Abstract
Genetic disorders due to mitochondrial dysfunction are not uncommon and the majority of these patients will have eye-related manifestations, including visual loss from the optic nerve and retinal disease, visual field loss from retrochiasmal visual pathway damage, and ptosis and ocular dysmotility from extraocular muscle involvement. Defects in both the nuclear and mitochondrial genomes cause mitochondrial dysfunction via several mechanisms, including impaired mitochondrial energy production, oxidative stress, mitochondrial DNA instability, abnormalities in the regulation of mitochondrial dynamics and mitochondrial quality control, and disturbed cellular interorganellar communication. Advances in our understanding of the molecular genetic basis of mitochondrial disease have not only improved genetic diagnosis, but they have provided important insights into the pathophysiologic basis of these disorders and potential therapeutic targets. In parallel, more sophisticated techniques for genetic manipulation are facilitating the development of animal and in vitro models that should prove powerful and versatile tools for disease modelling and therapeutic experimentation. Effective therapies for mitochondrial disorders are beginning to translate from bench to bedside along the paths of neuroprotection, gene replacement and stem cell-based regenerative paradigms. Additionally, preventing the transmission of pathogenic mtDNA mutations from mother to child is now a reality with in vitro fertilization mitochondrial replacement techniques.
Introduction
Genetic disorders whose primary pathogenesis occurs via disruption of mitochondrial structure or function may individually represent relatively uncommonly encountered conditions, but collectively are ubiquitous. A recent comprehensive assessment of the frequency of all forms of adult mitochondrial disease in the North East of England found a minimum prevalence of approximately 1 in 4,300, making these disorders among the most common adult forms of inherited neurological disease (1). Since both nuclear and mitochondrial DNA (mtDNA) encode proteins essential for mitochondrial function, mitochondrial disorders are theoretically and in practice the result of abnormalities of either genome (2,3). Disease inheritance will respect the rules of Mendelian genetics if the disrupted genes are chromosomal, and maternal inheritance if the mutations occur in mtDNA. Additional genetic disorders may not cause primary mitochondrial dysfunction, but the cascade of pathophysiologic events may ultimately compromise mitochondrial function and produce indistinguishable phenotypic expression (4). Clinical manifestations of these disorders typically reflect involvement of those tissues most reliant on mitochondrial function, especially the central nervous system and its sensory organs such as the eyes and ears, muscles, endocrine glands, and the cardiac conduction system. Therefore, ‘eye-related’ disorders due to mitochondrial dysfunction are common.
Neuro-Ophthalmologic Manifestations of Mitochondrial Disease
Many mitochondrial disorders have clinical symptoms and signs referable to the afferent and efferent pathways for vision and eye movements. The most frequently encountered manifestations include optic neuropathy, pigmentary retinopathy, retrochiasmal visual loss, and chronic progressive ptosis and external ophthalmoplegia (5). The occurrence of any of these findings in isolation, in combination, and, especially, in association with other signs suggestive of mitochondrial dysfunction, such as sensorineural hearing loss, muscle weakness, diabetes mellitus and cardiac conduction defects, should raise suspicion for a primary or secondary mitochondrial disorder.
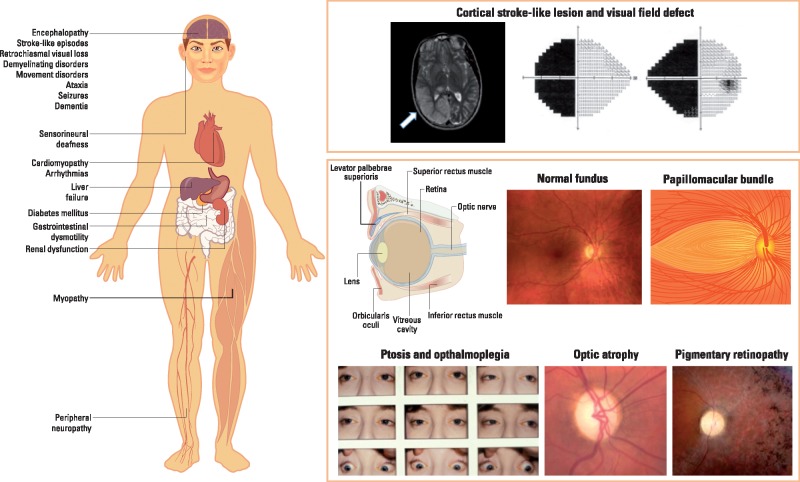
The optic neuropathy of mitochondrial dysfunction can be insidious and symmetric, as seen in patients with autosomal dominant optic atrophy (DOA), or subacute and sequential, as in Leber hereditary optic neuropathy (LHON) (6). The severity of visual compromise varies, but essentially all of these patients ultimately have bilateral loss of visual acuity. The retina is a multilayered structure and the axons of about 1.2 million retinal ganglion cells (RGCs) within the inner retina converge to form the optic nerve (Fig. 1). Intriguingly, there is a marked predilection for damage to the papillomacular bundle, which contains the highest density of RGCs, resulting in central vision loss and temporal pallor of the optic nerve (a reflection of optic atrophy) (7).
Figure 1.
Neuro-ophthalmologic and multisystemic manifestations of mitochondrial disease.
Involvement of the outer retina (retinal pigment epithelium and photoreceptor layers) is a more variable feature of mitochondrial disorders, ranging from asymptomatic peripheral ‘salt-and-pepper’ changes to frank ‘bone-spiculing’ of the peripheral and macular regions with severe visual field constriction and central visual acuity loss, respectively (8,9). The pattern and type of pigmentary changes are not specific to the particular genetic defect. Progression, albeit slow, is often noted in an individual patient, which can have a disabling impact when the neurodegenerative process starts to impinge on the perifoveal and foveal regions. Classical mitochondrial syndromes with prominent outer retinal involvement include: (i) neuropathy, ataxia and retinitis pigmentosa (NARP) secondary to the m.8993T > G mtDNA mutation in MT-ATP6 (10); (ii) mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) secondary to the m.3243A > G mtDNA mutation in MT-TL1, which encodes for the mitochondrial transfer RNA (tRNA) for leucine (9,11); and (iii) chronic progressive external ophthalmoplegia (CPEO), in particular the Kearns-Sayre syndrome (KSS) secondary to single large-scale mtDNA deletions (12,13). The latter is a particular severe phenotypic manifestation with disease onset before the age of 20 years old, frequently occurring in association with cardiac conduction defects leading to heart block.
The episodes of cerebral dysfunction lasting hours, weeks or months, accompanied by migraine-like prodromes, acute confusional states and focal seizures, that are characteristic of MELAS have a predilection for the occipital and parietal lobes, thereby resulting in homonymous hemianopic visual field defects (Fig. 1) (14). Lactic acidosis and a progressive dementing encephalopathy complete the classic presentation, with many other features contributing to varied phenoptypic expression, including pigmentary retinopathy (especially of the macula), sensorineural hearing loss, muscle weakness, peripheral neuropathy, cardiomyopathy, diabetes mellitus and growth failure (15).
Ptosis of the upper lids combined with a mostly generalized limitation of eye movements and facial muscle weakness are seen in over 40% of patients with genetically confirmed mitochondrial disease (PYWM, unpublished data). Ptosis typically precedes abnormalities of eye movements, and although the ophthalmoplegia is usually symmetrical, about a third of patients report significant diplopia, which can be managed in most cases with appropriately fitted prisms, usually for reading (16). The CPEO syndromes, which include KSS and mitochondrial neurogastrointestinal encephalomyopathy (MNGIE), the latter caused by recessive mutations in the thymidine phosphorylase TYMP gene (22q13.33) (17), can present with a wide variety of associated ophthalmologic, neurologic and systemic manifestations, including pigmentary retinopathy, optic neuropathy, cataracts, corneal opacities, myopathy, hearing loss, peripheral neuropathy, ataxia, encephalopathy, basal gangliar abnormalities, gastrointestinal dysmotility, cardiac conduction defects, short stature, diabetes mellitus, and other hormonal and metabolic imbalances (5,18).
Mitochondria in Health and Disease
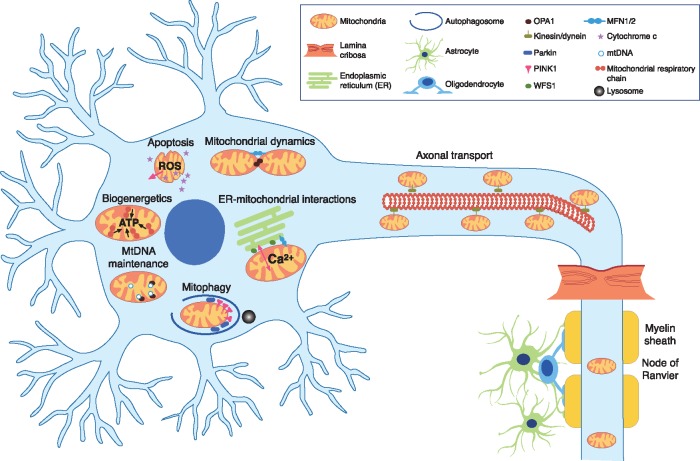
Mitochondria are ubiquitous organelles that serve multiple roles contributing to the cell’s survival under both physiological and pathological conditions. Although it is tempting to compartmentalise the development of specific mitochondrial phenotypes into specific mechanisms, the reality is that our knowledge is still imperfect and a critical review of the published literature reveals diverging opinions and inconsistencies that suggest a complex picture in which a genetic defect or the lack of a protein eventually contribute to cell death and tissue dysfunction through interrelated pathways (Fig. 2) (4,19).
Figure 2.
Mitochondrial disease mechanisms implicated in inherited eye-related disorders.
Central visual loss is a devastating complication of mitochondrial disease and much effort has been invested in understanding the processes that trigger RGC loss, especially because this neuronal cell type provides several attractive paradigms with relevance to other visual and neuromuscular manifestations of mitochondrial disease, and the pursuit of targeted therapeutics. Three of the most important causes of mitochondrial blindness, namely, DOA, linked to mutations in the nuclear genes OPA1 (3q28-q29) and MFN2 (1p36.2); Wolfram syndrome, secondary to WFS1 (4p16.1) and CISD2 (4q24) mutations; and LHON, most commonly caused by one of three pathogenic mtDNA mutations (m.3460G > A in MT-ND1, m.11778G > A in MT-ND4, and m.14484T > C in MT-ND6), which all encode for complex I subunits of the mitochondrial respiratory chain, illustrate the advances and challenges in this area of active research (20). Despite the genetic heterogeneity spanning both the mitochondrial and nuclear genomes, the neuropathological basis of mitochondrial optic neuropathies is strikingly similar to their preferential vulnerability of RGCs above other neuronal populations and a characteristic wave of RGC loss that starts within the papillomacular bundle (accounting for the central or caecocentral scotoma) before spreading to other retinal locations. What differs among them are the patient demographics and the course of visual loss, with LHON characteristically having an abrupt onset and a relatively rapid evolution among young adult men, whereas the visual loss in DOA and Wolfram syndrome usually starts insidiously in early childhood with a slower rate of progression and with no gender bias (5,20). Investigations are focusing on what contributes to RGC loss in these mitochondrial optic neuropathies and what are the overlapping disease mechanisms.
Mitochondrial Respiratory Chain Dysfunction
Mitochondria exist to produce energy in the form of ATP and this evolutionary adaption is both a necessity and a potential source of danger when the process is inhibited by whatever means. The mitochondrial respiratory chain consists of five complexes located along the inner mitochondrial membrane. In a finely choreographed series of biomechanical events, high-energy electrons are transferred serially to drive the transfer of protons from the matrix compartment into the intermembrane space. This proton motive force is then used by Complex V (ATP synthase) to synthesize ATP from ADP, resulting in a virtuous circle that can adapt to a cell’s demands. It is inevitable that the three primary LHON mutations will disrupt Complex I activity, but lack of the OPA1 protein, which is a mitochondrial inner membrane protein, also has a disruptive effect on the assembly of Complex I and its stability within mitochondrial supercomplexes (21).
Complexes I and III are major sources of superoxide () production and these potent molecules can damage proteins, lipids and DNA if left unchecked (22,23). To counter this, superoxide is converted to hydrogen peroxide by superoxide dismutase (Mn-SOD in the mitochondrial matrix and Cu/Zn-SOD in the cytosol), followed by breakdown of hydrogen peroxide to water and oxygen by catalase or glutathione peroxidase. Besides a reduction in ATP synthesis, the impaired flow of high-energy electrons along the mitochondrial respiratory chain has another dangerous consequence for the cell if the resulting increase in superoxide production overwhelms the cell’s constitutive antioxidant defences. There is often a circular debate about the contribution of impaired mitochondrial energy production and oxidative stress, and the relative primacy of these two factors, in driving a cell’s fate towards apoptosis. A pragmatic approach when it comes to therapeutic directions is that both are intrinsically related and any remedial process that improves mitochondrial electron flux should result in both an increased efficiency of ATP production and a decrease in free radical associated damage (24).
Mitochondrial DNA Instability
The mitochondrial genome is a relatively small, 16-kilobase-pair, circular molecule, but it is a very high copy number genome, with up to 100,000 mtDNA copies per cell depending on the cell’s overall metabolic requirements (2,3). Unlike nuclear DNA, mtDNA is constantly replicating even in resting post-mitotic cells and the cell’s copy number remains stable as mtDNA replication is closely matched to the rate of degradation. MtDNA maintenance disorders arise when this balance is disturbed and the two major subgroups are defined by either mtDNA depletion (due to a reduction in mtDNA copy number) or the accumulation of multiple mtDNA deletions (i.e. mtDNA molecules of variable size that lack the full mitochondrial gene complement) (25,26). Next-generation exome and whole-genome sequencing is contributing to a rapidly expanding list of nuclear genes that cause mtDNA instability and CPEO is a prominent clinical manifestation. For example, recessive TYMP mutations in MNGIE cause severe mtDNA depletion, whereas mutations in POLG and PEO1 are important causes of CPEO with cytochrome c oxidase (COX) deficient and ragged red fibres (RRFs) in muscle histopathological specimens, with the individual fibres harbouring high levels of somatic mtDNA deletions (17,27).
Until recently, OPA1-related DOA was widely regarded as an isolated optic neuropathy that starts in childhood with a relatively slow rate of visual deterioration (28,29). More detailed phenotypic characterisation and the greater availability of OPA1 genetic testing has expanded the clinical spectrum associated with OPA1 mutations to encompass additional varying combinations of sensorineural hearing loss, CPEO, myopathy, ataxia and peripheral neuropathy (30–32). Interestingly, muscle biopsies from patients with these so-called DOA ‘plus’ syndromes show evidence of multiple DNA deletions (33,34). Hence, OPA1 is emerging as a key regulator of mtDNA replication, possibly helping to anchor mtDNA nucleoids, the putative replicative units located within the matrix compartment, to the inner mitochondrial inner membrane (35). Due to the lack of human optic nerve specimens for analysis, it is still not clear whether the accumulation of mtDNA deletions represents an epiphenomenon or whether these also primarily contribute to RGC loss in DOA.
Mitochondrial Dynamics
Far from being static organelles, mitochondria are in a constant state of fusion and fission, producing a long interconnected network that is especially relevant for neuronal cells with axons stretching over long distances. Two important pro-fusion mediators are mitofusin 2 (MFN2) and OPA1, which localise to the mitochondrial outer and inner membrane, respectively (36,37). These two sister proteins belong to the GTPase dynamin family of mechanoenzymes and they operate in tandem to allow sequential fusion of the mitochondrial outer and inner membranes. Analogous to OPA1 mutations, MFN2 mutations have been associated with the syndromic form of DOA, with evidence of mtDNA instability in the form of multiple mtDNA deletions in skeletal muscle biopsies (38–40). Regulated fusion is critical not only for the structural integrity of the mitochondrial inner membrane, which contains the essential apparatus for oxidative phosphorylation (OXPHOS), but it also allows the sequestration of pro-apoptotic cytochrome c molecules within the mitochondrial cristae spaces and functional complementation; for example, a depolarised mitochondrial segment can merge with the larger network to reconstitute its normal complement and thus recover (41,42). The mediators involved in regulating mitochondrial dynamics have taken centre stage in the field of late-onset neurodegenerative diseases, such as Alzheimer disease and Parkinson disease, a perfect illustration of the insight that rare monogenic diseases can provide by clarifying multistep relationships in a simpler model (43,44).
Mitochondrial Quality Control
Mitochondrial dynamics is intrinsically linked to mitochondrial quality control, which is a powerful mechanism by which a cell can eliminate defective mitochondria before they can trigger deleterious pathways (45,46). As already emphasized, mitochondria are ‘metabolic time bombs’ that hold the cell’s fate with the imperative being cell destruction rather than an inefficient OXPHOS system contributing to elevated ROS levels or the accumulation of defective mtDNA molecules. Depolarised mitochondrial segments are detected by a complex machinery whose components are still being dissected, but OPA1, MFN2 and WFS1 all play a role, be it direct or indirect via the Parkin-Pink1 canonical pathways (Fig. 2) (4). It has been shown that mtDNA LHON and OPA1 mutations result in dysfunctional upregulation of mitophagy, which can be associated with mitochondrial network fragmentation and a reduction in mtDNA content, thereby producing a perfect storm for irreversible neuronal cell loss (44,47,48). A similar picture is emerging in the context of more complex central nervous system (CNS) neurodegenerative diseases, and it is therefore not surprising that some patients with mitochondrial optic neuropathies can develop more severe neurological ‘plus’ features (4).
Interorganellar Communication
Traditionally, mitochondria have been viewed in isolation as the cell’s powerhouse and as the effector arm of programmed cell death. In parallel with the greater appreciation of mitochondrial dynamics in health and disease states, the dynamic communications among different organelles have emerged as crucial factors that ensure the cell’s rapid adaptation to various stresses and the coordination of either increased or decreased synthesis of proteins and intermediary metabolites depending on the cell’s metabolic needs (49,50). The close juxtaposition of the mitochondrial network and the endoplasmic reticulum is particularly prominent at areas referred to as mitochondria-associated membranes (MAMs) where there is actually physical tethering mediated by a cluster of proteins that includes MFN2, WFS1 and CISD2 (51). WFS1 and CISD2 are transmembrane ER proteins that are either mutated or degraded prematurely in patients with Wolfram syndrome type 1 and type 2, respectively, with early-onset optic atrophy and diabetes mellitus being the defining hallmarks in addition to other more variable neurologic and endocrinologic manifestations (52,53). MAMs are hotspots for a number of important activities ranging from the regulation of ER stress and the unfolded protein response (UPR), to the careful buffering of calcium flux among the ER, mitochondria and the cytosol, and to the fine-tuning of insulin sensitivity and glucose homeostasis (54–56). There is mounting evidence that disturbed interorganellar cross-talk is involved in the pathogenesis of DOA and Wolfram syndrome, and it is equally compelling that serum samples from patients with LHON seem to carry a specific metabolomic signature indicative of elevated ER stress, thus providing another possible pathway amenable to therapeutic manipulation (57,58).
The Next Decade
Understanding tissue specificity and phenotypic heterogeneity
Our understanding of the molecular genetic basis of mitochondrial disease has expanded exponentially since the first mtDNA mutations associated with human disease were reported in 1988 (59,60). The advent of high-throughput next-generation sequencing will further accelerate the pace of new gene discovery until all patients with suspected mitochondrial disease will have a confirmed genetic diagnosis with the benefit of appropriate counselling. However, gene identification is only the first step in understanding disease evolution and progression. There is a daunting list of unanswered questions in the field of mitochondrial medicine, several of which are centred on the mystery surrounding tissue specificity and the wide phenotypic variability seen with the same genetic defect, frequently within the same family (3,61). For example, it remains unclear why there is such a marked incomplete disease penetrance and male bias in LHON, why some DOA patients develop ‘plus’ features in addition to optic atrophy, and why extraocular muscles are more vulnerable compared with skeletal muscle in CPEO? (6,62)
A major challenge for mitochondrial eye diseases is the lack of diseased human tissues available for analysis and the inherent anatomical complexity of RGCs and photoreceptors that cannot be substituted with more readily available in vitro models such as fibroblasts and immortalised cancerous cell lines. There is still a paucity of animal models, especially those that harbour bona fide pathogenic mtDNA mutations. Targeted gene editing with the CRISPR-Cas9 system is an exciting development that could help scientists generate various models (mouse, zebrafish and drosophila) carrying specific mutations, providing powerful tools not only for unravelling the chronology of cell loss and the underlying driving mechanisms, but also making available ideal pre-clinical models for drug screening, gene therapy and toxicology studies (63,64). Animal models, however, have their own set of limitations. Mice do not have a macula and hence a papillomacular bundle, which is the primary site of pathology in mitochondrial optic neuropathies, and mutant mice are invariably established from highly inbred colonies, dissimilar to the heterogeneous nuclear genetic background observed in human populations. An altogether different strategy is the so-called ‘disease in a dish’ model whereby somatic cells, such as skin fibroblasts, are transformed into induced pluripotent stem cells (iPSCs) by delivering a potent combination of transcription factors with viral vectors or plasmids (65,66). Although the generation of iPSCs is now a relatively straightforward process, their differentiation and maintenance into specific cell lineages are more challenging. Generating photoreceptors and RGCs, and the application of CRISPR-Cas9 technology, would open up a whole new avenue for disease modelling and therapeutic experimentation, especially crucial for rare disease-causing genes that affect only a few patients or families (67–69).
From bench to bedside—the long road ahead
A critical review of the published literature provides a rather sobering picture of the poor evidence base that underpins the use of a myriad of treatments for patients with mitochondrial disease (70). The majority of studies are small case series, often retrospective in nature, with no control groups, variable lengths of treatments and follow-ups, and inconsistencies in the reporting of outcome measures. The lack of effective disease-modifying treatments for patients with mitochondrial disorders will not be overcome until we have a better understanding of the missing links between a genetic defect and loss of a specific cell type. The promise of personalised genomic medicine could become a reality thanks to a confluence of advances in targeted drug discovery, gene replacement and stem cell-based regenerative paradigms, guided by a more complete understanding of the natural history of disease, and in the case of mitochondrial eye diseases, highly sophisticated non-invasive tools for assessing ocular structure and function both pre- and post-treatment. For example, in LHON, a number of neuroprotective drug molecules are in early-phase clinical studies and pivotal gene therapy trials are currently ongoing in Europe and North America for patients carrying the most common m.11778G > A mutation (71). Whilst daunting because of the prerequisite for accurate axonal guidance from the inner retina to the optic nerve, RGC replacement is being explored by a number of research programmes. A more viable approach, at least in the short term, could be the transplantation of stem cell progenitors that exert a local trophic influence and promote neuronal survival, not only for RGCs, but also for oligodendrocytes and other supporting glial cells, which are equally important in ensuring optimal optic nerve function (Fig. 2) (72,73). The development of more precise methods for genetic manipulation has ushered in the possibility of correcting the actual genetic defect in the cells that are most at risk or in the patient’s autologous-derived iPSCs with a view to future differentiation and transplantation. Lastly, preventing the transmission of a pathogenic mtDNA mutation from mother to child is also now a reality with the use of modified in vitro fertilisation (IVF) techniques that involve the use of a donor egg from a healthy woman (containing wild-type mitochondria) to accommodate the pronuclei of the biological parents (74). A child born from mitochondrial donation would therefore inherit a limited number of 37 mitochondrial genes from the female donor rather than the biological mother (75–77). There are risks attached to any experimental treatment, but this potentially groundbreaking approach remains controversial as it entails germline modification, with possible adverse effects not becoming apparent until much later in life (78). The report of the first live birth arising from the mitochondrial replacement therapy in a woman with the m.8993T > G mtDNA mutation has generated considerable scientific and media attention (79,80). The baby boy was reported to be healthy and a long-term follow-up plan is in place to fully assess the child’s neurodevelopmental progress.
Conclusions
After 30 years since its inception, mitochondrial medicine has matured into a multidisciplinary specialty that combines the latest genetic diagnostic technology with a better appreciation of the heterogeneous clinical manifestations and variable disease progression seen in this group of disorders. Neuro-ophthalmologic complications arise in over half of all patients with mitochondrial disease and the pattern of involvement can help direct the diagnostic work-up and, importantly, avoid undue diagnostic delays, a frequent source of distress for patients and their families. The cumulative knowledge gained from studying mitochondrial genes and their downstream effects is building a remarkable picture of the multiple pathways under direct mitochondrial control and the dynamic cross-talk between mitochondria and other cellular organelles. The translation of this knowledge into tangible patient benefit will take time and patience, but there is no doubt that we are currently entering an exciting phase in this journey from bench to bedside for mitochondrial diseases.
Acknowledgements
PYWM is supported by a Clinician Scientist Fellowship Award (G1002570) from the Medical Research Council (UK), and also receives funding from Fight for Sight (UK), the UK National Institute of Health Research (NIHR) as part of the Rare Diseases Translational Research Collaboration, and the NIHR Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. NJN is supported in part by an unrestricted departmental grant (Department of Ophthalmology, Emory University School of Medicine) from Research to Prevent Blindness, Inc. (New York, N.Y.) and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine).
Conflict of Interest statement. PYWM holds a consultancy agreement with GenSight Biologics (Paris, France). NJN holds a consultancy agreement with GenSight Biologics (Paris, France) and Santhera Pharmaceuticals (Liestal, Switzerland), and is a member of the Data Safety and Monitoring Board for the NAION trial sponsored by Quark Pharmaceuticals (Ness Ziona, Israel).
Funding
Medical Research Council (UK), Fight for Sight (UK), UK National Institute of Health Research (NIHR) as part of the Rare Diseases Translational Research Collaboration, and the NIHR Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. Department of Ophthalmology, Emory University School of Medicine) from Research to Prevent Blindness, Inc. (New York, N.Y.) and by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine).
References
- 1. Gorman G.S., Schaefer A.M., Ng Y., Gomez N., Blakely E.L., Alston C.L., Feeney C., Horvath R., Yu-Wai-Man P., Chinnery P.F.. et al. (2015) Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol., 77, 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koopman W.J.H., Willems P., Smeitink J.A.M. (2012) Monogenic mitochondrial disorders. N. Eng. J. Med., 366, 1132–1141. [DOI] [PubMed] [Google Scholar]
- 3. Lightowlers R.N., Taylor R.W., Turnbull D.M. (2015) Mutations causing mitochondrial disease: What is new and what challenges remain?. Science, 349, 1494–1499. [DOI] [PubMed] [Google Scholar]
- 4. Burte F., Carelli V., Chinnery P.F., Yu-Wai-Man P. (2015) Disturbed mitochondrial dynamics and neurodegenerative disorders. Na. Rev. Neurol., 11, 11–24. [DOI] [PubMed] [Google Scholar]
- 5. Fraser J.A., Biousse V., Newman N.J. (2010) The neuro-ophthalmology of mitochondrial disease. Surv. Ophthalmol., 55, 299–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu-Wai-Man P., Griffiths P.G., Chinnery P.F. (2011) Mitochondrial optic neuropathies - Disease mechanisms and therapeutic strategies. Prog. Retin. Eye Res., 30, 81–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan B.X., Ross-Cisneros F.N., Carelli V., Rue K.S., Salomao S.R., Moraes-Filho M.N., Moraes M.N., Berezovsky A., Belfort R. Jr., Sadun A.A. (2012) Mathematically modeling the involvement of axons in Leber's hereditary optic neuropathy. Invest. Ophthalmol. Vis. Sci., 53, 7608–7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith P.R., Bain S.C., Good P.A., Hattersley A.T., Barnett A.H., Gibson J.M., Dodson P.M. (1999) Pigmentary retinal dystrophy and the syndrome of maternally inherited diabetes and deafness caused by the mitochondrial DNA 3243 tRNA(Leu) A to G mutation. Ophthalmology, 106, 1101–1108. [DOI] [PubMed] [Google Scholar]
- 9. de Laat P., Smeitink J.A.M., Janssen M.C.H., Keunen J.E.E., Boon C.J.F. (2013) Mitochondrial retinal dystrophy associated with the m.3243A > G Mutation. Ophthalmology, 120, 2684–2696. [DOI] [PubMed] [Google Scholar]
- 10. Rojo A., Campos Y., Sanchez J.M., Bonaventura I., Aguilar M., Garcia A., Gonzalez L., Rey M.J., Arenas J., Olive M.. et al. (2006) NARP-MILS syndrome caused by 8993 T > G mitochondrial DNA mutation: a clinical, genetic and neuropathological study. Acta Neuropathol., 111, 610–616. [DOI] [PubMed] [Google Scholar]
- 11. Michaelides M., Jenkins S.A., Bamiou D.E., Sweeney M.G., Davis M.B., Luxon L., Bird A.C., Rath P.P. (2008) Macular dystrophy associated with the A3243G mitochondrial DNA mutation - Distinct retinal and associated features, disease variability, and characterization of asymptomatic family members. Arch. Ophthalmol., 126, 320–328. [DOI] [PubMed] [Google Scholar]
- 12. Kearns T.P., Sayre G.P. (1958) Retinitis pigmentosa, external ophthalmophegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA Arch. Ophthalmol., 60, 280–289. [PubMed] [Google Scholar]
- 13. Moraes C.T., Dimauro S., Zeviani M., Lombes A., Shanske S., Miranda A.F., Nakase H., Bonilla E., Werneck L.C., Servidei S.. et al. (1989) Mitochondrial-DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N. Eng. J. Med., 320, 1293–1299. [DOI] [PubMed] [Google Scholar]
- 14. Koo B., Becker L.E., Chuang S., Merante F., Robinson B.H., MacGregor D., Tein I., Ho V.B., McGreal D.A., Wherrett J.R.. et al. (1993) Mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes (MELAS): clinical, radiological, pathological, and genetic observations. Ann. Neurol., 34, 25–32. [DOI] [PubMed] [Google Scholar]
- 15. El-Hattab A.W., Adesina A.M., Jones J., Scaglia F. (2015) MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol. Genet. Metabol., 116, 4–12. [DOI] [PubMed] [Google Scholar]
- 16. Richardson C., Smith T., Schaefer A., Turnbull D., Griffiths P. (2005) Ocular motility findings in chronic progressive external ophthalmoplegia. Eye, 19, 258–263. [DOI] [PubMed] [Google Scholar]
- 17. Nishino I., Spinazzola A., Papadimitriou A., Hammans S., Steiner I., Hahn C.D., Connolly A.M., Verloes A., Guimaraes J., Maillard I.. et al. (2000) Mitochondrial neurogastrointestinal encephalomyopathy: An autosomal recessive disorder due to thymidine phosphorylase mutations. Ann. Neurol., 47, 792–800. [PubMed] [Google Scholar]
- 18. McClelland C., Manousakis G., Lee M.S. (2016) Progressive external ophthalmoplegia. Curr. Neurol. Neurosci. Rep., 16, [DOI] [PubMed] [Google Scholar]
- 19. Carelli V., Chan D.C. (2014) Mitochondrial DNA: impacting central and peripheral nervous systems. Neuron, 84, 1126–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu-Wai-Man P., Votruba M., Burte F., La Morgia C., Barboni P., Carelli V. (2016) A neurodegenerative perspective on mitochondrial optic neuropathies. Acta Neuropathol., 132, 789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zanna C., Ghelli A., Porcelli A.M., Karbowski M., Youle R.J., Schimpf S., Wissinger B., Pinti M., Cossarizza A., Vidoni S.. et al. (2008) OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain, 131, 352–367. [DOI] [PubMed] [Google Scholar]
- 22. Murphy M.P. (2009) How mitochondria produce reactive oxygen species. Biochem. J., 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zorov D.B., Juhaszova M., Sollott S.J. (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev., 94, 909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu-Wai-Man P., Soiferman D., Moore D.G., Burte F., Saada A. (2017) Evaluating the therapeutic potential of idebenone and related quinone analogues in Leber hereditary optic neuropathy. Mitochondrion, pii: S1567-7249(17)30012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu-Wai-Man P., Chinnery P.F. (2012) Dysfunctional mitochondrial maintenance: what breaks the circle of life?. Brain, 135, 9–11. [DOI] [PubMed] [Google Scholar]
- 26. Viscomi C., Zeviani M. (2017) MtDNA-maintenance defects: syndromes and genes. J. Inherit. Metabol. Dis., doi: 10.1007/s10545-017-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirano M., DiMauro S. (2001) ANT1, twinkle, POLG, and TP - New genes open our eyes to ophthalmoplegia. Neurology, 57, 2163–2165. [DOI] [PubMed] [Google Scholar]
- 28. Yu-Wai-Man P., Griffiths P.G., Burke A., Sellar P.W., Clarke M.P., Gnanaraj L., Ah-Kine D., Hudson G., Czermin B., Taylor R.W.. et al. (2010) The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology, 117, 1538–1546. 1546.e1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu-Wai-Man P., Shankar S.P., Biousse V., Miller N.R., Bean L.J.H., Coffee B., Hegde M., Newman N.J. (2011) Genetic screening for OPA1 and OPA3 mutations in patients with suspected inherited optic neuropathies. Ophthalmology, 118, 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hudson G., Amati-Bonneau P., Blakely E.L., Stewart J.D., He L., Schaefer A.M., Griffiths P.G., Ahlqvist K., Suomalainen A., Reynier P.. et al. (2008) Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain, 131, 329–337. [DOI] [PubMed] [Google Scholar]
- 31. Amati-Bonneau P., Valentino M.L., Reynier P., Gallardo M.E., Bornstein B., Boissiere A., Campos Y., Rivera H., de la Aleja J.G., Carroccia R.. et al. (2008) OPA1 mutations induce mitochondrial DNA instability and optic atrophy ′plus′ phenotypes. Brain, 131, 338–351. [DOI] [PubMed] [Google Scholar]
- 32. Yu-Wai-Man P., Griffiths P.G., Gorman G.S., Lourenco C.M., Wright A.F., Auer-Grumbach M., Toscano A., Musumeci O., Valentino M.L., Caporali L.. et al. (2010) Multi-system neurological disease is common in patients with OPA1 mutations. Brain, 133, 771–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stewart J.D., Hudson G., Yu-Wai-Man P., Blakeley E.L., He L., Horvath R., Maddison P., Wright A., Griffiths P.G., Turnbull D.M.. et al. (2008) OPA1 in multiple mitochondrial DNA deletion disorders. Neurology, 71, 1829–1831. [DOI] [PubMed] [Google Scholar]
- 34. Yu-Wai-Man P., Sitarz K.S., Samuels D.C., Griffiths P.G., Reeve A.K., Bindoff L.A., Horvath R., Chinnery P.F. (2010) OPA1 mutations cause cytochrome c oxidase deficiency due to loss of wild-type mtDNA molecules. Hum. Mol. Genet., 19, 3043–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elachouri G., Vidoni S., Zanna C., Pattyn A., Boukhaddaoui H., Gaget K., Yu-Wai-Man P., Gasparre G., Sarzi E., Delettre C.. et al. (2011) OPA1 links human mitochondrial genome maintenance to mtDNA replication and distribution. Genome Res., 21, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen H., Chan D.C. (2006) Critical dependence of neurons on mitochondrial dynamics. Curr. Opin. Cell Biol., 18, 453–459. [DOI] [PubMed] [Google Scholar]
- 37. Landes T., Leroy I., Bertholet A., Diot A., Khosrobakhsh F., Daloyau M., Davezac N., Miquel M.C., Courilleau D., Guillou E.. et al. (2010) OPA1 (dys)functions. Semin Cell Dev. Biol., 21, 593–598. [DOI] [PubMed] [Google Scholar]
- 38. Zuchner S., De Jonghe P., Jordanova A., Claeys K.G., Guergueltcheva V., Cherninkova S., Hamilton S.R., Van Stavern G., Krajewski K.M., Stajich J.. et al. (2006) Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann. Neurol., 59, 276–281. [DOI] [PubMed] [Google Scholar]
- 39. Rouzier C., Bannwarth S., Chaussenot A., Chevrollier A., Verschueren A., Bonello-Palot N., Fragaki K., Cano A., Pouget J., Pellissier J.F.. et al. (2012) The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy ′plus′ phenotype. Brain, 135, 23–34. [DOI] [PubMed] [Google Scholar]
- 40. Renaldo F., Amati-Bonneau P., Slama A., Romana C., Forin V., Doummar D., Barnerias C., Bursztyn J., Mayer M., Khouri N.. et al. (2012) MFN2, a new gene responsible for mitochondrial DNA depletion. Brain, 135, e223. 221-224; author reply e224, 221-223. [DOI] [PubMed] [Google Scholar]
- 41. Chen H., Vermulst M., Wang Y.E., Chomyn A., Prolla T.A., McCaffery J.M., Chan D.C. (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell, 141, 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frezza C., Cipolat S., de Brito O.M., Micaroni M., Beznoussenko G.V., Rudka T., Bartoli D., Polishuck R.S., Danial N.N., De Strooper B.. et al. (2006) OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell, 126, 177–189. [DOI] [PubMed] [Google Scholar]
- 43. Calkins M.J., Manczak M., Mao P., Shirendeb U., Reddy P.H. (2011) Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum. Mol. Genet., 20, 4515–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carelli V., Musumeci O., Caporali L., Zanna C., La Morgia C., Del Dotto V., Porcelli A.M., Rugolo M., Valentino M.L., Iommarini L.. et al. (2015) Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann. Neurol., 78, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen H., Chan D.C. (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum. Mol. Genet., 18, R169–1R176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Twig G., Shirihai O.S. (2011) The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal., 14, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dombi E., Diot A., Morten K., Carver J., Lodge T., Fratter C., Ng Y.S., Liao C.Y., Muir R., Blakely E.L.. et al. (2016) The m.13051G > A mitochondrial DNA mutation results in variable neurology and activated mitophagy. Neurology, 86, 1921–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liao C.Y., Ashley N., Diot A., Morten K., Phadwal K., Williams A., Fearnley I., Rosser L., Lowndes J., Fratter C.. et al. (2017) Dysregulated mitophagy and mitochondrial organization in optic atrophy due to OPA1 mutations. Neurology, 88, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Brito O.M., Scorrano L. (2010) An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. Embo J., 29, 2715–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murley A., Nunnari J. (2016) The emerging network of mitochondria-organelle contacts. Mol. Cell, 61, 648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., Voeltz G.K. (2011) ER tubules mark sites of mitochondrial division. Science, 334, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chaussenot A., Bannwarth S., Rouzier C., Vialettes B., Mkadem S.A., Chabrol B., Cano A., Labauge P., Paquis-Flucklinger V. (2011) Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann. Neurol., 69, 501–508. [DOI] [PubMed] [Google Scholar]
- 53. Rigoli L., Di Bella C. (2012) Wolfram syndrome 1 and Wolfram syndrome 2. Curr. Opin. Pediatr., 24, 512–517. [DOI] [PubMed] [Google Scholar]
- 54. Fonseca S.G., Ishigaki S., Oslowski C.M., Lu S.M., Lipson K.L., Ghosh R., Hayashi E., Ishihara H., Oka Y., Permutt M.A.. et al. (2010) Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Invest., 120, 744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wiley S.E., Andreyev A.Y., Divakaruni A.S., Karisch R., Perkins G., Wall E.A., van der Geer P., Chen Y.F., Tsai T.F., Simon M.I.. et al. (2013) Wolfram Syndrome protein, Miner1, regulates sulphydryl redox status, the unfolded protein response, and Ca2+ homeostasis. EMBO Mol. Med., 5, 904–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu S.M., Kanekura K., Hara T., Mahadevan J., Spears L.D., Oslowski C.M., Martinez R., Yamazaki-Inoue M., Toyoda M., Neilson A.. et al. (2014) A calcium-dependent protease as a potential therapeutic target for Wolfram syndrome. Proc. Natl Acad. Sci. U S A, 111, E5292–E5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de la Barca J.M.C., Simard G., Amati-Bonneau P., Safiedeen Z., Prunier-Mirebeau D., Chupin S., Gadras C., Tessier L., Gueguen N., Chevrollier A.. et al. (2016) The metabolomic signature of Leber's hereditary optic neuropathy reveals endoplasmic reticulum stress. Brain, 139, 2864–2876. [DOI] [PubMed] [Google Scholar]
- 58. Rouzier C., Moore D., Delorme C., Lacas-Gervais S., Ait-El-Mkadem S., Fragaki K., Burte F., Serre V., Bannwarth S., Chaussenot A.. et al. (2017) A novel CISD2 mutation associated with a classical Wolfram syndrome phenotype alters Ca2+ homeostasis and ER-mitochondria interactions. Hum. Mol. Genet., 26, 1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wallace D.C., Singh G., Lott M.T., Hodge J.A., Schurr T.G., Lezza A.M.S., Elsas L.J., Nikoskelainen E.K. (1988) Mitochondrial-DNA mutation associated with lebers hereditary optic neuropathy. Science, 242, 1427–1430. [DOI] [PubMed] [Google Scholar]
- 60. Holt I.J., Harding A.E., Morganhughes J.A. (1988) Deletions of muscle mitochondrial-DNA in patients with mitochondrial myopathies. Nature, 331, 717–719. [DOI] [PubMed] [Google Scholar]
- 61. Lombes A., Aure K., Bellanne-Chantelot C., Gilleron M., Jardel C. (2014) Unsolved issues related to human mitochondrial diseases. Biochimie, 100, 171–176. [DOI] [PubMed] [Google Scholar]
- 62. Greaves L.C., Yu-Wai-Man P., Blakely E.L., Krishnan K.J., Beadle N.E., Kerin J., Barron M.J., Griffiths P.G., Dickinson A.J., Turnbull D.M.. et al. (2010) Mitochondrial DNA defects and selective extraocular muscle involvement in CPEO. Invest. Ophthalmol. Vis. Sci., 51, 3340–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang H.Y., Yang H., Shivalila C.S., Dawlaty M.M., Cheng A.W., Zhang F., Jaenisch R. (2013) One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell, 153, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hsu P.D., Lander E.S., Zhang F. (2014) Development and applications of CRISPR-Cas9 for genome engineering. Cell, 157, 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Okita K., Ichisaka T., Yamanaka S. (2007) Generation of germline-competent induced pluripotent stem cells. Nature, 448, 313–317. [DOI] [PubMed] [Google Scholar]
- 66. Shi Y., Inoue H., Wu J.C., Yamanaka S. (2017) Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov., 16, 115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xie B.B., Zhang X.M., Hashimoto T., Tien A.H., Chen A., Ge J., Yang X.J. (2014) Differentiation of retinal ganglion cells and photoreceptor precursors from mouse induced pluripotent stem cells carrying an Atoh7/Math5 lineage reporter. Plos One, 9, e112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhong X.F., Gutierrez C., Xue T., Hampton C., Vergara M.N., Cao L.H., Peters A., Park T.S., Zambidis E.T., Meyer J.S.. et al. (2014) Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun., 5, 4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hockemeyer D., Jaenisch R. (2016) Induced pluripotent stem cells meet genome editing. Cell Stem Cell, 18, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pfeffer G., Horvath R., Klopstock T., Mootha V.K., Suomalainen A., Koene S., Hirano M., Zeviani M., Bindoff L.A., Yu-Wai-Man P.. et al. (2013) New treatments for mitochondrial disease-no time to drop our standards. Nat. Rev. Neurol., 9, 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yu-Wai-Man P., Votruba M., Moore A.T., Chinnery P.F. (2014) Treatment strategies for inherited optic neuropathies: past, present and future. Eye (Lond), 28, 521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Johnson T.V., Martin K.R. (2013) Cell transplantation approaches to retinal ganglion cell neuroprotection in glaucoma. Curr. Opin. Pharmacol., 13, 78–82. [DOI] [PubMed] [Google Scholar]
- 73. Flachsbarth K., Kruszewski K., Jung G., Jankowiak W., Riecken K., Wagenfeld L., Richard G., Fehse B., Bartsch U. (2014) Neural stem cell-based intraocular administration of ciliary neurotrophic factor attenuates the loss of axotomized ganglion cells in adult mice. Invest. Ophthalmol. Vis. Sci., 55, 7029–7039. [DOI] [PubMed] [Google Scholar]
- 74. Herbert M., Turnbull D. (2017) Mitochondrial donation - clearing the final regulatory hurdle in the United Kingdom. N. Eng. J. Med., 376, 171–173. [DOI] [PubMed] [Google Scholar]
- 75. Craven L., Elson J.L., Irving L., Tuppen H.A., Lister L.M., Greggains G.D., Byerley S., Murdoch A.P., Herbert M., Turnbull D. (2011) Mitochondrial DNA disease: new options for prevention. Hum. Mol. Genet., 20, R168–R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tachibana M., Amato P., Sparman M., Woodward J., Sanchis D.M., Ma H., Gutierrez N.M., Tippner-Hedges R., Kang E., Lee H.S.. et al. (2013) Towards germline gene therapy of inherited mitochondrial diseases. Nature, 493, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hyslop L.A., Blakeley P., Craven L., Richardson J., Fogarty N.M.E., Fragouli E., Lamb M., Wamaitha S.E., Prathalingam N., Zhang Q.. et al. (2016) Towards clinical application of pronuclear transfer to prevent mitochondrial DNA disease. Nature, 534, 383. +. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shoubridge E.A. (2016) Replacing the cell's power plants. Nature, 540, 210–211. [DOI] [PubMed] [Google Scholar]
- 79. Zhang J., Liu H., Luo S., Lu Z., Chavez-Badiola A., Liu Z., Yang M., Merhi Z., Silber S.J., Munne S.. et al. (2017) Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online, 34, 361–368. [DOI] [PubMed] [Google Scholar]
- 80. Alikani M., Fauser B.C., Garcia-Valesco J.A., Simpson J.L., Johnson M.H. (2017) First birth following spindle transfer for mitochondrial replacement therapy: hope and trepidation. Reprod. Biomed. Online, 34, 333–336. [DOI] [PubMed] [Google Scholar]