DOX’s ovarian toxicity on multilayered secondary follicle development and oocyte maturation but not the younger staged follicles, and the in vitro multiple follicle culture and the neonatal ovary organ culture provide good models to further study the chemical-induced ovarian toxicity on primordial, primary, and early secondary staged follicles (Hornick et al., 2013; Kim et al., 2013). Besides, it is also worth to test whether the DOX-induced damages on growing follicles will burnout the primordial follicle reserve and cause premature ovarian failure.
During steroidogenesis, luteinizing hormone (LH) stimulates theca cells to produce androstenedione, which will be further converted to estradiol under the control of FSH in granulosa cells. In our study, the E2 production was slightly increased from days 2 to 4 in both control and DOX-treated groups, suggesting the DOX induced theca and granulosa cell damage did not significantly inhibit the steroid production. Upon the follicles grow to antral stage and started to secret large amounts of steroid hormones (days 4–8 of eIVFG), the inhibited follicle growth and viability significantly disrupted the E2 production at both 100 and 200 nM treated groups. This supports the previous human study showing increased amenorrhea incidence after DOX treatment in female cancer patients and demonstrates the acute growing follicle death after receiving DOX for chemotherapy (Petrek et al., 2006). However, more studies are necessary to address the specific question that whether the altered steroid hormone production is directly resulted from DOX’s exposure or indirectly from the DOX’s toxicity on follicle growth and maturation.
Different from other in vitro toxicity testing of certain cell lines, ovarian follicles are composed of different cell types, including theca cells, granulosa cells, and oocytes. Based on DOX’s auto-fluorescence, we revealed that DOX reached follicle somatic cells (theca and granulosa cells) first, and then penetrated the oocyte zona pellucida and cell membrane, and bound to oocyte nuclear DNA (Figure 5), suggesting that the dynamic cell apoptosis in somatic and germ cells result from the time and cell type-dependent DOX distribution and accumulation. These results are consistent with the previously published data (Roti Roti et al., 2012), and the in vitro follicle culture model provides another option for monitoring the DOX or other xenobiotics’ ovarian cell-type and time-point distribution patterns. However, further studies are needed to investigate whether the dynamic DOX distribution is caused by the different cell uptake sensitivity or by the follicle architecture with somatic cells outside and oocyte in the center, and whether the oocyte DNA damage and apoptosis are caused by direct toxicity of DOX, or indirectly resulted from damaged granulosa cells.
In vivo studies have demonstrated that DOX induced follicle apoptosis and decreased ovulation rate at 10–20 mg/kg in mouse (Ben-Aharon et al., 2010; Morgan et al., 2013; Roti Roti et al., 2012). Further, denuded oocytes undergo degeneration when they are exposed to DOX at 200 nM and 10 μM in vitro (Bar-Joseph et al., 2010; Perez et al., 1997). In this study, we treated the intact follicle unit with DOX, not the denuded oocyte, which is more representative as pre-ovulated oocyte are not directly exposed to DOX during chemotherapy. The significantly disrupted oocyte maturation outcomes in follicles treated with DOX at 100 and 200 nM are correlated with the increased follicle apoptosis and death. At lower exposure level of DOX at 20 nM, there were slightly inhibited follicle growth and decreased oocyte MII percentage, but they are still comparable to the control group.
We have previously demonstrated that polar body extrusion was not a sufficient indicator for the oocyte quality, as the functional oocyte meiotic spindle is also essential for proper chromosome segregation and producing a haploid gamete (Li et al., 2006; Xiao et al., 2015a). After further examining the MII oocytes from 2 and 20 nM DOX-treated groups, we found a significantly higher percentage of oocytes with abnormal spindle morphology and chromosome misalignment when follicles were treated with DOX at 20 nM (Figure 6). Similarly, when mice were treated with low doses of DOX at 0.4 and 2 mg/kg in vivo, >50% of oocytes reached MII stage but showed a significantly higher percentage of oocytes with abnormal spindle morphology and chromosome misalignment (Figure 7B). These data demonstrate that the low dose of DOX does not significantly affect follicle growth and oocyte polar body extrusion, but adversely disrupts oocyte meiotic division and chromosome segregation, which should be given more awareness as the inappropriate meiotic division increases the risk of aneuploidy and birth defect, and it is suggested that cancer patients should avoid this window for oocyte and embryo cryopreservation and pregnancy during chemotherapy.
In conclusion, the eIVFG provides a good in vitro model to screen new gonadotoxic chemicals as well as help test the efficacy of the potential ovarian function and fertility protectants during chemotherapy, such as bortezimib and dexrazoxane, which have been reported to protected DOX induced ovarian toxicity (Kropp et al., 2015; Roti Roti et al., 2014). Besides, our study demonstrates the dose-dependent toxicity of DOX on ovarian follicle growth, survival, and hormone secretion, and that the low dose of DOX affects oocyte maturation and results in the abnormality of oocyte meiotic division. These findings provide significant implications in understanding how DOX disrupts ovarian functions and how to provide fertility protective options for patients who are at the risk of reproductive dysfunction and infertility during chemotherapy.
ACKNOWLEDGMENTS
We thank Chanel A. Arnold-Murray (Northwestern University Ovarian Histology: UH3TR001207 and P50HD076188) for sectioning our follicle samples. S.X. and T.K.W. conceived of the project, designed experiments, collected data, analyzed and interpreted data, wrote the article, and final approve the article. J.Z. was involved in experiment design, data collection and analysis. M.L., H.I., and H.R.R. contributed to data collection and analysis and article writing. The authors declare no conflict of interest.
FUNDING
National Institutes of Health NCATS/NIEHS/NICHD/ORWH (UH3TR001207); Center for Reproductive Health After Disease from the National Institutes of Health National Center for Translational Research in Reproduction and Infertility (NCTRI) (P50HD076188).
REFERENCES
- Ahn R. W., Barrett S. L., Raja M. R., Jozefik J. K., Spaho L., Chen H. M., Bally M. B., Mazar A. P., Avram M. J., Winter J. N., et al. (2013). Nano-encapsulation of arsenic trioxide enhances efficacy against murine lymphoma model while minimizing its impact on ovarian reserve in vitro and in vivo. PloS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Joseph H., Ben-Aharon I., Rizel S., Stemmer S. M., Tzabari M., Shalgi R. (2010). Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reprod. Toxicol. 30, 566–572. [DOI] [PubMed] [Google Scholar]
- Barpe D. R., Rosa D. D., Froehlich P. E. (2010). Pharmacokinetic evaluation of doxorubicin plasma levels in normal and overweight patients with breast cancer and simulation of dose adjustment by different indexes of body mass. Eur. J. Pharm. Sci. 41, 458–463. [DOI] [PubMed] [Google Scholar]
- Ben-Aharon I., Bar-Joseph H., Tzarfaty G., Kuchinsky L., Rizel S., Stemmer S. M., Shalgi R. (2010). Doxorubicin-induced ovarian toxicity. Reprod. Biol. Endocrinol. 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bines J., Oleske D. M., Cobleigh M. A. (1996). Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 14, 1718–1729. [DOI] [PubMed] [Google Scholar]
- Blum R. H., Carter S. K. (1974). Adriamycin. A new anticancer drug with significant clinical activity. Ann. Intern. Med. 80, 249–259. [DOI] [PubMed] [Google Scholar]
- Damodar G., Smitha T., Gopinath S., Vijayakumar S., Rao Y. (2014). An evaluation of hepatotoxicity in breast cancer patients receiving injection doxorubicin. Ann. Med. Health Sci. Res. 4, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio M., Basch E., Bryce J., Perrone F. (2016). Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat. Rev. Clin. Oncol. 13, 319–325. [DOI] [PubMed] [Google Scholar]
- Ferguson J. E., Dodwell D. J., Seymour A. M., Richards M. A., Howell A. (1993). High dose, dose-intensive chemotherapy with doxorubicin and cyclophosphamide for the treatment of advanced breast cancer. Br. J. Cancer 67, 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansler T., Ganz P. A., Grant M., Greene F. L., Johnstone P., Mahoney M., Newman L. A., Oh W. K., Thomas C. R. Jr, Thun M. J., et al. (2010). Sixty years of CA: A cancer journal for clinicians. CA Cancer J. Clin. 60, 345–350. [DOI] [PubMed] [Google Scholar]
- Gewirtz D. A. (1999). A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 57, 727–741. [DOI] [PubMed] [Google Scholar]
- Hofland K. F., Thougaard A. V., Sehested M., Jensen P. B. (2005). Dexrazoxane protects against myelosuppression from the DNA cleavage-enhancing drugs etoposide and daunorubicin but not doxorubicin. Clin. Cancer Res. 11, 3915–3924. [DOI] [PubMed] [Google Scholar]
- Hornick J. E., Duncan F. E., Shea L. D., Woodruff T. K. (2013). Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 145, 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen P. B. (1981). Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother. Pharmacol. 5, 267–270. [DOI] [PubMed] [Google Scholar]
- Kalich-Philosoph L., Roness H., Carmely A., Fishel-Bartal M., Ligumsky H., Paglin S., Wolf I., Kanety H., Sredni B., Meirow D. (2013). Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 5, 185ra62.. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Cordeiro M. H., Serna V. A., Ebbert K., Butler L. M., Sinha S., Mills A. A., Woodruff T. K., Kurita T. (2013). Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 20, 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp J., Roti Roti E. C., Ringelstetter A., Khatib H., Abbott D. H., Salih S. M. (2015). Dexrazoxane diminishes doxorubicin-induced acute ovarian damage and preserves ovarian function and fecundity in mice. PLoS One 10, e0142588.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo L. J., Yang L. X. (2008). Gamma-H2AX – a novel biomarker for DNA double-strand breaks. In Vivo 22, 305–309. [PubMed] [Google Scholar]
- Kurz E. U., Douglas P., Lees-Miller S. P. (2004). Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J. Biol. Chem. 279, 53272–53281. [DOI] [PubMed] [Google Scholar]
- Lee Y. T., Chan K. K., Harris P. A., Cohen J. L. (1980). Distribution of adriamycin in cancer patients: Tissue uptakes, plasma concentration after IV and hepatic IA administration. Cancer 45, 2231–2239. [DOI] [PubMed] [Google Scholar]
- Li M., Zhao H. C., Li R., Yu Y., Qiao J. (2014). Chromosomal aberrations in in-vitro matured oocytes influence implantation and ongoing pregnancy rates in a mouse model undergoing intracytoplasmic sperm injection. Plos One 9, e103347.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Feng H. L., Cao Y. J., Zheng G. J., Yang Y., Mullen S., Critser J. K., Chen Z. J. (2006). Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil. Steril. 85, 827–832. [DOI] [PubMed] [Google Scholar]
- Mahalingam S., Gao L., Eisner J., Helferich W., Flaws J. A. (2016). Effects of isoliquiritigenin on ovarian antral follicle growth and steroidogenesis. Reprod. Toxicol. 66, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaeili S., Rashidi B. H., Safa M., Najafi A., Sobhani A., Asadi E., Abbasi M. (2016). Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch. Gynecol. Obstet. 294, 185–192. [DOI] [PubMed] [Google Scholar]
- Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. (2004). Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 56, 185–229. [DOI] [PubMed] [Google Scholar]
- Moon J. H., Hyun C. S., Lee S. W., Son W. Y., Yoon S. H., Lim J. H. (2003). Visualization of the metaphase II meiotic spindle in living human oocytes using the Polscope enables the prediction of embryonic developmental competence after ICSI. Hum. Reprod. 18, 817–820. [DOI] [PubMed] [Google Scholar]
- Morgan S., Anderson R. A., Gourley C., Wallace W. H., Spears N. (2012). How do chemotherapeutic agents damage the ovary?. Hum. Reprod. Update 18, 525–535. [DOI] [PubMed] [Google Scholar]
- Morgan S., Lopes F., Gourley C., Anderson R. A., Spears N. (2013). Cisplatin and doxorubicin induce distinct mechanisms of ovarian follicle loss; imatinib provides selective protection only against cisplatin. PloS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (2015). SEER Cancer Statistics Review 1975-2012. Available at: http://seer.cancer.gov/archive/csr/1975_2012/.
- Patel S., Peretz J., Pan Y. X., Helferich W. G., Flaws J. A. (2016). Genistein exposure inhibits growth and alters steroidogenesis in adult mouse antral follicles. Toxicol. Appl.Pharmacol. 293, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G. I., Knudson C. M., Leykin L., Korsmeyer S. J., Tilly J. L. (1997). Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat. Med. 3, 1228–1232. [DOI] [PubMed] [Google Scholar]
- Petrek J. A., Naughton M. J., Case L. D., Paskett E. D., Naftalis E. Z., Singletary S. E., Sukumvanich P. (2006). Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J. Clin. Oncol. 24, 1045–1051. [DOI] [PubMed] [Google Scholar]
- Robker R. L., Richards J. S. (1998). Hormonal control of the cell cycle in ovarian cells: Proliferation versus differentiation. Biol. Reprod. 59, 476–482. [DOI] [PubMed] [Google Scholar]
- Roti Roti E. C., Leisman S. K., Abbott D. H., Salih S. M. (2012). Acute doxorubicin insult in the mouse ovary is cell- and follicle-type dependent. PloS One 7, e42293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti Roti E. C., Ringelstetter A. K., Kropp J., Abbott D. H., Salih S. M. (2014). Bortezomib prevents acute doxorubicin ovarian insult and follicle demise, improving the fertility window and pup birth weight in mice. PLoS One 9, e108174.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheithauer W., Zielinksi C., Ludwig H. (1985). Weekly low dose doxorubicin monotherapy in metastatic breast cancer resistant to previous hormonal and cytostatic treatment. Breast Cancer Res. Treat. 6, 89–93. [DOI] [PubMed] [Google Scholar]
- Shen M., Liu Z., Li B., Teng Y., Zhang J., Tang Y., Sun S. C., Liu H. (2014). Involvement of FoxO1 in the effects of follicle-stimulating hormone on inhibition of apoptosis in mouse granulosa cells. Cell Death Dis. 5, e1475.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2016). Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30. [DOI] [PubMed] [Google Scholar]
- Slot K. A., Voorendt M., de Boer-Brouwer M., van Vugt H. H., Teerds K. J. (2006). Estrous cycle dependent changes in expression and distribution of Fas, Fas ligand, Bcl-2, Bax, and pro- and active caspase-3 in the rat ovary. J. Endocrinol. 188, 179–192. [DOI] [PubMed] [Google Scholar]
- Speth P. A., van Hoesel Q. G., Haanen C. (1988). Clinical pharmacokinetics of doxorubicin. Clin. Pharmacokinet. 15, 15–31. [DOI] [PubMed] [Google Scholar]
- Sturgill M. G., August D. A., Brenner D. E. (2000). Hepatic enzyme induction with phenobarbital and doxorubicin metabolism and myelotoxicity in the rabbit. Cancer Invest. 18, 197–205. [DOI] [PubMed] [Google Scholar]
- Xiao S., Duncan F. E., Bai L., Nguyen C. T., Shea L. D., Woodruff T. K. (2015a). Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction 150, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Zhang J., Romero M. M., Smith K. N., Shea L. D., Woodruff T. K. (2015b). In vitro follicle growth supports human oocyte meiotic maturation. Sci. Rep. 5, 17323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Kreeger P. K., Shea L. D., Woodruff T. K. (2006a). Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 12, 2739–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., West E., Shea L. D., Woodruff T. K. (2006b). Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol. Reprod. 75, 916–923. [DOI] [PubMed] [Google Scholar]
- Xu Y., Duncan F. E., Xu M., Woodruff T. K. (2015). Use of an organotypic mammalian in vitro follicle growth assay to facilitate female reproductive toxicity screening. Reprod. Fertil. Dev. doi: 10.1071/RD14375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liu X., Bawa-Khalfe T., Lu L. S., Lyu Y. L., Liu L. F., Yeh E. T. (2012). Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642. [DOI] [PubMed] [Google Scholar]