Abstract
STUDY QUESTION
What are the separate and combined effects of mild hyperandrogenemia and consumption of a high-fat Western-style diet (WSD) on white adipose tissue (WAT) morphology and function in young adult female nonhuman primates?
SUMMARY ANSWER
Combined exposure to mild hyperandrogenemia and WSD induces visceral omental (OM-WAT) but not subcutaneous (SC-WAT) adipocyte hypertrophy that is associated with increased uptake and reduced mobilization of free fatty acids.
WHAT IS KNOWN ALREADY
Mild hyperandrogenemia in females, principally in the context of polycystic ovary syndrome, is often associated with adipocyte hypertrophy, but the mechanisms of associated WAT dysfunction and depot specificity remain poorly understood.
STUDY DESIGN, SIZE AND DURATION
Female rhesus macaques were randomly assigned at 2.5 years of age (near menarche) to receive either cholesterol (C; n = 20) or testosterone (T; n = 20)-containing silastic implants to elevate T levels 5-fold above baseline. Half of each of these groups was then fed either a low-fat monkey chow diet or WSD, resulting in four treatment groups (C, control diet; T alone; WSD alone; T + WSD; n = 10/group) that were maintained until the current analyses were performed at 5.5 years of age (3 years of treatment, young adults).
PARTICIPANTS/MATERIALS, SETTING AND METHODS
OM and SC-WAT biopsies were collected and analyzed longitudinally for in vivo changes in adipocyte area and blood vessel density, and ex vivo basal and insulin-stimulated fatty acid uptake and basal and isoproterenol-stimulated lipolysis.
MAIN RESULTS AND THE ROLE OF CHANCE
In years 2 and 3 of treatment, the T + WSD group exhibited a significantly greater increase in OM adipocyte size compared to all other groups (P < 0.05), while the size of SC adipocytes measured at the end of the study was not significantly different between groups. In year 3, both WAT depots from the WSD and T + WSD groups displayed a significant reduction in local capillary length and vessel junction density (P < 0.05). In year 3, insulin-stimulated fatty acid uptake in OM-WAT was increased in the T + WSD group compared to year 2 (P < 0.05). In year 3, basal lipolysis was blunted in the T and T + WSD groups in both WAT depots (P < 0.01), while isoproterenol-stimulated lipolysis was significantly blunted in the T and T + WSD groups only in SC-WAT (P < 0.01).
LIMITATIONS, REASONS FOR CAUTION
At this stage of the study, subjects were still relatively young adults, so that the effects of mild hyperandrogenemia and WSD may become more apparent with increasing age.
WIDER IMPLICATIONS OF THE FINDINGS
The combination of mild hyperandrogenemia and WSD accelerates the development of WAT dysfunction through T-specific (suppression of lipolytic response by T), WSD-dependent (reduced capillary density) and combined T + WSD (increased fatty acid uptake) mechanisms. These data support the idea that combined hyperandrogenemia and WSD increases the risk of developing obesity in females.
STUDY FUNDING/COMPETING INTEREST(S)
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number P50 HD071836 to C.T.R. and award number OD 011092 from the Office of the Director, National Institutes of Health, for operation of the Oregon National Primate Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Keywords: PCOS, Western-style diet, nonhuman primates, hyperandrogenemia, obesity, androgen, testosterone, adipose tissue, lipolysis, fatty acid
Introduction
Polycystic ovary syndrome (PCOS) is a common disorder of reproductive age associated with ovarian dysfunction and infertility, which results in metabolic disturbances (Solomon, 1999) such as obesity and insulin resistance (IR) (Wagenknecht et al., 2003; Escobar-Morreale and San Millan, 2007; Diamanti-Kandarakis and Dunaif, 2012). The diagnosis of PCOS has evolved over time, with the original NIH criteria requiring clinical or biochemical hyperandrogenism and evidence of oligo-ovulation and the exclusion of other disorders. The subsequent Rotterdam criteria required two out of three symptoms (hyperandrogenemia, oligo- or anovulation or polycystic ovaries), while the later Androgen Excess/PCOS Society criteria specified excess androgen levels, altered ovulation and/or polycystic ovaries and exclusion of other disorders (Rosenfield and Ehrmann, 2016).
Hyperandrogenemia is the most common primary diagnostic parameter in all cases, however. Mild (3 to 4-fold elevation) hyperandrogenemia can present as early as adolescence and persists into adulthood (Burt Solorzano et al., 2012). While hyperandrogenemia is reported to play a central role in the pathogenesis of IR in PCOS women (Azziz et al., 2009; Diamond et al., 1998; Polderman et al., 1994), its mechanisms with regard to female metabolism remain poorly understood. Weight loss can reduce ovarian and metabolic dysfunction (Barber and Franks, 2013; Lim et al., 2013), resulting in improved insulin sensitivity and reduced hyperandrogenemia (Pasquali et al., 1989; Holte et al., 1995; Gambineri et al., 2004; Mehrabani et al., 2012; Thomson et al., 2008 ), suggesting that obesity may exaggerates hyperandrogenemia-related symptoms in PCOS patients.
Clinical and animal studies demonstrated that PCOS and hyperandrogenemia are associated with the development of white adipose tissue (WAT) dysfunction characterized by local IR (Ciaraldi et al., 1992; Ciaraldi et al., 2009; Dunaif et al., 1992 ), low-grade inflammation (Spritzer et al., 2015), reduced insulin-mediated inhibition of lipolysis (Marsden et al., 1994; Moro et al., 2009), increased intra-abdominal fat mass (Dumesic et al., 2016) and abdominal subcutaneous (SC) adipocyte hypertrophy (Manneras-Holm et al., 2011; Dumesic et al., 2016). The WAT present in PCOS women exhibits increased expression of fatty acid transporters and reduced levels of hormone-sensitive lipase (HSL) (Seowet al., 2009) and the insulin-dependent glucose transporter-4 (GLUT-4) (Chen et al., 2013; Wu et al., 2014). PCOS-related WAT dysfunction also includes altered adipokine secretion (Spritzer et al., 2015), reduced expression of lipogenic and developmental genes (Chazenbalk et al., 2012) and altered adipogenesis (Keller et al., 2014). Although these studies demonstrated the development of multiple WAT alterations in the presence of hyperandrogenemia, it remains to be determined whether the observed changes are primarily the result of increased visceral adiposity, which is also associated with the PCOS phenotype (Manneras-Holm et al., 2011; Dumesic et al., 2016).
The development of nonhuman primate (NHP) models of PCOS (Abbott et al., 2013) has provided valuable translational tools for delineating tissue-specific mechanisms contributing to PCOS-related pathologies in humans. Pilot studies employing chronic, mild elevation of androgen in prepubertal rhesus macaques maintained through young adulthood demonstrated that these female monkeys developed metabolic and ovarian dysfunction when exposed to a high-fat/calorie-dense Western-style diet (WSD) as young adults (Varlamov et al., 2013; McGee et al., 2014). These findings revealed, for the first time, that diet can directly modulate reproductive and metabolic symptoms associated with hyperandrogenemia. In the present study, prepubertal female rhesus macaques were maintained on a control diet or WSD in the presence or absence of chronically elevated testosterone (T) levels similar to those in young women predisposed to PCOS through puberty and into early reproductive age. We used this study design to address the hypothesis that androgen excess would accelerate WSD-induced WAT dysfunction and to delineate diet- and androgen-specific mechanisms of metabolic dysfunction.
Materials and Methods
Animals
This study was approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee (IACUC) and conforms to current Office of Laboratory Animal Welfares (OLAW) regulations as stipulated in assurance number A3304-01. Female rhesus macaques were pair-housed, with the cage size adjusted to animal weight according to the USDA Cage Size Guide, eighth Edition, beginning at 2.5 years of age (n = 40). The experimental design is described in Fig. 1. Females were maintained on either a typical chow diet (n = 20) consisting of two daily meals of fiber-balanced monkey diet (15% calories from fat, 27% from protein and 59% from carbohydrates; no. 5052; Lab Diet, St. Louis, MO), supplemented with fruits and vegetables, or a WSD (n = 20), containing 36% calories from fat, 18% from protein and 45% from carbohydrates (TAD Primate Diet 5LOP, 5A1F, Lab Diet), as previously reported (Grayson et al., 2010). Animals consumed chow or WSD ad libitum. Daily caloric intake was not statistically different between experimental groups at any time point examined in the study (True et al., 2017); thus, the WSD is isocaloric. Subsets of females consuming each diet were randomly selected to receive testosterone (T; n = 20) or cholesterol (C; n = 20) implants as reported elsewhere (McGee et al., 2014). These treatments resulted in experimental groups designated chow + C (C), chow + T (T), C + WSD (WSD) and T + WSD (n = 10/ group). Androgen levels of T-treated animals were maintained 5-fold higher than control monkeys, which exceeds the 2-fold increase in T levels observed in post-pubertal women (Diamanti-Kandarakis and Panidis, 2007; Dumesic et al., 2016). However, the T values achieved in the present study are similar to those observed in obese PCOS women (Eagleson et al., 2003) (True et al., 2017).
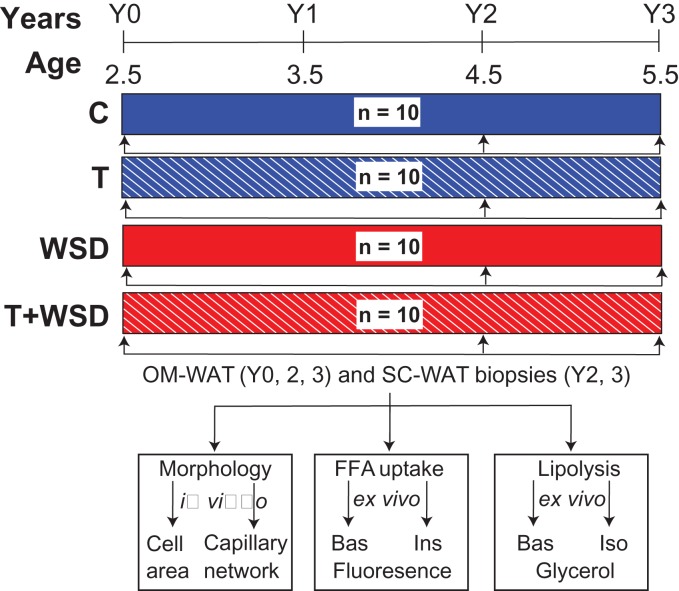
Figure 1.
Study design. Female rhesus macaques were started at 2.5 years of age and maintained for 3 years on a standard chow or Western-style diet (WSD). The study involved four experimental groups: C, control animals; T, animals with chronically elevated testosterone (T) levels; WSD and T + WSD; n = 10/group (see the ‘Results’ section for details). Visceral omental-white adipose tissue (OM-WAT) biopsies were collected in years 0, 2 and 3, and sub-cutaneous (SC)-WAT biopsies in years 2 and 3. WAT biopsies were subjected to morphological analysis for the assessment of adipocyte size (cell area) and capillary network properties (see ‘Materials and Methods’ for details), and also analyzed ex vivo to quantify basal (Bas) and insulin-stimulated (Ins) fatty acid (FFA) uptake and lipolysis (glycerol release) under basal (Bas) and isoproterenol (Iso)-stimulated conditions.
Wat biopsies
WAT biopsies were collected at the beginning of the study (year 0) and repeated annually (Fig. 1). In years 0 and 1, animals had small volumes of OM-WAT and had undeveloped SC-WAT, imposing some restrictions on a number of assays that could be conducted (see ‘Results’ for details). At the year 1 time point, animals exhibited no metabolic phenotype so WAT samples were not analyzed. WAT biopsies were collected by laparoscopy as previously described (Cameron et al., 2016). In brief, anesthetized animals were positioned in dorsal recumbency followed by sterile preparation and draping of the abdomen. A Verres needle was inserted via a 1-cm subumbilical skin incision, followed by insufflation to 15 mm Hg pressure with CO2 gas. The needle was removed and an 11-mm trocar/sheath and 10-mm telescope was inserted by puncture at the same site. A right paralumbar 5-mm accessory port was placed, through which a cutting biopsy grasper was inserted. Pinch-biopsy forceps were used to retrieve an OM-WAT biopsy from the falciform ligament. Grasping forceps were used to grab a small section of omentum, which was pulled through the side port and a 1 × 2 × 1 cm3 tissue block was removed via sharp and blunt dissection. Abdominal SC-WAT biopsies were retrieved from the site of the scope incision. The abdomen was rinsed with warm saline, and the laparoscopic instruments were removed. The incision was closed with interrupted 4-0 Monocryl in the rectus fascia and skin.
Histological staining
At biopsy, 200–500-mg fragments of SC-WAT and OM-WAT were fixed in zinc formalin (Fisher Scientific, Hampton, NH, USA) at 4°C for 48 h, transferred to 70% (v/v) ethanol, embedded in Paraplast wax (Leica, Wetzlar, Germany) using a Tissue-Tek VIP six automatic tissue processor and embedding center (Sakura Finetek USA, Inc., Torrance, CA, USA), and 5-μm sections were prepared using on a micron rotary microtome. Slides were stained with a Masson Trichrome Stain Kit (IMEB Inc., San Marcos, CA, USA) according to the manufacturer's instructions. Images were acquired using an Olympus BX61VS slide scanner (Olympus, Tokyo, Japan) equipped with a ×20 UPLANSAPO NA 0.75 dry objective.
Lipolysis assay
At biopsy, 100 to 500-mg fragments of SC-WAT and OM-WAT were placed in 10 ml of M199 media (General Electric Company, Boston, MA, USA) at room temperature and transported to the laboratory within 30 min. 50 ± 5-mg WAT explants (three basal and three isoproterenol-stimulated replicates) were placed into a 48-well plate containing 0.2 ml incubation medium (Hank's Salt [HBSS], 0.2% BSA [Sigma-Aldrich, St, Louis, MO, USA] and 5 mM glucose), and incubated at 37°C free-floating for 2 h with or without 10 μM isoproterenol (Sigma-Aldrich) in an atmosphere with 5% CO2 at 37°C. Glycerol release was determined using a glycerol detection kit (Sigma-Aldrich). Glycerol concentrations were calculated using glycerol standards (Sigma-Aldrich) and normalized to wet tissue weight.
Fluorescent fatty acid uptake and isolectin staining
At biopsy, 100–200 mg of WAT were collected in M199 media at room temperature and separated into smaller explants for two basal and two insulin-stimulated samples. WAT explants were incubated free-floating in a 48-well plate filled with 0.4 ml incubation medium (M199 medium, 0.1% (w/v) fatty acid-free BSA [Sigma-Aldrich], 20 mM HEPES [pH 7.4], supplemented with penicillin, streptomycin and fungizone, with or without 10 nM human insulin [Sigma-Aldrich]) for 2 h. Fluorescently labeled fatty acid BODIPY-500/510 C1, C12 (BODIPY-C12; Life Technologies, Waltham, MA, USA) was prepared in advance by diluting a 2.5 mM methanol stock solution in incubation medium to a final concentration of 10 μM and incubated for 15 min, protected from light, in a 37°C water bath. One hundred μl of diluted BODIPY-C12 and 2 μl of live-cell staining dye Calcein Red-Orange AM (Life Technologies) were added to each well containing WAT explants and incubated for 15 min at 37°C. Media was removed by aspiration and explants were rapidly washed three times with incubation medium at 37°C and incubated in 0.4 ml basal or insulin-containing incubation medium for additional 30 min. This incubation step allows the translocation of BODIPY-C12 fluorescence associated with micro-lipid droplets (mLDs) into the interior of central lipid droplets (cLDs) and the incorporation of BODIPY-C12 into cellular triglycerides (Chu et al., 2014; Varlamov et al., 2015). Medium was removed by aspiration, the WAT explants were fixed at room temperature with 4% paraformaldehyde (Sigma-Aldrich) in PBS for 20 min, washed four times with PBS, and stained, protected from light, for 1 h on a rocking platform with 20 μg/ml Isolectin GS-IB4, Alexa FluorTM 647 conjugate (Life Technologies). Explants were washed four times with PBS and immediately analyzed by confocal microscopy. Each WAT explant triple-labeled with BODIPY-C12, calcein and isolectin was placed into a 35-mm glass-bottom imaging culture dish (MatTek, Ashland, MA) containing 100–200 μl of glycerol and sandwiched with the upper coverslip, allowing a tight contact between the outer layer of WAT explant and the bottom coverslip. Confocal microscopy was performed using a Leica SP5 AOBS spectral confocal system as described (Chu et al., 2014) with the following modifications. Three-channel optical sections were collected in a sequential mode, at 2-μm intervals, using HC PL FLUOTAR 10.0 × 0.30 and ×20 PL APO NA 0.70 dry objectives.
Image analysis
Confocal optical sections were merged to generate the sum of z-projections using FIJI (https://imagej.net/Fiji/Downloads). Adipocyte area and single-cell BODIPY fluorescence (integrated density) were calculated semi-automatically using an open source Cell Magic Wand plugin for FIJI. Average adipocyte area in each animal was calculated as average values of 20–50 adipocytes per each depot. Blood vessel characteristics were assessed by isolectin channel using an open source software AngioTool as described (Zudaire et al., 2011). Total vessel length in a WAT volume of 1550 μm × 1550 μm × 100 μm was calculated using ×10 images (sum of z-projections). Junction densities were calculated as a number of junctions in μm2 of WAT.
Statistical analysis
OM-WAT adipocyte area and OM-WAT fatty acid uptake were analyzed using the Mixed Models function of SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). Factors interrogated included time, steroid, diet, steroid by diet and steroid by diet by time. OM and SC-WAT glycerol release in years 2 and 3 of treatment was analyzed using the Linear Models function of SAS for each year individually. Factors interrogated included isoproterenol, diet, steroid and diet by steroid. SC-WAT adipocyte area, OM- and SC-WAT vessel and capillary junction density at year 3 were also analyzed using the Linear Models function of SAS. Factors interrogated included diet, steroid and diet by steroid. When significant (P < 0.05) factors were identified, all post-hoc analyses were then performed between relevant treatment groups using the least squared means function of SAS.
Results
Adipocyte size and wat morphology
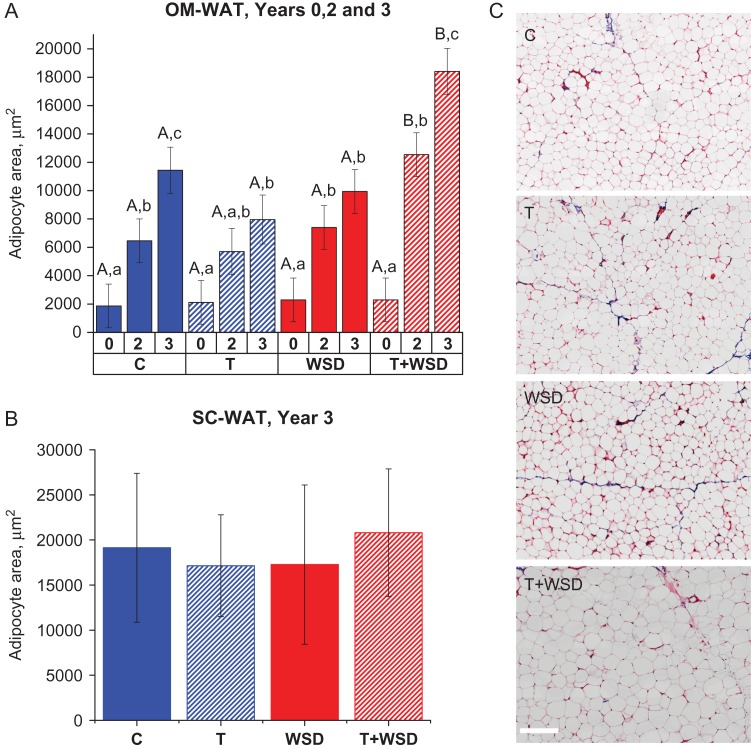
The four treatment groups started with equally sized OM adipocytes that displayed progressive cellular hypertrophy over the course of the study (Fig. 2A). The C and T + WSD groups exhibited statistically significant increases in adipocyte size in years 2 and 3, while in the T group the increase in adipocyte size was only statistically significant when year 3 was compared to year 0. In the WSD group the increase in adipocyte size was statistically significant in year 2 but not in year 3. In years 2 and 3, the T + WSD group had statistically significantly larger OM adipocytes compared to all other groups (Fig. 2A). The morphological analysis of OM-WAT collected in year 2 showed no obvious signs of WAT fibrosis, with the exception of blood vessel-associated collagen beds (Fig. 2C). There was no significant difference in SC adipocyte size in year 3 (Fig. 2B). In year 3, in every experimental group, SC adipocytes were larger than OM adipocytes (Fig. 2A and B), which is consistent with previous human studies (Tchernof et al., 2006; Michaud et al., 2014; Muir et al., 2016). Importantly, the relative difference in SC and OM adipocyte size was minimal in the T + WSD group compared to other groups (Fig. 2A and B).
Figure 2.
Adipocyte area and WAT morphology. Omental (OM) (A) and sub-cutaneous (SC) (B) adipocyte area was quantified in years 0, 2 and 3 for OM-white adipose tissue (WAT), and in year 3 for SC-WAT, as described in ‘Materials and Methods.’ Average cell area per animal was calculated for 20–50 adipocytes. (C) Representative examples of Trichrome-stained histological sections of OM-WAT (year 2). Pink color demarcates cellular membranes and cytoplasm, and blue color labels fibrotic structures (scale bar = 200 μm). (A) Uppercase letters denote differences (P < 0.05) by treatment within year, and lowercase letters indicate differences (P < 0.05) within a given treatment by year. (B) Analysis of SC-WAT area data was done for year 3 only with no difference detected by treatments (P > 0.8). Bars are means ± SEM.
Vessel density in WAT
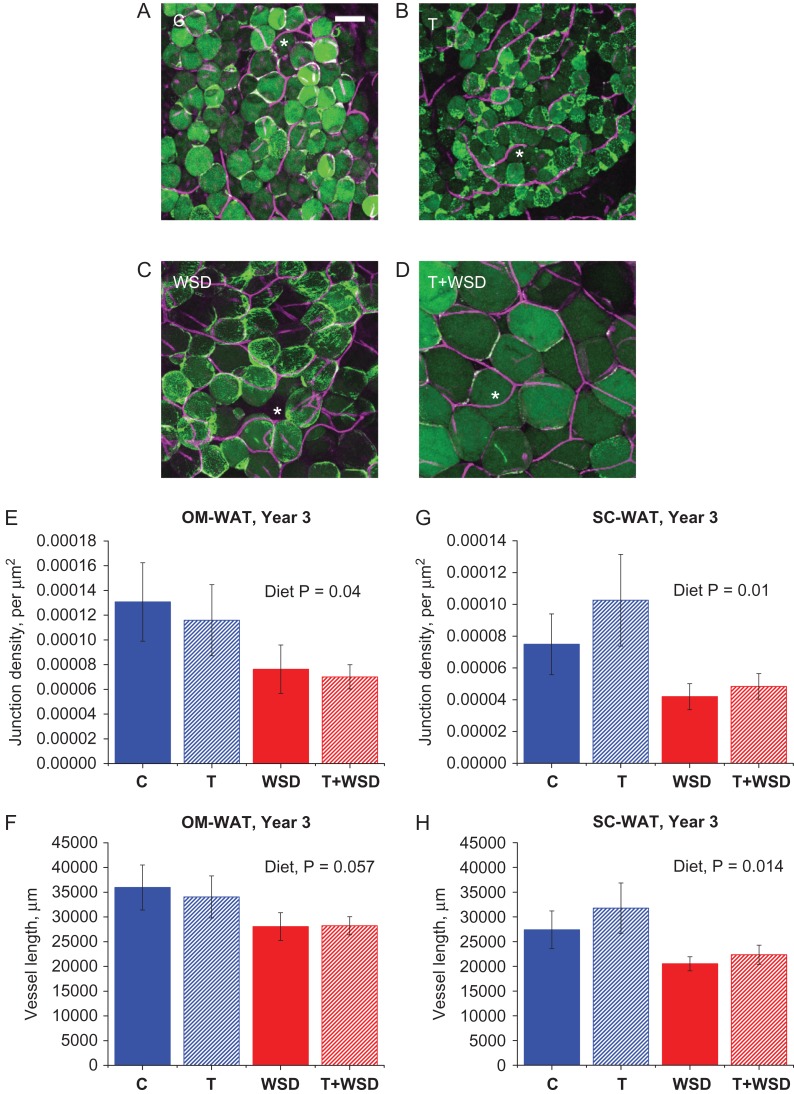
Double-staining of BODIPY-C12-labeled (see next section) WAT explants collected in year 3 with Isolectin GS-IB4 revealed an extensive capillary network surrounding individual OM (Fig. 3A–D) and SC (data not shown) adipocytes. Typically, blood vessels formed junctions demarcating the boundaries between adjacent adipocytes (Fig. 3A–D, asterisks). Quantification of capillary length and junctions revealed that the overall effect of diet (WSD and T + WSD) on vessel length and junction densities was significant or a strong trend for both OM-WAT and SC-WAT and the WSD groups displayed reduced vessel length and junctional densities (Fig. 3E–H). Although OM adipocytes in the T + WSD group were larger compared to the WSD group (Figs 2A and 3 C and D), their capillary network properties, including vessel junction densities and vessel length, were similar (Fig. 3E and F). Similarly, WSD reduced vessel length and junction densities in SC-WAT (Fig. 3G and H).
Figure 3.
White adipose tissue (WAT) capillary networks. Omental (OM)-WAT biopsies collected in year 3 were co-labeled with BODIPY-C12 and Isolectin GS-IB4, Alexa FluorTM 647 conjugate and analyzed by confocal microscopy as described in ‘Materials and Methods.’ (A–D) Representative images of OM-WAT; scale bar = 50 μm; asterisks, vessel junctions. Vessel junction density (E and G) and vessel length (F and H) were calculated for OM-WAT (E and F) and SC-WAT (G and H). Total vessel length in each WAT depot was calculated using ×10 images representing confocal slice with a 1550 × 1550 × 100-μm3 dimension. Junction density is the number of junctions populating 1 μm2 of a confocal projection. Overall effects of diet are indicated in inset above graphs.
Free fatty acid uptake in WAT
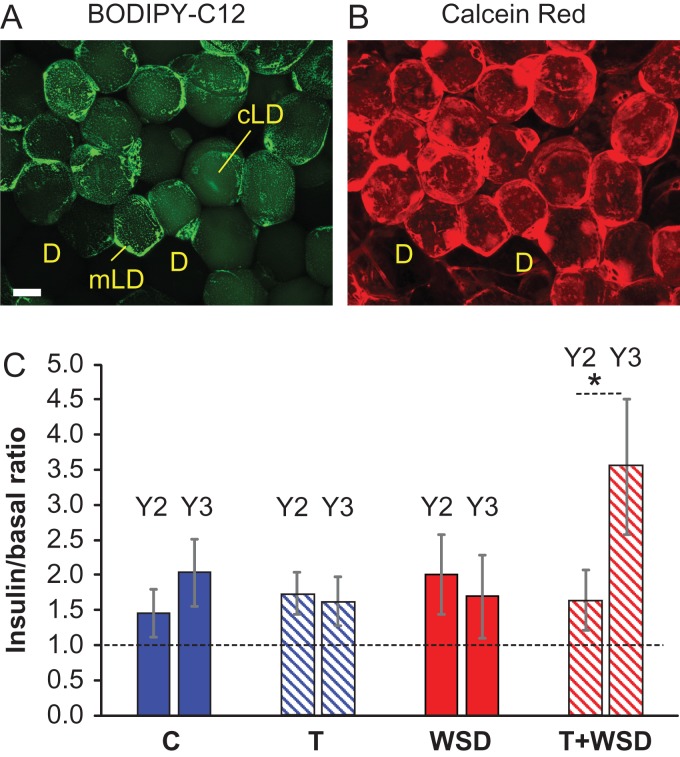
To analyze the uptake and incorporation of free fatty acids into cellular triglycerides, WAT explants were incubated ex vivo with basal or insulin-containing media and labeled with the green fluorescent fatty acid tracer BODIPY-C12, which is incorporated into cellular triglycerides (Chu, et al., 2014; Varlamov et al., 2015). Co-staining of BODIPY-C12-labeled OM-WAT explants with the live-cell marker Calcein Red-Orange AM revealed that only red, live adipocytes accumulated green fluorescent dye, while dead adipocytes (D) appeared dark (Fig. 4A and B). In live adipocytes, intracellular BODIPY fluorescence was distributed to clusters of small micro-lipid droplets (mLDs, Fig. 4A, punctate peripheral staining) and the central lipid droplet (cLD, Fig. 4A). An apparent intercellular heterogeneity in BODIPY-C12 uptake and mLDs (Figs 4A and 3A–D) were previously reported (Chu, et al., 2014; Varlamov et al., 2015). The quantification of basal and insulin-stimulated BODIPY-C12 fluorescence taken up by individual adipocytes showed no differences between treatment groups in year 2. However, only the T + WSD group demonstrated a statistically significant increase in the level of fatty acid uptake in year 3 compared to year 2 (Fig. 4C).
Figure 4.
Free fatty acid uptake in omental white adipose tissue (OM-WAT). OM-WAT explants collected in years 2 and 3 were treated with basal or insulin-containing media, labeled with BODIPY-C12 (A) and live cell marker Calcein Red-Orange AM (B), fixed and processed for microscopy analysis as described in ‘Materials and Methods.’ (A and B) Representative confocal images of insulin-treated OM-WAT explants (scale bar = 50 μm). BODIPY-C12 is distributed to micro-lipid droplets (mLDs) and the central lipid droplet (cLD). Green fluorescence is excluded from red-negative dead adipocytes (‘D’). (C) Quantification of insulin effects on BODIPY-C12 incorporation as a ratio of insulin-stimulated vs basal fluorescence. Bars are means ± SEM, n = 6–10 animals per group (average fluorescence per animal was calculated for 20–50 adipocytes). Asterisk, pair-wise comparison shows a statistically significant difference (P < 0.05) between years 2 and 3.
Lipolysis
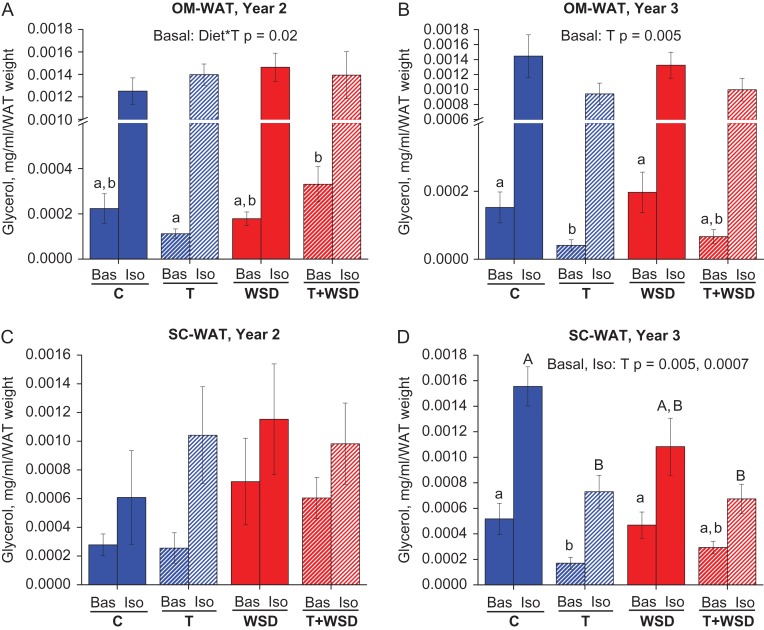
The lipolytic response of WAT to the β-adrenergic agonist isoproterenol was studied ex vivo using WAT biopsies collected in years 2 and 3. In year 2, there were no statistically significant differences between treatment groups in isoproterenol-stimulated glycerol release in OM-WAT and SC-WAT (Fig. 5A and C, ‘Iso’). In OM-WAT, there was a significant combined effect of diet and T on basal lipolysis, with the T + WSD group showing elevated basal glycerol release compared to the T group (Fig. 5A). In SC-WAT, due to a small number of biopsy samples collected, there were no group differences in basal lipolysis at year 2, although both WSD and T + WSD groups displayed a trend towards elevated basal lipolysis (Fig. 5C, ‘Bas’). By year 3, the patterns of lipolytic responses changed dramatically compared to year 2. In both WAT depots and under both dietary conditions, there was a significant inhibitory effect of T on basal glycerol release (Fig. 5B and D, ‘Bas’). Also, both T and T + WSD groups displayed significantly reduced isoproterenol-stimulated glycerol release in SC-WAT and a trend towards reduction in OM-WAT (Fig. 5D and B, ‘Iso’).
Figure 5.
White adipose tissue (WAT) lipolysis. Omental (OM)-WAT (A and B) and sub-cutaneous (SC)-WAT (C and D) biopsies were collected in year 2 (A and C) and year 3 (B and D), divided into 50-mg explants, and treated for 2 h with basal (Bas) or isoproterenol (Iso)-containing media, in duplicate or triplicate. Conditioned media was used to determine glycerol release as described in ‘Materials and Methods.’ Lower-case letters indicate differences (P < 0.05) between treatments at Bas, and uppercase letters indicate differences between treatments cultured with Iso (P < 0.05). Bars are means ± SEM, n = 6–10 animals per group.
Discussion
The present study was designed to discern the effects of WSD versus hyperandrogenemia on WAT function in young female NHPs, using a comprehensive approach of ex vivo and in vitro analyses of fat biopsies from two distinct fat depots. OM-WAT biopsies were collected longitudinally from the same anatomical sites, allowing the monitoring of the natural age-dependent progression of adipose hypertrophy, as well as the longitudinal assessment of various adipose-specific functions. The data from this study and a companion study (True et al., 2017) suggest that the combination hyperandrogenemia and WSD accelerates the development of obesity (increase in BMI) and visceral WAT hypertrophy (increase in OM adipocyte area), while there were no significant effects of either treatment on SC-WAT hypertrophy (Fig. 2). Metabolic and WAT-specific changes became pronounced after 3 years of treatment, revealing several T-specific, diet-specific and combined effects of treatment, in that: (i) T inhibited basal and isoproterenol-stimulated lipolysis under both control diet and WSD conditions; (ii) WSD significantly reduced local WAT blood vessel length and junction densities, in the presence and absence of hyperandrogenemia; and (iii) only the combination of T and WSD resulted in increased insulin-dependent fatty acid uptake ex vivo (Table I).
Table I.
Significant effects of hyperandrogenemia and Western-Style Diet (WSD) on white adipose tissue (WAT) morphology and function.
| WAT-related process | T-specific | WSD-specific | T + WSD combined |
|---|---|---|---|
| Reduced basal lipolysis | + | ||
| Reduced stimulated lipolysis | + | ||
| Reduced blood vessel density | + | ||
| Increased FFA uptake | + | ||
| Visceral adipocyte hypertrophy | + |
Omental (OM)-WAT and sub-cutaneous (SC)-WAT display significantly reduced basal lipolysis after 3 years of exposure to hyperandrogenemia, and these effects were apparent on both chow and WSD. SC-WAT developed β-adrenergic lipolytic resistance following 3 years of treatment, which was also a diet-independent effect. In year 3, both WAT depots demonstrated reduced vessel length and junction densities in response to WSD, which was independent of hyperandrogenemia. The combination of hyperandrogenemia and WSD results in increased free fatty acid (FFA) uptake in OM-WAT (in year 3) and visceral OM-WAT hypertrophy (increase in adipocyte area). The combinatorial effect of hyperandrogenemia and WSD is associated with the greatest WAT dysfunction, including impaired lipolytic response, reduced capillary development and increased FFA uptake. These factors contribute to visceral obesity associated with the polycystic ovary syndrome (PCOS) phenotype.
Adipocyte size
The present study indicates that hyperandrogenemia in the context of an obesogenic diet is associated with the development of enlarged visceral adipocytes. Although previous studies demonstrated that women with PCOS have increased visceral adiposity (Manneras-Holm et al., 2011; Gourgari et al., 2015; Dumesic et al., 2016), the effects of PCOS on the size of visceral adipocytes in humans is not yet reported. Consistent with our studies, rodent models of PCOS (McNeilly and Duncan, 2013) indicate that visceral adipocytes are also enlarged (Manneras et al., 2008; Caldwell et al., 2014; Kauffman et al., 2015; Nohara et al., 2013; Nikolic et al., 2015). Studies in sheep (Padmanabhan and Veiga-Lopez, 2013) similarly reported enlarged adipocytes in intra-abdominal depots of prenatally androgenized female offspring (Veiga-Lopez et al., 2013). Previous primate studies (Abbott et al., 2013) did not observe significant effects of androgen excess on adipocyte size. However, this is potentially due the shorter duration of T + WSD treatment in one study (Varlamov et al., 2013; McGee et al., 2014) and a separate study employed a nonobesogenic, low-fat monkey chow diet in combination with prenatal T excess (Keller et al., 2014).
We did not detect significant differences between treatment groups in the size of SC adipocytes. Although several studies indicate that PCOS is associated with enlarged SC adipocytes (Ek et al., 1997; Faulds et al., 2003; Manneras-Holm et al., 2011), a recent human study showed no significant effect of PCOS on the average size of SC adipocytes (Dumesic et al., 2016). However, the latter study reported the presence of a subpopulation of smaller SC adipocytes without a change in mean adipocyte size, which correlated with a degree of IR in lean PCOS patients (Dumesic et al., 2016). Other studies also demonstrated that IR in normal obese (McLaughlin et al., 2014) and PCOS obese (Keller et al., 2014) patients is associated with accumulation of small SC adipocytes. Consequently, subtle changes in SC adipocyte fat storage due to androgen-mediated inhibition of preadipocyte differentiation with an increased proportion of small SC adipocytes (Chazenbalk et al., 2012; Dumesic et al., 2016; Keller et al., 2014 ) could theoretically favor redistribution of free fatty acids to the visceral compartment, linking SC abdominal fat functional abnormalities with increased visceral adipocyte size.
WAT capillary system
WSD induced a significant reduction in capillary density in both WAT depots. The reduction in blood vessel length and junction densities in the WSD and T + WSD groups suggests that an obesogenic diet may impair WAT vascular development and capillary network expansion. Inadequate expansion of WAT capillary networks during diet-induced obesity is the principal factor responsible for the development of local hypoxia, inflammation and a major contributor to IR (Hosogai et al., 2007; Pasarica et al., 2009; Hodson et al., 2013; Rausch et al., 2008 ). The levels of the proinflammatory biomarker C-reactive protein (Pou et al., 2007; Neeland et al., 2013) were significantly elevated in both WSD groups (True et al., 2017). This increase in C-reactive protein was correlated with reduced WAT capillary densities, which is thought to contribute to systemic inflammation and hypoxia (Pasarica et al., 2009). Increased angiogenesis can protect against WAT hypoxia and metabolic complications in obese mice (Michailidou et al., 2012; Robciuc et al., 2016). There are no recent reports directly addressing vascular properties of WAT in PCOS patients and in related animal models; however, there are in vitro studies suggesting an increase in systemic angiogenesis in PCOS women (Tan et al., 2010). Alterations in ovarian angiogenesis and vascularization are hypothesized to contribute to ovarian dysfunction in PCOS, including the manifestation of the PCO phenotype (Di Pietro et al., 2015). Future studies employing functional in vivo and in vitro-based assays may distinguish whether endothelial and vascular properties of WAT are differentially affected by hyperandrogenemia and WSD, and if these effects are depot-specific.
Free fatty acid uptake in WAT
The present report suggests that hyperandrogenemia is associated with increased fatty acid uptake in OM-WAT, confirming a previous pilot study that female NHPs exposed to chronic hyperandrogenemia and acute WSD challenge display increased insulin-stimulated fatty acid uptake and increased insulin signaling in visceral WAT (Varlamov et al., 2013). Our findings are also consistent with published data showing increased expression of the fatty acid transporter CD36 in OM-WAT of PCOS women (Seow et al., 2009). Thus, increased fatty acid influx may contribute to the increased visceral obesity observed in PCOS women consuming obesogenic diets. It is also possible that increased fatty acid uptake is secondary to or concomitant with visceral adipocyte hypertrophy, which may develop as a result of suppressed lipolysis in the background of lipid-rich obesogenic diets.
WAT lipolysis
Hyperandrogenemia had an inhibitory effect on basal and isoproterenol-stimulated lipolysis in both fat depots under both dietary conditions. The suppression of fat cell lipolysis by androgen excess may represent the principal pathophysiological mechanism contributing to increased obesity in PCOS patients. Earlier studies employing SC-WAT biopsies obtained from women with PCOS and BMI-matched controls demonstrated that the former developed catecholamine-induced lipolytic resistance and had reduced β2-adrenergic receptor levels (Ek et al., 1997). Other studies also reported a reduction in β2-adrenergic receptors expressed in SC adipocytes of PCOS patients and revealed additional defects such as a reduced capacity of endogenous protein kinase A to activate HSL (Faulds et al., 2003), the principal lipase responsible for the β-adrenergic lipolytic response in WAT (Ryden et al., 2007).
The data on visceral WAT lipolysis are more controversial. Ek et al. (2002) reported that OM-WAT obtained from PCOS patients had increased catecholamine-induced lipolysis, although our and other groups reported reduced HSL levels in visceral WAT in T-treated female NHPs (Varlamov et al., 2013), as well as in WAT of PCOS patients (Seow et al., 2009), respectively. Interestingly, OM-WAT from the T + WSD group obtained in year 2 displayed elevated basal lipolysis, while during the transition to year 3 this effect was reversed. These differences may be related to the duration of treatment or age-dependent remodeling of the local sympathetic innervation and ultimately the β-adrenergic lipolytic response in WAT might be different in younger versus older female primates (Zeng et al., 2015; Zhu et al., 2016).
One possible mechanism driving the development of catecholamine resistance in WAT is that elevated androgens induce the dysregulation of sympathetic tone in WAT in PCOS patients. Developmental androgen excess is associated with increased sympathetic tone in SC and visceral WAT in mice (Nohara et al., 2013). Alterations in WAT sympathetic tone may represent a secondary effect of obesity and require further investigations, including defining the distribution of the local sympathetic innervation and the association with blood vessels, combined with functional assessment of sympathetic function in WAT.
Conclusions and future directions
In the United States, 80% of women with PCOS suffer from obesity and related metabolic complications (Legro et al., 1999; Dumesic et al., 2015), while the mechanisms contributing to excess body fat in these patients remain poorly understood. The present NHP study sheds light on the pathogenesis of obesity and adipose dysfunction observed in women with PCOS. The combined effects of hyperandrogenemia and consumption of WSD on WAT identified in the present study include impaired basal and β-adrenergic-stimulated lipolysis, increased insulin-stimulated fatty acid uptake and reduced blood vessel density (Table I), which collectively may contribute to the increased WAT hypertrophy and obesity observed in PCOS women.
Future studies will address the depot-specific biochemical, transcriptional and epigenetic effects of hyperandrogenemia and WSD. Specifically, we will elucidate the mechanisms underlying visceral fat hypertrophy and the potential development of adipocyte hyperplasia in response to hypoandrogenemia and WSD. We will also evaluate the effects of these treatments on the epigenetic profiles and differentiation and functional properties of adipose mesenchymal stem cells in this cohort of monkeys.
Acknowledgments
We thank the NCTRI Nonhuman Primate core, directed by Dr. Ov Slayden, the ONPRC Imaging and Morphology Support Core for technical assistance, Division of Comparative Medicine's Surgical Services Unit for performing all surgical procedures described in this manuscript and post-surgical monitoring of macaques, and Dr. Richard Stouffer and Dr. Daniel Dumesic for critical reading of the article.
Authors’ roles
O.V. designed the study, conducted experiments and wrote the article, C.V.B. and S.S. analyzed the data, M.H., D.T. and A.W. conducted experiments, C.T.R. designed the study and wrote the article.
Funding
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number P50 HD071836 to CTR and award number OD 011092 from the Office of the Director, National Institutes of Health, for operation of the Oregon National Primate Research Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Abbott DH, Nicol LE, Levine JE, Xu N, Goodarzi MO, Dumesic DA. Nonhuman primate models of polycystic ovary syndrome. Mol Cell Endocrinol 2013;373:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 2009;91:456–488. [DOI] [PubMed] [Google Scholar]
- Barber TM, Franks S. Adipocyte biology in polycystic ovary syndrome. Mol Cell Endocrinol 2013;373:68–76. [DOI] [PubMed] [Google Scholar]
- Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids 2012;77:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell AS, Middleton LJ, Jimenez M, Desai R, McMahon AC, Allan CM, Handelsman DJ, Walters KA. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014;155:3146–3159. [DOI] [PubMed] [Google Scholar]
- Cameron JL, Jain R, Rais M, White AE, Beer TM, Kievit P, Winters-Stone K, Messaoudi I, Varlamov O. Perpetuating effects of androgen deficiency on insulin resistance. Int J Obes (Lond) 2016;40:1856–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazenbalk G, Chen YH, Heneidi S, Lee JM, Pall M, Chen YD, Azziz R. Abnormal expression of genes involved in inflammation, lipid metabolism, and Wnt signaling in the adipose tissue of polycystic ovary syndrome. J Clin Endocrinol Metab 2012;97:E765–E770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, Chen BS, Chazenbalk G, Azziz R. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 2013;62:2278–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M, Sampath H, Cahana DY, Kahl CA, Somwar R, Cornea A, Roberts CT Jr, arlamov O. Spatiotemporal dynamics of triglyceride storage in unilocular adipocytes. Mol Biol Cell 2014;25:4096–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi TP, Aroda V, Mudaliar S, Chang RJ, Henry RR. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab 2009;94:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi TP, el-Roeiy A, Madar Z, Reichart D, Olefsky JM, Yen SS. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab 1992;75:577–583. [DOI] [PubMed] [Google Scholar]
- Di Pietro M, Parborell F, Irusta G, Pascuali N, Bas D, Bianchi MS, Tesone M, Abramovich D. Metformin regulates ovarian angiogenesis and follicular development in a female polycystic ovary syndrome rat model. Endocrinology 2015;156:1453–1463. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012;33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin Endocrinol (Oxf) 2007;67:735–742. [DOI] [PubMed] [Google Scholar]
- Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab 1998;83:4420–4425. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA et al. Hyperandrogenism accompanies increased intra-abdominal fat storage in normal weight polycystic ovary syndrome women. J Clin Endocrinol Metab 2016;101:4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev 2015;36:487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 1992;41:1257–1266. [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Bellows AB, Hu K, Gingrich MB, Marshall JC. Obese patients with polycystic ovary syndrome: evidence that metformin does not restore sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by ovarian steroids. J Clin Endocrinol Metab 2003;88:5158–5162. [DOI] [PubMed] [Google Scholar]
- Ek I, Arner P, Bergqvist A, Carlstrom K, Wahrenberg H. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance. J Clin Endocrinol Metab 1997;82:1147–1153. [DOI] [PubMed] [Google Scholar]
- Ek I, Arner P, Ryden M, Holm C, Thorne A, Hoffstedt J, Wahrenberg H. A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes 2002;51:484–492. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, San Millan JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab 2007;18:266–272. [DOI] [PubMed] [Google Scholar]
- Faulds G, Ryden M, Ek I, Wahrenberg H, Arner P. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab 2003;88:2269–2273. [DOI] [PubMed] [Google Scholar]
- Gambineri A, Pelusi C, Genghini S, Morselli-Labate AM, Cacciari M, Pagotto U, Pasquali R. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60:241–249. [DOI] [PubMed] [Google Scholar]
- Gourgari E, Lodish M, Shamburek R, Keil M, Wesley R, Walter M, Sampson M, Bernstein S, Khurana D, Lyssikatos C et al. Lipoprotein particles in adolescents and young women with PCOS provide insights into their cardiovascular risk. J Clin Endocrinol Metab 2015;100:4291–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 2010;151:1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes 2013;62:1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 1995;80:2586–2593. [DOI] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56:901–911. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Thackray VG, Ryan GE, Tolson KP, Glidewell-Kenney CA, Semaan SJ, Poling MC, Iwata N, Breen KM, Duleba AJ et al. A novel letrozole model recapitulates both the reproductive and metabolic phenotypes of polycystic ovary syndrome in female mice. Biol Reprod 2015;93:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E, Chazenbalk GD, Aguilera P, Madrigal V, Grogan T, Elashoff D, Dumesic DA, Abbott DH. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology 2014;155:2696–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 1999;84:165–169. [DOI] [PubMed] [Google Scholar]
- Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev 2013;14:95–109. [DOI] [PubMed] [Google Scholar]
- Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, Holm G, Hellstrom M, Lonn L, Olivecrona G, Stener-Victorin E et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 2011;96:E304–E311. [DOI] [PubMed] [Google Scholar]
- Manneras L, Jonsdottir IH, Holmang A, Lonn M, Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology 2008;149:3559–3568. [DOI] [PubMed] [Google Scholar]
- Marsden PJ, Murdoch A, Taylor R. Severe impairment of insulin action in adipocytes from amenorrheic subjects with polycystic ovary syndrome. Metabolism 1994;43:1536–1542. [DOI] [PubMed] [Google Scholar]
- McGee WK, Bishop CV, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. Am J Physiol Endocrinol Metab 2014;306:E1292–E1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, Cushman SW. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity (Silver Spring) 2014;22:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AS, Duncan WC. Rodent models of polycystic ovary syndrome. Mol Cell Endocrinol 2013;373:2–7. [DOI] [PubMed] [Google Scholar]
- Mehrabani HH, Salehpour S, Amiri Z, Farahani SJ, Meyer BJ, Tahbaz F. Beneficial effects of a high-protein, low-glycemic-load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr 2012;31:117–125. [DOI] [PubMed] [Google Scholar]
- Michailidou Z, Turban S, Miller E, Zou X, Schrader J, Ratcliffe PJ, Hadoke PW, Walker BR, Iredale JP, Morton NM et al. Increased angiogenesis protects against adipose hypoxia and fibrosis in metabolic disease-resistant 11beta-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. J Biol Chem 2012;287:4188–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud A, Boulet MM, Veilleux A, Noel S, Paris G, Tchernof A. Abdominal subcutaneous and omental adipocyte morphology and its relation to gene expression, lipolysis and adipocytokine levels in women. Metabolism 2014;63:372–381. [DOI] [PubMed] [Google Scholar]
- Moro C, Pasarica M, Elkind-Hirsch K, Redman LM. Aerobic exercise training improves atrial natriuretic peptide and catecholamine-mediated lipolysis in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009;94:2579–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 2016;24:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 2013;21:E439–E447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M, Macut D, Djordjevic A, Velickovic N, Nestorovic N, Bursac B, Antic IB, Macut JB, Matic G, Vojnovic Milutinovic D. Possible involvement of glucocorticoids in 5alpha-dihydrotestosterone-induced PCOS-like metabolic disturbances in the rat visceral adipose tissue. Mol Cell Endocrinol 2015;399:22–31. [DOI] [PubMed] [Google Scholar]
- Nohara K, Waraich RS, Liu S, Ferron M, Waget A, Meyers MS, Karsenty G, Burcelin R, Mauvais-Jarvis F. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am J Physiol Endocrinol Metab 2013;304:E1321–E1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol 2013;373:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009;58:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Antenucci D, Casimirri F, Venturoli S, Paradisi R, Fabbri R, Balestra V, Melchionda N, Barbara L. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J Clin Endocrinol Metab 1989;68:173–179. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 1994;79:265–271. [DOI] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF Jr., Meigs JB, Lipinska I, Kathiresan S et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 2007;116:1234–1241. [DOI] [PubMed] [Google Scholar]
- Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6 J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. [DOI] [PubMed] [Google Scholar]
- Robciuc MR, Kivela R, Williams IM, de Boer JF, van Dijk TH, Elamaa H, Tigistu-Sahle F, Molotkov D, Leppanen VM, Kakela R et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab 2016;23:712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev 2016;37:467–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden M, Jocken J, van Harmelen V, Dicker A, Hoffstedt J, Wiren M, Blomqvist L, Mairal A, Langin D, Blaak E et al. Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. Am J Physiol Endocrinol Metab 2007;292:E1847–E1855. [DOI] [PubMed] [Google Scholar]
- Seow KM, Tsai YL, Hwang JL, Hsu WY, Ho LT, Juan CC. Omental adipose tissue overexpression of fatty acid transporter CD36 and decreased expression of hormone-sensitive lipase in insulin-resistant women with polycystic ovary syndrome. Hum Reprod 2009;24:1982–1988. [DOI] [PubMed] [Google Scholar]
- Solomon CG. The epidemiology of polycystic ovary syndrome. Prevalence and associated disease risks. Endocrinol Metab Clin North Am 1999;28:247–263. [DOI] [PubMed] [Google Scholar]
- Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 2015;149:R219–R227. [DOI] [PubMed] [Google Scholar]
- Tan BK, Adya R, Farhatullah S, Chen J, Lehnert H, Randeva HS. Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes 2010;59:3023–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernof A, Belanger C, Morisset AS, Richard C, Mailloux J, Laberge P, Dupont P. Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes 2006;55:1353–1360. [DOI] [PubMed] [Google Scholar]
- Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:3373–3380. [DOI] [PubMed] [Google Scholar]
- True CA, Takahashi DL, Burns SE, Mishler EC, Bond KR, Wilcox MC, Calhoun AR, Bader LA, Dean TA et al. Chronic combined hyperandrogenemia and western-style diet in young female rhesus macaques causes greater metabolic impairments compared to either treatment alone. Hum Reprod 2017;32:1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlamov O, Chu M, Cornea A, Sampath H, Roberts CT Jr. Cell-autonomous heterogeneity of nutrient uptake in white adipose tissue of rhesus macaques. Endocrinology 2015;156:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlamov O, Chu MP, McGee WK, Cameron JL, O'Rourke RW, Meyer KA, Bishop CV, Stouffer RL, Roberts CT Jr. Ovarian cycle-specific regulation of adipose tissue lipid storage by testosterone in female nonhuman primates. Endocrinology 2013;154:4126–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Moeller J, Patel D, Ye W, Pease A, Kinns J, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology 2013;154:1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 2003;52:2490–2496. [DOI] [PubMed] [Google Scholar]
- Wu HL, Heneidi S, Chuang TY, Diamond MP, Layman LC, Azziz R, Chen YH. The expression of the miR-25/93/106b family of micro-RNAs in the adipose tissue of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2014;99:E2754–E2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu YH, Kozlova A, Voss H, Martins GG et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 2015;163:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Gao Y, Tao C, Shao M, Zhao S, Huang W, Yao T, Johnson JA, Liu T, Cypess AM et al. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab 2016;24:420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One 2011;6:e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]