Abstract
Objective. Despite modern antiretroviral therapy, HIV-associated neuropathy is one of the most prevalent, disabling and treatment-resistant complications of HIV disease. The presence and intensity of distal neuropathic pain is not fully explained by the degree of peripheral nerve damage. A better understanding of brain structure in HIV distal neuropathic pain may help explain why some patients with HIV neuropathy report pain while the majority does not. Previously, we reported that more intense distal neuropathic pain was associated with smaller total cerebral cortical gray matter volumes. The objective of this study was to determine which parts of the cortex are smaller.
Methods. HIV positive individuals with and without distal neuropathic pain enrolled in the multisite (N = 233) CNS HIV Antiretroviral Treatment Effects (CHARTER) study underwent structural brain magnetic resonance imaging. Voxel-based morphometry was used to investigate regional brain volumes in these structural brain images.
Results. Left ventral posterior cingulate cortex was smaller for HIV positive individuals with versus without distal neuropathic pain (peak P = 0.017; peak t = 5.15; MNI coordinates x = −6, y = −54, z = 20). Regional brain volumes within cortical gray matter structures typically associated with pain processing were also smaller for HIV positive individuals having higher intensity ratings of distal neuropathic pain.
Conclusions. The posterior cingulate is thought to be involved in inhibiting the perception of painful stimuli. Mechanistically a smaller posterior cingulate cortex structure may be related to reduced anti-nociception contributing to increased distal neuropathic pain.
Keywords: HIV Distal Neuropathic Pain, Structural Magnetic Resonance Imaging, Posterior Cingulate Cortex, Voxel-Based Morphometry
Introduction
HIV-associated distal neuropathic pain (DNP) affects approximately 20% of individuals with HIV and is one of the most prevalent neurological complications of HIV infection in the era of combination antiretroviral therapy (CART) [1]. HIV DNP is typically refractory to current chronic pain therapies [2,3]; and is associated with unemployment, impairment in activities of daily living, and significantly diminished quality of life [1].
Similar to other common peripheral neuropathies such as diabetic and alcoholic peripheral neuropathy [4–7], it is unclear why some individuals with HIV peripheral neuropathy experience DNP and others do not [1,5,6,8]. Approximately half of HIV-infected (HIV+) individuals have sensory neuropathy by physical examination or nerve conduction studies [1,9]. About 40% of them report chronic DNP, while the remainder reports only numbness or paresthesiae or no symptoms at all [1,9]. Most research on HIV DNP mechanisms has focused on the direct effects of HIV or antiretroviral drugs on peripheral nerves (e.g., exposure to dideoxynucleoside reverse transcriptase inhibitors such as stavudine or didanosine) [10,11] and on clinical risk factors for neuropathy (age, height, and lower CD4 nadir) [1]. This research suggests the intensity of DNP is not fully explained by the extent of HIV damage to peripheral nerve fibers [11–14], nor by clinical risk factors [1,8], leaving it unclear why some HIV neuropathy individuals experience DNP and others do not.
Central nervous system pathways exert a major influence on the clinical expression of peripherally-induced pain [15–17] and contribute to the transition from acute to chronic pain states [18,19]. Brain imaging has revealed the impact of peripheral neuropathy on the central nervous system. For example, in diabetic neuropathy the somatosensory cortex is smaller [7] while in painful diabetic neuropathy resting state network connectivity is altered [20–22].
Previously, using conventional morphometric methods which included hand segmentation techniques [23], we reported that total cortical volumes are smaller for HIV+ individuals with higher DNP ratings, when controlling for clinical and imaging covariates associated with changes in total cerebral cortical gray matter volume [24]. This research leaves unresolved which parts of the cortex are smaller for HIV+ individuals with larger DNP ratings. This research also does not address if parts of the cortex are smaller for HIV+ individuals with DNP compared to those without DNP.
The first purpose of this investigation was to use the structural imaging data set from our previous investigation [24] to determine if regional cortical volumes are smaller for HIV+ individuals with DNP compared to those without DNP. The second purpose is to determine if regional cortical volumes are smaller for HIV+ individuals with larger DNP ratings, that is determine if regional cortical volumes are negatively correlated with DNP. Given that it is too laborious to parcelate the total cortex for the hundreds of individuals in this structural imaging data set [24] using hand segmentation methods [23], here we use automated voxel-based morphometry (VBM) to investigate which regions of the cortex are smaller for HIV+ individuals with DNP compared to those without DNP, and whether regions of the cortex are smaller for HIV+ individuals with larger DNP intensity ratings.
In other chronic pain conditions regional cortical gray matter volumes within cortical pain processing structures (such as the anterior cingulate cortex, insula cortex, and prefrontal cortex) and within resting state default mode cortical structures (such as the posterior cingulate cortex) are smaller [25–37]. We therefore hypothesized that regional cortical gray matter volumes within these same structures would be smaller in HIV+ individuals with DNP compared to those without DNP, and would be smaller for those with larger DNP intensity ratings.
Methods
Participants
The Central Nervous System HIV Anti-Retroviral Therapy Effects Research Study (CHARTER), examined 1,556 HIV+ individuals at five United States academic medical centers with a comprehensive battery of neuromedical and neuropsychiatric assessments [1,23]. A subset of CHARTER HIV+ enrollees (N = 699) were recruited to participate in a longitudinal arm of the study which involved follow-up appointments every 6 months. The primary selection criterion for the longitudinal arm was that people were willing to do lumbar punctures at longitudinal visits. Additionally, subgroups were recruited into the longitudinal arm for: 1) presence or absence of peripheral neuropathy; 2) being eligible for magnetic resonance imaging (MRI); and 3) presence of lipodystrophy or lipoatrophy. Of the 699 individuals entering the longitudinal arm, 241 met criteria for MRI and agreed to participate in a sub-study which involved undergoing brain structural MRI studies [23]. Since VBM methods require a relatively normally shaped brain, eight brains with gross structural abnormalities out of the original 241 brains were excluded, so the total number of individuals included in this study was 233. Data reported here are from their first adequate MRI at their second CHARTER visit, which occurred between 2004 and 2007. The sites performing MRI included: Johns Hopkins University (Baltimore, MD; N = 47); Mount Sinai School of Medicine (New York, NY; N = 48); University of California at San Diego (San Diego, CA; N = 70); University of Texas Medical Branch (Galveston, TX; N = 46); and University of Washington (Seattle, WA; N = 30).
Standard Protocol Approvals and Patient Consents
The Human Subjects Protection Committees of each participating institution approved these procedures. Written informed consent was obtained from all study participants as part of enrollment into the CHARTER MRI sub-study.
Measures and Assessments
Diagnosis of HIV Distal Neuropathic Pain
HIV DNP was defined as a specific pattern of bilateral burning, aching, or shooting pain in the lower extremities, which is a standard definition of distal neuropathic pain that has been validated in prior work [1,9]. Recognizing that this specific pattern of pain may occur in small fiber-predominant neuropathies in which abnormalities on clinical examination are sometimes absent due to the relative paucity of large fiber involvement, we included in the diagnosis of DNP those cases that did not have signs of neuropathy (e.g., diminished vibratory sensation). Patients were further diagnosed with sensory neuropathy if examination revealed at least one sign of neuropathy bilaterally (e.g., reduced ankle reflexes, diminished vibratory sense or sharp-dull discrimination). Based on patients’ reports of pain intensity, impact on everyday function, and treatment-seeking, study clinicians further classified DNP into five categories of magnitude: none; slight (occasional, fleeting); mild (frequent); moderate (frequent, disabling); and severe (constant, daily, disabling, requiring analgesic medication or other treatment). The DNP grading system used here has been validated against a variety of indices of impact on overall health and daily functioning [1] as well as neuropathy severity as assessed by neurological examination and electrophysiological measures [9]. Because neuropathic pain in HIV is known to wax and wane in severity, and may even remit and then recur, we also asked participants to estimate the duration of their DNP in spans of time: 4 days to 4 weeks, 1 month to 1 year, 1 to 10 years, greater than 10 years. The average time between the DNP clinical assessment and the MRI brain imaging was 10 days. Data related to the validation of the DNP grading system [1,9] as well as DNP clinical case report forms and an associated instruction manual, are available at www.charternntc.org. Alternatively, the DNP clinical case report forms and associated instruction manual can be obtained from the corresponding author for this manuscript.
Neuromedical and Neuropsychiatric Evaluation
Clinicians conducting the neurological examination also performed semi-structured interviews and standardized examinations to ascertain HIV disease status, HIV treatment history, psychotropic medications, and pain treatments. Plasma and cerebrospinal fluid (CSF) HIV concentration were determined [23]. Plasma hepatitis C and plasma CD4 were also determined [23,38].
An extensive treatment history queried for prior and current anti-HIV treatment since exposure to anti-HIV dideoxynucleoside reverse transcriptase inhibitors (didanosine, stavudine, and zalcitabine) may cause neuropathy. Performance on neuropsychological testing was assessed, and a global deficit score was calculated to control for the impact of HIV cognitive deficits on brain structure [13,23,39].
Because psychiatric and substance use disorders may be associated with neuroimaging and cognitive abnormalities, history of major depression (and current Beck depression inventory scores) and psychoactive substance use disorders were assessed using the Composite International Diagnostic Interview and DSM-IV criteria [40–42]. History of abuse and dependence was collected for alcohol, cannabis, cocaine, hallucinogens, inhalants, methamphetamine, opioids, and sedatives.
Structural Imaging
As described previously [23], structural MRI volumes were acquired on 1.5 Tesla GE scanners at the five participating sites. Image acquisition parameters were sagittal acquisitions with section thickness = 1.3 mm, FOV 24 cm, matrix size 256 x 256 x 124, voxel dimensions 0.94 mm x 0.94 mm x 1.94 mm, 3D T1-weighted SPGR with TR = 20 ms, TE = 6ms, flip angle = 30.
Voxel-Based Morphometric Analysis
Structural data was analyzed with FSL-VBM [43] (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimised VBM protocol [44] carried out with FSL tools [45]. Since the transformation from native brain space to standard space requires a relatively normally shaped native space brain, eight of the original 241 brains were excluded due to gross brain anatomical abnormalities (such as brain tumor, focal encephalomalacia, and hydrocephalus); so the total number of individuals included in this study was 233. The gross brain abnormalities were identified through careful inspection during image analysis and a consensus of the investigators. Not all normal images were reviewed by a neruoradiologist, but all abnormal images were reviewed by a neuroradiologist. Native space structural images were brain-extracted using automated FSL BET with further manual refinement conducted with AFNI [46] as necessary. The native space structural images were then gray matter-segmented [47] before being registered to the MNI 152 standard space using an affine transform [48] that was supplemented by non-linear refinement [49]. The resulting images were averaged to create a study-specific template. In order to eliminate white matter abnormalities in the study-specific template, which have the same intensity as gray matter, we masked the white matter portion of the study-specific template with a mask generated from the avg152T1_gray tissue prior. All native gray matter images were non-linearly registered to this study-specific template and modulated to correct for local expansion (or contraction) due to the non-linear component of the spatial transformation [44]. The modulated gray matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 (12.25 mm). Finally, voxelwise general linear modeling was applied using permutation-based non-parametric testing that corrected for multiple comparisons across the whole brain using the Threshold-Free Cluster Enhancement method [50]. The corrected alpha was set to 0.05.
Statistical Methods
Demographic and clinical statistical differences between the DNP group and the non-DNP group were determined using the independent samples t-test (age, education, Beck depression inventory, global deficit score) [51], Fisher’s exact test (gender, ethnicity, hepatitis C, plasma and CSF viral load, antiretroviral use, dideoxynucleoside reverse transcriptase inhibitors (D-drug) use, history of major depression, history of inhalant use, history of methamphetamine use) [52], and the Goodman and Kruskal ordinal gamma test (CD4) [53].
Two statistical models were investigated for each regional brain volume: First, we used a general linear model to compare regional brain volumes between the HIV+ individuals with and without DNP; second, we used an alternate general linear model to investigate which regional brain volumes were negatively correlated with DNP (volumes are smaller for HIV subjects with larger DNP ratings). DNP was treated as a continuous variable: none (DNP = 0), slight (DNP = 1), mild (DNP = 2), moderate (DNP = 3), and severe (DNP = 4). This is not a region of interest (ROI) analysis where we investigated a priori-defined ROI. Instead, the VBM methods used here investigate the whole brain gray matter to identify clusters of voxels such that the voxel clusters have smaller volumes for HIV+ individuals with DNP compared to individuals without DNP or the voxel clusters have smaller volumes for HIV+ individuals with larger DNP ratings. As described in the Voxel-Based Morphometric Analysis Methods above, all reported P values are corrected for multiple comparisons across space [50].
For each of these statistical models an unadjusted and an adjusted analysis was performed (Table 1). In the unadjusted analysis we did not control for clinical and imaging covariates associated with changes in total cerebral cortical gray matter volume, while in the adjusted analysis we did control for clinical and imaging covariates associated with changes in total cerebral cortical gray matter volume.
Table 1.
Four statistical models used to investigate which regional brain volumes are smaller for HIV+ individuals with DNP compared to HIV+ individuals without DNP as well as HIV+ individuals with larger DNP ratings. Models #2 and #4 control for covariates previously associated with changes in total cortical volume [24]—these control covariates are listed in Table 2
| Unadjusted models not controlling for covariates associated with changes in total cortical volume | Adjusted models controlling for covariates associated with changes in total cortical volume | |
|---|---|---|
| Statistical models investigating regional brain volumes smaller for individuals with DNP vs individuals without DNP | Model #1 | Model #2 |
| Statistical models investigating smaller regional brains in HIV+ individuals with larger DNP ratings | Model #3 | Model #4 |
The clinical and imaging covariates which have been previously associated with changes in total cortical volume [24] include age, ethnicity, gender, cerebral-vault volume, scanner, history of D-drug use, history of inhalant abuse, history of methamphetamine abuse, and Global Deficit Score – see Table 2. There were two reasons for controlling for these clinical and imaging covariates in the adjusted analysis. First, these covariates may be confounders of the association between DNP and regional brain volumes and thus need to be included to reduce bias in estimating the effect of DNP. Second, these covariates are independent predictors of brain volumetrics. Thus, their inclusion in the model may in fact help to increase the power of our comparisons of interest. To control for bias resulting from inter-scanner variability [54–57], we controlled for scanner as a covariate in the adjusted analysis.
Table 2.
List of clinical and imaging covariates that are associated with changes in total cortical volume [24]. Models #2 and #4 in Table 1 control for these covariates. The definition for each covariate includes its type and range
| Variable | Type | Range |
|---|---|---|
| Age | Continuous | 23 yo–67 yo |
| Ethnicity | Nominal | See Table 3 |
| Gender | Binary | See Table 3 |
| Log-cerebral vault | Continuous | 13.5–14.3 |
| Scanner (# of scanners at site) | Nominal |
|
| D-drug* ever | Nominal |
|
| Inhalant abuse ever | Binary | Yes or no |
| Methamphetamine abuse ever | Binary | Yes or no |
| Global deficit score | Continuous | 0–3.47 |
D-drug = dideoxynucleoside reverse transcriptase inhibitors.
Finally, we performed an additional adjusted analysis to investigate if the pain component of neuropathic pain is associated with changes in regional brain volume independent of contributions of HIV peripheral neuropathy and independent of possible systemic disease associated with HIV peripheral neuropathy. In this additional adjusted analysis, we controlled for clinical and imaging covariates associated with changes in total cerebral cortical gray matter volume and we also controlled for number of signs of HIV neuropathy. We controlled for signs of HIV neuropathy in both statistical models: the general linear model comparing regional brain volumes between the HIV+ individuals with and without DNP as well as the general linear model investigating which regional brain volumes were negatively correlated with HIV DNP. We controlled for signs of HIV neuropathy using two different methods: 1) we controlled for the number of signs of HIV neuropathy by treating the number of signs as a continuous variable, and 2) we controlled for the number of signs of HIV neuropathy by controlling for the effect of having one or two signs of HIV neuropathy compared to having no signs of HIV neuropathy.
Results
Signs of Neuropathy
Of the 233 evaluable individuals, 132 (57%) met criteria for distal sensory neuropathy by virtue of having at least one objective sign of neuropathy (for example, reduced ability to discriminate vibration sense in the feet and toes bilaterally [58]). Sixty-five individuals (28%) met a more stringent definition of at least two objective signs of neuropathy. The DNP and non-DNP proportions of signs of neuropathy were significantly different using the Goodman and Kruskal ordinal gamma test [53], P < 0.001. The proportion of zero signs of neuropathy was higher in the non-DNP group (9/64 = 14% for individuals with DNP and 92/169 = 54% for individuals without DNP). The proportion of two signs of neuropathy was higher in the DNP group (35/64 = 55% for individuals with DNP and 30/169 = 18% for individuals without DNP).
Demographic and Clinical Characteristics
The demographic and clinical characteristics of the study participants (Table 3) show that typical individuals were middle-aged men of Caucasian, African-American, or Hispanic ethnicity who had AIDS and were currently on antiretrovirals, with good immune recovery and fair virologic control.
Table 3.
Demographic and clinical characteristics of the participants. Column 5 lists the P values showing if the characteristic proportions or mean values are significantly different for individuals with DNP compared to individuals without DNP
| DNP | All individuals | DNP | No DNP | P values DNP vs no DNP |
|---|---|---|---|---|
| No. of individuals | 233 | 64 | 169 | |
| Neuropathic pain rating, no. (%) | ||||
| No pain | 169 (73) | 0 (0) | 169 (100) | |
| Slight | 18 (8) | 18 (11) | 0 (0) | |
| Mild | 22 (9) | 22 (13) | 0 (0) | |
| Moderate | 9 (4) | 9 (5) | 0 (0) | |
| Severe | 15 (6) | 15 (90) | 0 (0) | |
| Age, mean (SD) | 44 (8) | 46 (8) | 43 (8) | <0.01* |
| Education yrs, mean (SD) | 13 (3) | 13 (2) | 13 (2) | 0.734 |
| Male, no. (%) | 187 (80) | 50 (78) | 137(81) | 0.712 |
| Race/ethnicity, no. (%) | ||||
| Caucasian | 92 (40) | 32 (50) | 60 (36) | 0.211 |
| African American | 110 (47) | 24 (38) | 86 (61) | |
| Hispanic | 26 (11) | 7 (11) | 19 (11) | |
| Other | 5 (2) | 1 (2) | 4 (2) | |
| Hepatitis C Virus seropositive, no. (%) | 64 (28) | 21 (33) | 43 (25) | 0.324 |
| AIDS, no. (%) | 147(63) | 41 (64) | 106(63) | 0.880 |
| CD4, no. (%) | ||||
| Current < 200; nadir < 200 | 34 (15) | 7 (11) | 27 (16) | 0.530 |
| Current ≥ 200, nadir < 200 | 101(43) | 29 (45) | 72 (43) | |
| Current ≥ 200, nadir ≥ 200 | 95 (41) | 27 (42) | 68 (40) | |
| Plasma HIV RNA detectable, no. (%) | ||||
| All individuals | 121(52) | 31 (49) | 90 (53) | 0.558 |
| ON CART**, no. (%) | 61 (26) | 19 (30) | 42 (36) | 0.505 |
| OFF CART**, no. (%) | 60 (26) | 12 (19) | 48 (28) | 0.179 |
| Cerebrospinal fluid HIV RNA detectable, no. (%) | ||||
| All individuals | 68 (30) | 16 (25) | 52 (31) | 0.423 |
| ON CART,** no. (%) | 24 (10) | 7 (11) | 17 (14) | 0.813 |
| OFF CART,** no. (%) | 44 (19) | 9 (14) | 35 (21) | 0.268 |
| CART** use, no. (%) | ||||
| Current | 170 (73) | 52 (81) | 118 (70) | 0.107 |
| Past | 37 (16) | 9 (14) | 28 (17) | |
| Never | 26 (11) | 3 (5) | 23 (14) | |
| D-drug,** no. (%) | ||||
| Current | 39 (17) | 11 (17) | 28 (17) | 0.230 |
| Past | 87 (37) | 29 (45) | 58 (34) | |
| Never | 107 (46) | 24 (38) | 83 (50) | |
| Beck depression inventory II, mean (SD) | 12 (11) | 16 (11) | 11 (11) | 0.001* |
| Lifetime major depressive disorder,*** no. (%) | 51 (22) | 21 (33) | 30 (18) | 0.020* |
| History of inhalant abuse, no. (%) | 5 (2) | 3 (5) | 2 (1) | 0.129 |
| History of methamphetamine abuse, no. (%) | 8 (3) | 2 (3) | 6 (4) | 1.000 |
| Global deficit score, mean (SD) | 0.4 (0.4) | 0.4 (0.4) | 0.4 (0.5) | 0.991 |
DNP and non-DNP characteristics are statistically different.
D-drug = dideoxynucleoside reverse transcriptase inhibitors; CART = combination antiretroviral therapy.
Major depressive disorder = Meets DSM-IV criteria for a major depressive episode within the last 30 days.
The average age of the DNP group was older than the non-DNP group (t = 3.24, degrees of freedom = 231, P < 0.001). In addition, the proportion with a history of depression was higher in the DNP group compared to the non-DNP group (P = 0.02) and the average Beck depression rating was higher for the DNP group compared to the non-DNP group (t = 3.23, degrees of freedom = 231, P < 0.001).
VBM Results
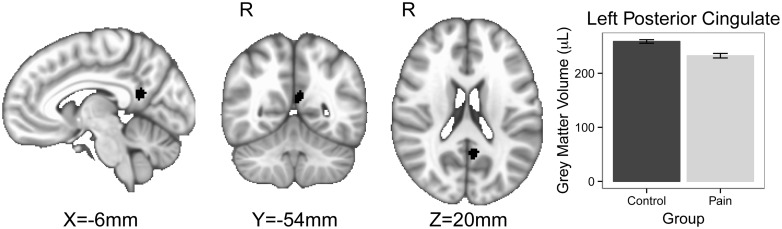
The left ventral posterior cingulate cortex (PCC) was smaller for HIV+ individuals with DNP compared to those without DNP (see Table 4). Figure 1 shows the part of the ventral PCC which is smaller for HIV+ individuals with DNP compared to those without DNP (peak P = 0.017; peak t = 5.15; MNI coordinates x = −6, y = −54, z = 20, cluster volume = 65 voxels; cluster average P = 0.032; cluster average t-score = 4.38). In addition, the ventral PCC volume trends towards statistical significance for being smaller for HIV+ individuals with larger DNP ratings (see Table 4). Regions within the right precentral gyrus, bilateral insula cortex, bilateral putamen, right orbital frontal cortex, and right caudate are smaller for HIV+ individuals with larger DNP ratings (see Table 4). No regional brain volumes were larger for HIV+ individuals with DNP compared to those without DNP nor for those with larger DNP ratings.
Table 4.
Results for which regional brain volumes are smaller for HIV+ individuals with DNP compared to HIV+ individuals without DNP and HIV+ individuals with larger DNP ratings. As described in Table 1, statistical models #1 and #2 investigate regional brain volumes smaller for HIV+ individuals with DNP compared to HIV+ individuals without DNP, while statistical models #3 and #4 investigate regional brain volumes which are smaller for HIV+ individuals with larger DNP ratings
| Statistical model | Brain region | P value (peak) | T score | MNI coordinates | Cluster volume # voxels P values < 0.05 |
|---|---|---|---|---|---|
| #1 Unadjusted analysis of brain regions smaller for DNP compared to no DNP | Left ventral PCC | 0.034 | 4.71 | X = −6, Y = −52, Z = 18 | 43 |
| #2 Adjusted analysis of brain regions smaller for DNP compared to no DNP | Left ventral PCC | 0.017 | 5.15 | X = −6, Y = −54, Z = 20 | 65 |
| #3 Unadjusted analysis of brain regions smaller for HIV+ individuals with larger DNP ratings | Left ventral PCC | 0.058 (Trend) | 3.83 | X = −6, Y = −54, Z = 20 | 0 |
| R PCG | 0.012 | 4.41 | X = 32, Y = −22, Z = 46 | 174 | |
| R caudate | 0.050 | 2.80 | X = 16, Y = 40, Z = 40 | 18 | |
| R putamen | 0.042 | 3.00 | X = 26, Y = 10, Z = 6 | 91 | |
| L putamen | 0.045 | 3.00 | X = −24, Y = 6, Z = 6 | 84 | |
| R insula cortex | 0.038 | 3.71 | X = 30, Y = 28, Z = 4 | 82 | |
| L insula cortex | 0.049 | 3.00 | X = -28, Y = 22, Z = 10 | 42 | |
| L OFC | 0.043 | 3.93 | X = −48, Y = 30, Z = −12 | 17 | |
| #4 Adjusted analysis of brain regions smaller for HIV+ individuals with larger DNP ratings | Left ventral PCC | 0.049 | 4.22 | X = −6, Y = −54, Z = 20 | 3 |
DNP = distal neuropathic pain; PCC = posterior cingulate cortex; PCG = precentral gyrus cortex; OFC = orbital frontal cortex.
Figure 1.
The shaded region in the brain figure is the part of the left ventral posterior cingulate cortex where the gray matter volume is smaller for the group of HIV+ individuals with distal neuropathic pain (DNP) compared to the HIV+ individuals without DNP using the adjusted analysis (see Table 4, statistical model #2). The bar chart shows that the average gray matter volume for the left posterior cingulate cortex cluster is smaller for the HIV DNP group compared with the HIV group without DNP. In the brain figure the shaded volume P values threshold are less than 0.05, cluster volume = 65 voxels, with an average t = 4.38, with an average P = 0.032. The peak P values is 0.017 and the peak t-value is 5.15 with MNI coordinates X = −6, Y = −54, Z = 20. The error bars in the bar chart represent the standard error of the mean.
When controlling for signs of HIV neuropathy as well as the covariates listed in Table 2, the PCC is no longer smaller for HIV+ individuals with DNP compared to those without DNP. Also, when controlling for signs of HIV neuropathy as well as the covariates listed in Table 2, the PCC volume is no longer smaller for HIV+ individuals with larger DNP ratings.
Discussion
We hypothesized that regional cortical gray matter volumes within pain processing structures and within default mode network structures would be smaller in HIV+ individuals with DNP compared to those without DNP, and would be smaller for those with larger DNP intensity ratings. Consistent with our hypotheses, regional cortical brain volumes in the left ventral posterior cingulate cortex were smaller in HIV+ individuals with DNP compared to those without DNP. In addition regional brain volumes within the bilateral insula cortex, left orbital frontal cortex, subcortex, and precentral gyrus were smaller for HIV+ individuals with larger DNP ratings. Contrary to our hypotheses, regions within the anterior cingulate cortex, the insula cortex, and prefrontal cortex are not smaller for individual with DNP compared to individuals without DNP.
These observed smaller regional brain volumes in individuals with HIV DNP is consistent with other chronic pain conditions that are associated with smaller regional brain volumes [59–62]. The mechanisms for these smaller regional brain volumes in individuals with chronic pain are not well understood. Possible mechanisms for smaller regional brain volumes in chronic pain include changes in neuronal volume, axonal white matter volume, synaptic volume, glial volume, or vascular volume [60,63–65].
Reduced brain volumes in the posterior cingulate cortex are widely reported in chronic pain: headache [25,33,36], phantom limb pain [26], fibromyalgia [27,28,34], pain disorder [29], temporomandibular pain [30–32], and trigeminal neuralgia [35]. These observations suggest that the PCC could have a role in the development of chronic pain.
Historically it was believed that the PCC was not involved in pain processing [66–68]; however, recent investigations suggest that the PCC may play a role in anti-nociception [69,70]. PCC gray matter density has been shown to be inversely related to cutaneous thermal pain sensitivity [70]. PCC cortical thickness trends towards being inversely related to cutaneous thermal pain thresholds [71] and is inversely related to rectal sensitivity thresholds [72]. An interpretation of these results is that increased PCC gray matter density/cortical thickness may reflect entrenched introspective thought patterns which function as competitors to processes that contribute to the construction of an experience of pain. That is rumination about non-pain related issues may function to inhibit the experience of pain [70]. This possible antinociceptive role for the PCC is consistent with the PCC participating in attentional disengagement from painful experiences during mind wandering [69,73,74]. A proposed mechanism for the PCC participating in antinociception may involve the default mode network circuit (connectivity between the PCC and medial prefrontal cortex) [37] interacting with descending analgesia brain circuits (connectivity between the periaqueductal gray matter and the rostral ventral medulla) [69,74,75]. This proposed antinociceptive role for the PCC suggests a possible mechanism for chronic pain: Some chronic pain may result from impairment of the PCC participating in antinociception which would make an individual less able to attentionally disengage from the experience of pain.
Reduced volumes in the orbital-frontal cortex and the insula cortex have also been widely reported in chronic pain [59–62]. Chronic pain reduction in orbital-frontal cortex volume may be related to chronic pain changes in reward processing [19,76], while chronic pain reduction in insula cortex volume may be related to chronic pain increased functional connectivity between the salience network [69,74,77,78] and the default mode network [22,75,79–83]. Our observed smaller regional brain volumes in the caudate, putamen, and the precentral gyrus for HIV+ individuals with larger DNP ratings are less commonly reported in other chronic pain conditions [59,60]. These smaller subcortical and precentral gyrus regional brain volumes may be related to behavioral changes in HIV+ individuals with larger DNP ratings [61].
There are several reports of different pain processing between left and right cortical structures involving the insula [84,85], amygdala [86,87], dorsolateral prefrontal cortex [88], and anterior cingulate [89,90]. In this regard it is interesting that the PCC has reduced regional volume on the left side but not the right side. This difference may suggest different function between the left and right sides of the PCC. Although we are not aware of studies investigating or reporting different function between the left and right PCC, the left region of the PCC has been shown to have abnormal resting state functional connectivity with the insula in another chronic pain condition, ankylosing spondylitis [91].
When we control for signs of HIV neuropathy, the PCC is no longer smaller for HIV+ individuals with HIV DNP compared to individuals without HIV DNP, and the PCC volume is no longer smaller for HIV+ individuals with larger DNP ratings. Interpretation of these results is complicated by two issues. First, it is problematic to attempt to control for a sign of disease when the research variable of interest is a symptom of the same disease. Presence of HIV neuropathy is defined as presence of at least one, or a combination, of three possible symptoms (presence of pain, presence of tingling, loss of sensation) and/or three possible signs (decreased sensation of vibration, decreased sensation of sharpness, decreased ankle reflex) [1]. Second, the purpose of controlling for signs of HIV neuropathy is to attempt to separate the pain aspect of HIV DNP from the neuropathy part of HIV DNP. In fact, the definition of HIV distal neuropathic pain is a pattern of bilateral burning, aching, or shooting pain in the lower extremities that occurs in specific conditions such as HIV peripheral nerve damage. The pain quality of HIV DNP is intimately related to the underlying disease process of damaged peripheral nerves so that it can be argued that it does not make sense to attempt to divide HIV distal neuropathic pain into a separate pain component and a separate neuropathy component. Attempting to control for signs of HIV neuropathy may actually be removing variance fundamental to the group of HIV+ individuals with distal neuropathic pain [92].
The PCC no longer being smaller for HIV+ individuals with HIV DNP compared to individuals without HIV DNP after controlling for signs of HIV neuropathy suggests that PCC volume, HIV distal neuropathic pain, and signs of HIV neuropathy are inter-related. There are multiple possible causal relationships between these three variables: HIV DNP may contribute to smaller PCC volume, smaller PCC volume may contribute to HIV DNP, HIV neuropathy may contribute to smaller PCC volume, HIV neuropathy may contribute to HIV DNP, and/or systemic HIV disease may contribute to smaller PCC volume and HIV DNP and HIV neuropathy. The cross-sectional nature of this study prevents determining which of these possible causal relationships, if any, are true.
This study has several limitations. These include its cross-sectional design, which leaves us unable to explore possible cause and effect relationships between PCC volume and HIV DNP. Our cohort was a convenience sample of individuals enrolled in HIV care at academic clinics, rather than from a random sample of HIV+ individuals in care. Other limitations are that we do not have resting state functional magnetic imaging (fMRI) images, or detailed information about other pain conditions which could confound our results. We also did not include an HIV negative control group. Since we did not collect skin biopsies, we cannot confirm that the DNP individuals without signs of neuropathy have HIV small fiber peripheral neuropathy. Finally, using brain structural MRI images from different scanners may introduce systematic noise [54–57] which could have contributed to why brain volumes for cortical pain processing regions (such as the anterior cingulate cortex, insula cortex, and prefrontal cortex) were not smaller for individuals with DNP compared to individuals without DNP.
Given that HIV DNP is prevalent, treatment resistant, and adversely impacts quality of life [1], additional research is warranted to investigate the mechanisms leading to smaller regional brain volumes in HIV DNP. Future possible studies to investigate mechanistic relationships among regional gray matter volumes, HIV DNP, and signs of HIV neuropathy include cross-sectional VBM studies to examine if associations exist between regional gray matter volume changes and symptoms of HIV neuropathy other than HIV DNP or between regional gray matter volume changes and signs of HIV neuropathy. In addition, cross-sectional functional and diffusion tensor MRI imaging may provide insight into alterations of PCC brain circuit connectivity in participants with HIV neuropathy symptoms and/or signs. Finally, it has recently been shown that approximately 25% of HIV+ individuals in a HIV+ but DNP negative cohort developed new onset HIV DNP in a 2-year period [93]. This suggests that a longitudinal multimodal MRI study may be suited to investigate if changes in PCC regional volumes or changes in PCC circuit connectivity precede onset of HIV DNP. Furthermore, it may also permit examination of whether change in PCC volume over time may be related to variation in HIV DNP over time.
References
- 1. Ellis RJ, Rosario D, Clifford DB, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: The CHARTER study. Arch Neurol 2010;67(5):552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simpson DM, Brown S, Tobias J, Group N-CS.. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology 2008;70(24):2305–13. [DOI] [PubMed] [Google Scholar]
- 3. Verma S, Estanislao L, Mintz L, Simpson D.. Controlling neuropathic pain in HIV. Curr Infect Dis Rep 2004;6(3):237–42. [DOI] [PubMed] [Google Scholar]
- 4. Sommer C. Painful neuropathies. Curr Opin Neurol 2003;16(5):623–8. [DOI] [PubMed] [Google Scholar]
- 5. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B.. Burden of illness in painful diabetic peripheral neuropathy: The patients' perspectives. J Pain 2006;7(12):892–900. [DOI] [PubMed] [Google Scholar]
- 6. Sadosky A, Schaefer C, Mann R, et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: Results from a retrospective chart review and cross-sectional survey. Diabetes Metab Syndr Obes 2013;6:79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selvarajah D, Wilkinson ID, Maxwell M, et al. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care 2014;37(6):1681–8. [DOI] [PubMed] [Google Scholar]
- 8. Ellis RJ, Marquie-Beck J, Delaney P, et al. Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol 2008;64(5):566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson-Papp J, Morgello S, Vaida F, et al. Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy. Pain 2010;151(3):732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore RD, Wong WM, Keruly JC, McArthur JC.. Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. Aids 2000;14(3):273–8. [DOI] [PubMed] [Google Scholar]
- 11. Cherry CL, McArthur JC, Hoy JF, Wesselingh SL.. Nucleoside analogues and neuropathy in the era of HAART. J Clin Virol 2003;26(2):195–207. [DOI] [PubMed] [Google Scholar]
- 12. Dorsey SG, Morton PG.. HIV peripheral neuropathy: Pathophysiology and clinical implications. AACN Clin Issues 2006;17(1):30–6. [DOI] [PubMed] [Google Scholar]
- 13. Herrmann DN, McDermott MP, Henderson D, et al. Epidermal nerve fiber density, axonal swellings and QST as predictors of HIV distal sensory neuropathy. Muscle Nerve 2004;29(3):420–7. [DOI] [PubMed] [Google Scholar]
- 14. Skopelitis E, Aroni K, Kontos AN, et al. Early detection of subclinical HIV sensory polyneuropathy using intraepidermal nerve fibre density quantification: Association with HIV stage and surrogate markers. Int J STD AIDS 2007;18(12):856–60. [DOI] [PubMed] [Google Scholar]
- 15. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK.. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9(4):463–84. [DOI] [PubMed] [Google Scholar]
- 16. Lee MC, Tracey I.. Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr Pain Headache Rep 2010;14(2):124–31. [DOI] [PubMed] [Google Scholar]
- 17. Ossipov MH, Dussor GO, Porreca F.. Central modulation of pain. J Clin Invest 2010;120(11):3779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borsook D. Neurological diseases and pain. Brain 2012;135(Pt 2):320–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15(8):1117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cauda F, Sacco K, Duca S, et al. Altered resting state in diabetic neuropathic pain. PloS One 2009;4(2):e4542.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cauda F, Sacco K, D'Agata F, et al. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. BMC Neurosci 2009;10:138.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cauda F, D'Agata F, Sacco K, et al. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry 2010;81(7):806–11. [DOI] [PubMed] [Google Scholar]
- 23. Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Clinical factors related to brain structure in HIV: The CHARTER study. J Neurovirol 2011;17(3):248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keltner JR, Fennema-Notestine C, Vaida F, et al. HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter. J Neurovirol 2014;20:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt-Wilcke T, Leinisch E, Straube A, et al. Gray matter decrease in patients with chronic tension type headache. Neurology 2005;65(9):1483–6. [DOI] [PubMed] [Google Scholar]
- 26. Draganski B, Moser T, Lummel N, et al. Decrease of thalamic gray matter following limb amputation. Neuroimage 2006;31(3):951–7. [DOI] [PubMed] [Google Scholar]
- 27. Kuchinad A, Schweinhardt P, Seminowicz DA, et al. Accelerated brain gray matter loss in fibromyalgia patients: Premature aging of the brain? J Neurosci 2007;27(15):4004–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wood PB, Glabus MF, Simpson R, Patterson JC. 2nd.. Changes in gray matter density in fibromyalgia: Correlation with dopamine metabolism. J Pain 2009;10(6):609–18. [DOI] [PubMed] [Google Scholar]
- 29. Valet M, Gundel H, Sprenger T, et al. Patients with pain disorder show gray-matter loss in pain-processing structures: A voxel-based morphometric study. Psychosomatic Med 2009;71(1):49–56. [DOI] [PubMed] [Google Scholar]
- 30. Younger JW, Shen YF, Goddard G, Mackey SC.. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain 2010;149(2):222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gerstner G, Ichesco E, Quintero A, Schmidt-Wilcke T.. Changes in regional gray and white matter volume in patients with myofascial-type temporomandibular disorders: A voxel-based morphometry study. J Orofac Pain 2011;25(2):99–106. [PubMed] [Google Scholar]
- 32. Ceko M, Bushnell MC, Fitzcharles MA, Schweinhardt P.. Fibromyalgia interacts with age to change the brain. Neuroimage Clin 2013;3:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Absinta M, Rocca MA, Colombo B, et al. Selective decreased grey matter volume of the pain-matrix network in cluster headache. Cephalalgia 2012;32(2):109–15. [DOI] [PubMed] [Google Scholar]
- 34. Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R.. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain 2011;12(4):436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DaSilva AF, Becerra L, Pendse G, et al. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PloS One 2008;3(10):e3396.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holle D, Naegel S, Krebs S, et al. Hypothalamic gray matter volume loss in hypnic headache. Ann Neurol 2011;69(3):533–9. [DOI] [PubMed] [Google Scholar]
- 37. Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102(27):9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Munoz-Moreno JA, Fumaz CR, Ferrer MJ, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses 2008;24(10):1301–7. [DOI] [PubMed] [Google Scholar]
- 39. Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004;26(3):307–19. [DOI] [PubMed] [Google Scholar]
- 40. Wittchen HU, Robins LN, Cottler LB, et al. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA field trials. Br J Psychiatry 1991;159:645–53, 658. [DOI] [PubMed] [Google Scholar]
- 41. Beck AT, Steer RA, Brown GK.. BDI-II, Beck depression inventory: Manual, 2nd edition San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 42. Lipps GE, Lowe GA, De La Haye W, et al. Validation of the Beck depression inventory II in HIV-positive patients. West Indian Med J 2010;59(4):374–9. [PubMed] [Google Scholar]
- 43. Douaud G, Smith S, Jenkinson M, et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 2007;130(Pt 9):2375–86. [DOI] [PubMed] [Google Scholar]
- 44. Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 45. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23 Suppl 1:S208–19. [DOI] [PubMed] [Google Scholar]
- 46. Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29(3):162–73. [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, Brady M, Smith S.. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001;20(1):12.. [DOI] [PubMed] [Google Scholar]
- 48. Jenkinson M, Bannister P, Brady M, Smith S.. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 2002;17(2):825–41. [DOI] [PubMed] [Google Scholar]
- 49. Andersson JLR, Jenkinson M, Smith S Non-linear registration, aka Spatial normalization Periodical: FMRIB technical report TR07JA2 Place Published: www.fmrib.ox.ac.uk/analysis/techrep. [Google Scholar]
- 50. Smith SM, Nichols TE.. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 51. Fisher BJ. Guinness, Gosset, Fisher, and small samples. Stat Sci 1987;2(1):7. [Google Scholar]
- 52. Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc 1922;85(1):7. [Google Scholar]
- 53. Goodman LAK, William H.. Measures of association for cross classifications. J Am Stat Assoc 1954;49(268):12. [Google Scholar]
- 54. Fennema-Notestine C, Gamst AC, Quinn BT, et al. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics 2007;5(4):235–45. [DOI] [PubMed] [Google Scholar]
- 55. Stonnington CM, Tan G, Kloppel S, et al. Interpreting scan data acquired from multiple scanners: A study with Alzheimer's disease. NeuroImage 2008;39(3):1180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moorhead TW, Gountouna VE, Job DE, et al. Prospective multi-centre voxel based morphometry study employing scanner specific segmentations: Procedure development using CaliBrain structural MRI data. BMC Med Imag 2009;9:8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takao HH, Naoto, Ohtomo Kuni.. Effects of the use of multiple scanners and scanner upgrade in longitudinal voxel-based morphometry studies. J Magn Reson Imaging 2013;38:8. [DOI] [PubMed] [Google Scholar]
- 58. Gonzalez-Duarte A, Robinson-Papp J, Simpson DM.. Diagnosis and management of HIV-associated neuropathy. Neurol Clin 2008;26(3):821–32, x. [DOI] [PubMed] [Google Scholar]
- 59. May A. Structural brain imaging: A window into chronic pain. Neuroscientist 2011;17(2):209–20. [DOI] [PubMed] [Google Scholar]
- 60. Davis KD, Moayedi M.. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 2013;8(3):518–34. [DOI] [PubMed] [Google Scholar]
- 61. Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV.. Brain morphological signatures for chronic pain. PloS One 2011;6(10):e26010.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smallwood RF, Laird AR, Ramage AE, et al. Structural brain anomalies and chronic pain: A quantitative meta-analysis of gray matter volume. J Pain 2013;14(7):663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Apkarian AV, Hashmi JA, Baliki MN.. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 2011;152(3 Suppl):S49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zatorre RJ, Fields RD, Johansen-Berg H.. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci 2012;15(4):528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H.. The effects of aerobic activity on brain structure. Front Psychol 2012;3:86.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vogt BA, Vogt L, Laureys S.. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage 2006;29(2):452–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tracey I, Mantyh PW.. The cerebral signature for pain perception and its modulation. Neuron 2007;55(3):377–91. [DOI] [PubMed] [Google Scholar]
- 68. Peyron R, Laurent B, Garcia-Larrea L.. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 2000;30(5):263–88. [DOI] [PubMed] [Google Scholar]
- 69. Kucyi A, Salomons TV, Davis KD.. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A 2013;110(46):18692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Emerson NM, Zeidan F, Lobanov OV, et al. Pain sensitivity is inversely related to regional grey matter density in the brain. Pain 2014;155(3):566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schwedt TJ, Chong CD.. Correlations between brain cortical thickness and cutaneous pain thresholds are atypical in adults with migraine. PloS One 2014;9(6):e99791.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Elsenbruch S, Schmid J, Kullmann JS, et al. Visceral sensitivity correlates with decreased regional gray matter volume in healthy volunteers: A voxel-based morphometry study. Pain 2014;155(2):244–9. [DOI] [PubMed] [Google Scholar]
- 73. Kucyi A, Moayedi M, Weissman-Fogel I, et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 2014;34(11):3969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kucyi A, Davis KD.. The dynamic pain connectome. Trends Neurosci 2015;38(2):86–95. [DOI] [PubMed] [Google Scholar]
- 75. Loggia ML, Kim J, Gollub RL, et al. Default mode network connectivity encodes clinical pain: An arterial spin labeling study. Pain 2013;154(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Baliki MN, Geha PY, Fields HL, Apkarian AV.. Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010;66(1):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wiech K, Lin CS, Brodersen KH, et al. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 2010;30(48):16324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Legrain V, Iannetti GD, Plaghki L, Mouraux A.. The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol 2011;93(1):111–24. [DOI] [PubMed] [Google Scholar]
- 79. Napadow V, LaCount L, Park K, et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62(8):2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR.. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett 2010;485(1):26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xue T, Yuan K, Zhao L, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One 2012;7(12):e52927.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013;119(6):1453–64. [DOI] [PubMed] [Google Scholar]
- 83. Napadow V, Kim J, Clauw DJ, Harris RE.. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum 2012;64(7):2398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kucyi A, Hodaie M, Davis KD.. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol 2012;108(12):3382–92. [DOI] [PubMed] [Google Scholar]
- 85. Craig AD. How Do You Feel?: An Interoceptive Moment with Your Neurobiological Self. Princeton: Princeton University Press; 2015. [Google Scholar]
- 86. Ji G, Neugebauer V.. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 2009;102(4):2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tran L, Greenwood-Van Meerveld B.. Lateralized amygdala activation: Importance in the regulation of anxiety and pain behavior. Physiol Behav 2012;105(2):371–5. [DOI] [PubMed] [Google Scholar]
- 88. Rego GG, Lapenta OM, Marques LM, et al. Hemispheric dorsolateral prefrontal cortex lateralization in the regulation of empathy for pain. Neurosci Lett 2015;594:12–6. [DOI] [PubMed] [Google Scholar]
- 89. Symonds LL, Gordon NS, Bixby JC, Mande MM.. Right-lateralized pain processing in the human cortex: An FMRI study. J Neurophysiol 2006;95(6):3823–30. [DOI] [PubMed] [Google Scholar]
- 90. Watanabe H, Fitting S, Hussain MZ, et al. Asymmetry of the endogenous opioid system in the human anterior cingulate: A putative molecular basis for lateralization of emotions and pain. Cerebral Cortex 2015;25(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD.. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct 2015. doi:10.1007/s00429-015-1161-1. [DOI] [PubMed] [Google Scholar]
- 92. Miller GA, Chapman JP.. Misunderstanding analysis of covariance. J Abnorm Psychol 2001;110(1):40–8. [DOI] [PubMed] [Google Scholar]
- 93. Malvar J, Vaida F, Sanders CF, et al. Predictors of new-onset distal neuropathic pain in HIV-infected individuals in the era of combination antiretroviral therapy. Pain 2015;156(4):731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]