Abstract
Objectives
The objective of this study was to determine our institution's compliance with 2010 Society for Healthcare Epidemiology of America and IDSA Clostridium difficile infection (CDI) treatment guidelines and their respective outcomes.
Methods
We collected clinical parameters, laboratory values, antibiotic therapy and clinical outcomes from the electronic medical records for all patients hospitalized at our institution with a diagnosis of CDI from December 2012 to November 2013. We specifically evaluated whether SHEA-IDSA treatment guidelines were followed and evaluated the associations between guideline adherence and severe outcomes including mortality.
Results
We identified 230 patients with CDI meeting inclusion criteria during the study period. Of these, 124 (54%) were appropriately treated, 46 (20%) were under-treated and 60 (26%) were over-treated. All-cause 90 day mortality was 17.4% overall; 43.5% in the under-treated group versus 12.9% in those appropriately treated (P < 0.0001) and 10.9% in those appropriately treated plus over-treated (P < 0.0001). Similarly, 90 day mortality attributed to CDI was 21.7% in those under-treated versus 8.9% in those appropriately treated (P = 0.03) and 8.2% in those either appropriately treated or over-treated (P = 0.015). Severe-complicated CDI occurred in 46 patients. In this subgroup, there was a non-significant trend towards increased mortality in under-treated patients (56.7%) compared with appropriately treated patients (37.5%, P = 0.35). Under-treatment was also associated with a higher rate of CDI-related ICU transfer (17.4% versus 4.8% in those appropriately treated, P = 0.023).
Conclusions
Adherence to CDI treatment guidelines is associated with improved outcomes especially in those with severe disease. Increased emphasis on provision of appropriate, guideline-based CDI treatment appears warranted.
Introduction
Clostridium difficile is a Gram-positive, spore-forming anaerobic bacillus first isolated in 1935 from the stool of a healthy neonate.1 However, it was not until the 1970s that a link between antibiotic-associated colitis and C. difficile was established.2 The diagnosis of C. difficile infection (CDI) is based on a combination of clinical and laboratory findings. A case definition for CDI includes the following findings: (i) the presence of diarrhoea, defined as passage of three or more unformed stools within 24 h; and (ii) a stool test result positive for the presence of toxigenic C. difficile or its toxins or colonoscopic or histopathological findings demonstrating pseudomembranous colitis.3
CDI is a highly morbid condition and places a heavy burden on the healthcare system. In the USA, as much as $3 billion are consumed annually for prevention, diagnosis and management of this infection.4 CDI has surpassed MRSA as the most common hospital-associated bacterial infection.5 It is also the most common known cause of infectious diarrhoea in hospitalized patients in industrialized countries.6C. difficile is also the most common cause of infectious diarrhoea in nursing homes and in 2008, CDI was ranked the 18th leading cause of death in individuals aged ≥65 years.3,7,8 Furthermore, in 2011, C. difficile had an incidence of nearly half a million infections and was responsible for ∼29 000 deaths, making it by far the most deadly enteric pathogen.9
In 2010, clinical practice guidelines for CDI in adults were released by the Society for Healthcare Epidemiology of America (SHEA) and the IDSA. These guidelines were designed to improve the diagnosis and management of CDI in adults.10 Guideline documents, with very similar treatment recommendations, have been produced by other national and international bodies.11,12
Studies have documented deficiencies in the knowledge and awareness of CDI guidelines amongst medical professionals as well as a lack of conformation to recommended treatment approaches.13,14 Thus, we elected to perform a quality improvement study at our hospital with two main goals: (i) to determine the degree of adherence to clinical practice guidelines among healthcare providers; and (ii) to determine whether deviation from the guidelines may have an impact on patient outcomes.
Methods
This study retrospectively examined the medical records of patients with symptoms and signs of CDI and a positive test for toxigenic C. difficile on an unformed stool sample between December 2012 and November 2013.
Research was conducted in compliance with the Declaration of Helsinki and national and institutional standards. The institutional review board of Beth Israel Deaconess Medical Center (BIDMC) approved this study and waived the requirement of informed consent. All patient identifiers were coded and stored securely on a password-protected network. This study involved no animal or procedure.
Patients were assigned a CDI disease severity rating according to the SHEA-IDSA guidelines.10 Mild-moderate CDI is defined by the presence of a white blood cell count ≤15 000/mm3 and a serum creatinine level ≤1.5× the premorbid level. Severe CDI is defined by the presence of a white blood cell count ≥15 000/mm3 or a serum creatinine level ≥1.5× the premorbid level. Severe-complicated CDI is defined by the presence of hypotension, shock, ileus or megacolon.10
SHEA-IDSA guidelines recommend treating mild-moderate CDI with 500 mg of metronidazole orally three times per day for 10–14 days. For severe CDI, the recommended treatment is 125 mg of vancomycin orally four times per day for 10–14 days. For severe-complicated CDI, the recommended treatment is 500 mg of vancomycin orally four times per day in addition to 500 mg of metronidazole intravenously three times per day. In the presence of ileus, rectal administration of vancomycin should be considered. Patients with a first episode of recurrent CDI should receive the same treatment as for an initial episode of CDI based on disease severity as described above. For a second recurrence, vancomycin in a tapered and/or pulsed regimen is recommended.10
BIDMC institutional guidelines for the treatment of CDI were also referenced during the analysis. Mild-moderate CDI is defined as diarrhoea (i) ≥3 but ≤6 unformed stools/24 h and (ii) fever <101°F and (iii) white blood cell count <15 000/mm3 and (iv) not significantly immunocompromised and (v) not a patient with known inflammatory bowel disease and (vi) normal albumin (>2.5 mg/dL) or unchanged from baseline and (vii) normal serum creatinine (≤1.2 mg/dL) or not significantly changed (<1.5× baseline). Severe CDI is defined as (i) diarrhoea (>6 unformed stools/24 h) or (ii) fever ≥101°F or (iii) white blood cell count ≥15 000/mm3 or (iv) immunocompromised or (v) patient with known inflammatory bowel disease or (vi) hypoalbuminaemia (≤2.5 mg/dL) or (vii) serum creatinine increase of 1.5× baseline or (viii) transfer from outside hospital for treatment of CDI or (ix) CT scan or X-ray reveals evidence of pan-colitis. Severe-complicated CDI is defined as (i) ICU-level care for CDI or (ii) evidence of sepsis or (iii) presence of ileus or (iv) elevated serum lactate (>2.5 mmol/L) or (v) white blood cell count ≥50 000/mm3 or (vi) haemodynamic instability or pressor requirement or (vii) severe abdominal pain or distention or abdominal rigidity or (viii) CT scan or X-ray reveals evidence of complication such as megacolon or (ix) confluent pseudomembranous colitis seen on colonoscopy.15
BIDMC institutional guidelines recommend treating mild-moderate CDI with 500 mg of metronidazole orally every 8 h. If there is no clinical response in 3–5 days, then treatment should be switched to 125 mg of vancomycin orally every 6 h. For severe CDI, the recommended treatment is 125 mg of vancomycin orally every 6 h. If there is no clinical response after 48 h, then 500 mg of metronidazole intravenously every 8 h should be added. For severe-complicated CDI, the recommended treatment is 500 mg of vancomycin every 6 h plus 500 mg of metronidazole intravenously every 8 h. The vancomycin dose should be decreased to 125 mg every 6 h upon clinical improvement. In the setting of ileus or a proximal diverting ostomy, rectal vancomycin should be added if this is safe from a surgical and gastroenterological perspective.15 These institutional guidelines are available on the online portal system for access by all BIDMC-affiliated individuals.
Patient demographics, clinical information, initial treatment for CDI and the clinical outcomes of the infection were recorded. Specific information collected included age, gender, relevant laboratory values and clinical data for assigning CDI severity by SHEA-IDSA criteria. Assessment of baseline serum creatinine was based on clinical judgement and availability of prior creatinine levels. The time period ranged from a few months to a few years. The initial treatment given for CDI was noted and categorized as under-treated (if the initial treatment was less than that recommended for the patient's CDI severity), appropriately treated (if the initial treatment matched the recommended treatment) or over-treated (if the initial treatment was more aggressive than that recommended for the patient's CDI disease severity). Outcomes recorded included 90 day all-cause mortality, 90 day CDI-related mortality, CDI-related ICU transfer and CDI-related colectomy. The relatedness of these outcomes to CDI was determined independently by two physician reviewers as previously described. The physicians were not blinded during analysis. In cases of disagreement (10 cases in this cohort), further review was performed by a third reviewer and the final determination was made by consensus.
A two-tailed Fisher exact test was used to compare categorical data including clinical outcomes between the three treatment guideline adherence groups (under-treated, appropriately treated or over-treated). Student's t-test was used to compare continuous data.
Results
We identified 286 patients hospitalized at our institution who had positive tests for toxigenic C. difficile on unformed stool between December 2012 and November 2013. Of these, 56 were excluded because we were unable to access their post-discharge medical records to evaluate outcomes (45) or because they were experiencing a third or later episode of CDI recurrence (11) and so did not come within the guidelines' specific treatment recommendations. No additional patients were excluded due to neutropenia, hospice care or other criteria.
The 230 patients who met our inclusion criteria had a mean age of 63.2 ± 16.2 years; 131 (57%) were male. See Table 1. By SHEA-IDSA guidelines, 124 patients (54%) were appropriately treated, 46 (20%) were under-treated and 60 (26%) were over-treated (Table 2). Adherence to SHEA-IDSA treatment guidelines in different CDI disease severity groups is shown in Table 2. The most common under-treatment in the severe CDI group was use of metronidazole instead of vancomycin in 14 of 15 cases. In the severe-complicated CDI group, the most common deviations from guidelines were use of vancomycin (125 mg every 6 h) in combination with intravenous metronidazole instead of vancomycin (500 mg every 6 h) in combination with intravenous metronidazole in 17 of 30 cases. Other deviations from treatment guidelines in severe-complicated cases included vancomycin alone at any dose without the use of intravenous metronidazole in 8 of 30 cases and metronidazole alone in 5 of 30 cases. The most common deviation resulting in over-treatment in the mild-moderate CDI group was the use of oral vancomycin in 52 of 54 cases. The most frequent over-treatment in the severe CDI group was the use of a combination of metronidazole and vancomycin in four of six cases.
Table 1.
Comparison of demographics and clinical factors between guideline-adherent and guideline-non-adherent groups
| Under-treated | Appropriately treated | Over-treated | P | |
|---|---|---|---|---|
| Demographic data | ||||
| subjects, n (%) | 46 (20%) | 124 (54%) | 60 (26%) | — |
| age (years), mean ± SD | 68.7 ± 15.5 | 65.3 ± 16.6 | 55.5 ± 16.5 | 0.0001a; 0.17b; 0.01c |
| male, n (%) | 25 (54.34) | 73 (58.87) | 33 (55) | 0.82a; 0.60b; 0.70c |
| Clinical data | ||||
| white blood cell count (103/mL), mean±SD. | 16.4 ± 12.5 | 10.9 ± 6.8 | 8.4 ± 5.8 | <0.0001a; 0.0004b; <0.0001c |
| absolute serum creatinine (mg/dL), mean±SD. | 3.2 ± 3 | 1.6 ± 1.7 | 1.1 ± 0.7 | <0.0001a; 0.0001b; <0.0001c |
aComparison across three groups.
bUnder-treated versus appropriately treated.
cUnder-treated versus appropriately treated plus over-treated.
Table 2.
Adherence to the SHEA-IDSA recommendations for the initial treatment of CDI stratified by severity of disease
| Treatment adherence according to disease severity (n) | Under-treated | Appropriately treated | Over-treated |
|---|---|---|---|
| Mild-moderate (136) | 1 (0.7%)a | 81 (59.6%) | 54 (39.7%)b |
| Severe (48) | 15 (31.2%)c | 27 (56.3%) | 6 (12.5%)d |
| Severe-complicated (46) | 30 (65.2%)e | 16 (34.8%) | 0 (0%) |
| Total (230) | 46 (20%) | 124 (54%) | 60 (26%) |
aReason for under-treatment was short duration (<10 days) of therapy with metronidazole.
bThe most common finding of over-treatment in the mild-moderate CDI group was use of oral vancomycin (52 of 54).
cThe most common finding of under-treatment in the severe CDI group was use of metronidazole instead of vancomycin (14 of 15).
dThe most common finding of over-treatment in the severe CDI group was use of a combination of metronidazole and vancomycin (four of six).
eThe most common findings of under-treatment in the severe-complicated CDI group was use of metronidazole alone (5 of 30) or use of vancomycin alone without intravenous metronidazole (8 of 30) or use of vancomycin at 125 mg every 6 h instead of at 500 mg every 6 h in combination with intravenous metronidazole (17 of 30).
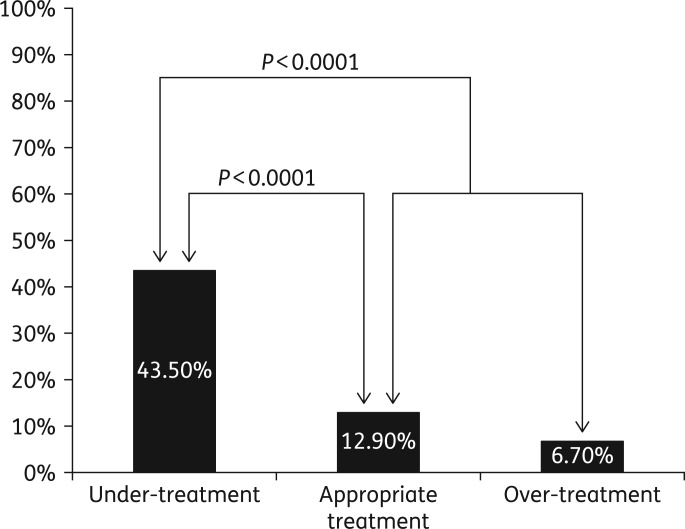
In total, 40 of the 230 patients died during the study period, yielding a 90 day all-cause mortality rate of 17.4% (Table 3). Mortality rates were highest in the under-treated patients (43.5%), lower in the appropriately treated (12.9%) and lowest in the over-treated (6.7%) (Figure 1). Under-treatment was associated with a higher all-cause 90 day mortality (43.5% versus 12.9% in those appropriately treated, P < 0.0001; and 43.5% versus 10.9% in those either appropriately treated or over-treated, P < 0.0001) (Figure 1). Hence, these outcomes represented differences in mortality rates that were highly significant both clinically and statistically with a >4-fold excess of deaths in the under-treated group when compared with all other patients (43.5% versus 10.9%, P < 0.001). See Table 3.
Table 3.
Comparison of negative outcomes between patients managed in accordance or not in accordance with the CDI treatment guidelines
| Negative patient outcomes | Under-treated | Appropriately treated | Over-treated | Pa | Pb |
|---|---|---|---|---|---|
| Total | 46 | 124 | 60 | ||
| All-cause 90 day mortality | 20 (43.5%) | 16 (12.9%) | 4 (6.7%) | <0.0001 | <0.0001 |
| CDI-related 90 day mortality | 10 (21.7%) | 11 (8.9%) | 4 (6.7%) | 0.03 | 0.02 |
| CDI-related ICU transfer | 8 (17.4%) | 6 (4.8%) | 1 (1.7%) | 0.02 | 0.003 |
| CDI-related colectomy | 1 (2.2%) | 4 (3.2%) | 0 (0%) | 1.00 | 1.00 |
| Combined CDI-related complications | 39 (84.8%) | 37 (29.8%) | 9 (15.0%) | <0.0001 | <0.0001 |
aUnder-treated versus appropriately treated.
bUnder-treated versus appropriately treated plus over-treated.
Figure 1.
All-cause 90 day mortality rate was 17.4% overall (40 of 230 patients). It was 43.5% (20 of 46) in the under-treated group, 12.9% (16 of 124) in the appropriately treated group and 6.7% (4 of 60) in the over-treated group.
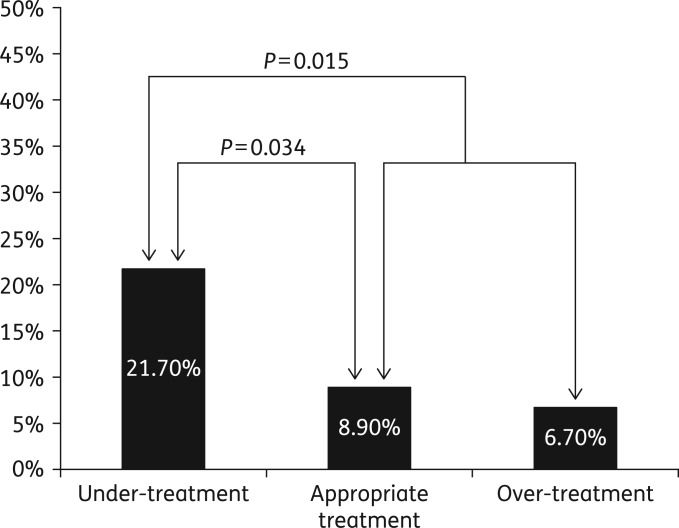
Although CDI-related deaths were fewer (20 in all), they were also disproportionately more common in the under-treated group (21.7%) as compared with the appropriately treated group (8.9%) or over-treated group (6.7%) (Figure 2). Under-treatment was associated with significantly higher CDI-related 90 day mortality (21.7% versus 8.9% in those appropriately treated, P = 0.034; and 21.7% versus 8.2% in those either appropriately treated or over-treated, P = 0.015) (Figure 2). Overall, CDI-related deaths were 2.6 times more common in the under-treated group when compared with all other patients combined (21.7% versus 8.2%, P = 0.015). See Table 3.
Figure 2.
CDI-related 90 day mortality rate was 10.9% overall (25 of 230 patients). It was 21.7% (10 of 46) in the under-treated group, 8.9% (11 of 124) in the appropriately treated group and 6.7% (4 of 60) in the over-treated group.
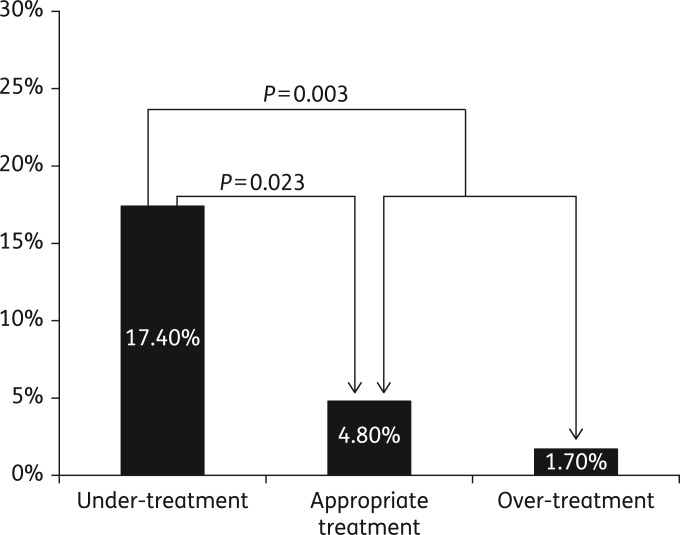
CDI-related ICU transfers were also more common in the under-treated group (17.4%) as compared with the appropriately treated group (4.8%) or over-treated group (1.7%) and were 4.6 times more common overall in the under-treated patients (17.4% versus 3.8%, P = 0.003) (Figure 3). There were few colectomies (five in total) and no statistically significant intergroup differences were evident. See Table 3.
Figure 3.
CDI-related ICU transfer rate was 6.5% overall (15 of 230 patients). It was 17.4% (8 of 46) in the under-treated group, 4.8% (6 of 124) in the appropriately treated group and 1.7% (1 of 60) in the over-treated group.
As part of our analysis, treatment appropriateness was also evaluated within each disease severity grouping. In patients with mild-moderate disease, 1 (0.7%) patient was under-treated, 81 (59.6%) patients were appropriately treated and 54 (39.7%) patients were over-treated. Among patients with severe disease, 15 (31.2%) were under-treated, 27 (56.3%) were appropriately treated and 6 (12.5%) patients were over-treated. Finally, in patients with severe-complicated disease, 30 (65.2%) patients received under-treatment, 16 (34.8%) patients were appropriately treated and 0 patients received over-treatment. These data demonstrate that patients with severe-complicated disease received under-treatment the most often compared with other disease categories (Table 2).
Given the low likelihood of under-treating mild-moderate CDI or over-treating severe-complicated CDI, we also analysed patient outcomes based on disease severity grouping and treatment appropriateness. In mild-moderate disease, only one patient was under-treated and this patient did not experience any CDI-related complications (Table 4). Of the 81 patients with mild-moderate disease who were appropriately treated, all-cause mortality was seen in 5 (6.2%) patients, 2 (2.5%) patients had a CDI-associated death, 1 (1.2%) patient had a CDI-related ICU transfer and 2 (2.5%) patients had CDI-related colectomy. Among 54 patients with mild-moderate disease who were over-treated, all-cause mortality was seen in 3 (5.6%) patients, 3 (5.6%) patients had CDI-related deaths, 1 (1.9%) patient had a CDI-related ICU transfer and 0 patients had colectomy (Table 4). These findings were not statistically significant.
Table 4.
Statistical analysis for mild-moderate disease based on treatment appropriateness and patient outcomes
| Negative patient outcomes | Under-treated | Appropriately treated | Over-treated | Pa |
|---|---|---|---|---|
| Total | 1 | 81 | 54 | |
| Death | 0 | 5 (6.2%) | 3 (5.6%) | 1.00 |
| CDI death | 0 | 2 (2.5%) | 3 (5.6%) | 0.39 |
| CDI ICU transfer | 0 | 1 (1.2%) | 1 (1.9%) | 1.00 |
| CDI-related colectomy | 0 | 2 (2.5%) | 0 | 0.52 |
aAppropriately treated versus over-treated.
In patients with severe disease, under-treated patients experienced more CDI-related complications, though these findings were not statistically significant (Table 5). In the 15 patients with severe disease who were under-treated, all-cause mortality was seen in 3 (20.0%) patients, 1 (6.7%) patient died of CDI-related causes, 3 (20.0%) patients had a CDI-related ICU transfer and 0 patients underwent a CDI-related colectomy. Among patients with severe disease who received appropriate treatment (27 in total), all-cause mortality was seen in 5 (18.5%) patients, 4 (14.8%) patients had a CDI-related death, 2 (7.4%) patients had a CDI-related ICU transfer and 1 (3.7%) patient had a CDI-related colectomy. Finally, among the six patients with severe disease who were over-treated, all-cause mortality was seen in one (16.7%) patient, one (16.7%) patient had a CDI-associated death and no patients experienced a CDI-related ICU transfer or colectomy (Table 5). While these findings are not statistically significant, certain trends can be appreciated. Under-treated patients had more CDI-related ICU transfers, at 20% of patients, compared with those who were appropriately treated (7.4%) (P = 0.33). Though not statistically significant, these results complement our findings that under-treated patients, regardless of disease severity, experienced more CDI-related ICU transfers (17.4%) as compared with the appropriately treated (4.8%) or over-treated (1.7%) patients (P = 0.0028 and P = 0.0031).
Table 5.
Statistical analysis for severe disease based on treatment appropriateness and patient outcomes
| Negative patient outcomes | Under-treated | Appropriately treated | Over-treated | Pa | Pb | Pc |
|---|---|---|---|---|---|---|
| Total | 15 | 27 | 6 | |||
| Death | 3 (20%) | 5 (18.5%) | 1 (16.7%) | 1.00 | 1.00 | 1.00 |
| CDI death | 1 (6.7%) | 4 (14.8%) | 1 (16.7%) | 0.70 | 0.64 | 0.65 |
| CDI ICU transfer | 3 (20%) | 2 (7.4%) | 0 | 0.38 | 0.33 | 0.31 |
| CDI-related colectomy | 0 (0%) | 1 (3.7%) | 0 | 1.00 | 1.00 | 1.00 |
aComparison across three groups.
bUnder-treated versus appropriately treated.
cUnder-treated versus appropriately treated plus over-treated.
Patients with severe-complicated disease were the most likely to be under-treated at 30 out of 46 patients (65.2%) (Table 6). Among these 30 patients, all-cause mortality was seen in 17 (56.7%) patients, 9 (30.0%) had a CDI-related death, 5 (16.7%) had a CDI-related ICU transfer and 1 (3.3%) patient had a CDI-related colectomy. Sixteen patients with severe-complicated disease were appropriately treated. Of these 16 patients, all-cause mortality was seen in 6 (37.5%) patients, 5 (31.3%) had CDI-associated deaths, 3 (18.8%) patients had a CDI-related ICU transfer and 1 (6.3%) patient had a CDI-related colectomy (Table 6). Patient outcomes based on treatment appropriateness in severe-complicated disease were not statistically significant. However, a trend towards increased mortality in under-treated patients can be appreciated.
Table 6.
Statistical analysis for severe-complicated disease based on treatment appropriateness and patient outcomes
| Negative patient outcomes | Under-treated | Appropriately treated | Over-treated | Pa |
|---|---|---|---|---|
| Total | 30 | 16 | 0 | |
| Death | 17 (56.7%) | 6 (37.5%) | 0 | 0.35 |
| CDI death | 9 (30%) | 5 (31.3%) | 0 | 1.00 |
| CDI ICU transfer | 5 (16.7%) | 3 (18.8%) | 0 | 1.00 |
| CDI-related colectomy | 1 (3.3%) | 1 (6.3%) | 0 | 1.00 |
aUnder-treated versus appropriately treated.
Discussion
This study sought to determine the adherence rate to SHEA-IDSA treatment guidelines for CDI at our institution and to evaluate the potential influence of these treatment decisions on patient outcomes. In this cohort of 230 patients with CDI, 20% were under-treated, 54% were appropriately treated and 26% were over-treated based on SHEA-IDSA guidelines (Table 2). These adherence data are similar to the findings of Curtin et al.,14 who observed 43.4% adherence to SHEA-IDSA guidelines among 290 patients with CDI.
Ultimately, these observations raise the question of whether appropriate risk stratification and treatment can improve patient outcomes. A prospective, randomized trial to address this question is not feasible for ethical reasons. Hence, observational studies, such as ours, are necessary.
Our study found that under-treatment of CDI was associated with a higher 90 day all-cause mortality at 43.5% versus 12.9% in those appropriately treated (P < 0.0001). Under-treatment was also associated with a higher rate of CDI-related ICU transfers at 17.4% versus 4.8% in patients who received appropriate treatment (P = 0.023) (Table 3). Patients with severe-complicated cases of CDI were the most likely to be under-treated at 65% of all under-treated cases. These patients had the highest all-cause 90 day mortality rate and rates of CDI-related ICU transfer. Over-treated patients, on the other hand, had the best outcomes.
Researchers at Texas Tech University found similar results in a retrospective case–control study analysing whether IDSA guideline-concordant therapy reduced rates of complications.16 Potential complications included infection recurrence, surgery to reduce complications of CDI, toxic megacolon and 30 day mortality. The study found that guideline-concordant treatment was higher in mild-moderate CDI and decreased as CDI complexity increased.16 Additionally, adherence to IDSA treatment guidelines was associated with a reduction in complications.16 These treatment adherence patterns and patient outcomes data reflect our study findings as well. Taken together, they suggest that adherence to treatment guidelines can help improve patient outcomes and lower healthcare costs.16
The findings from the above study and our research suggest that closer adherence to treatment guidelines may lead to better patient outcomes. Therefore, increased clinician education and quality improvement should be implemented to improve adherence to treatment guidelines. Jury et al.17 demonstrated that educating clinicians regarding treatment guidelines is possible and can lead to improved adherence. The research team initiated a hospital-wide education programme to improve adherence to SHEA-IDSA practice guidelines, which led to a significant reduction in non-adherence to SHEA-IDSA treatment guidelines. These findings provide support for continued clinician education that can improve treatment and patient care.
Several factors may be associated with the lack of adherence to SHEA-IDSA guidelines. Severe-complicated cases of CDI are fewer in number compared with mild-moderate and severe cases, which inherently leads to less everyday experience in treatment. The dual, oral plus intravenous, antibiotic therapy recommended for the treatment of severe-complicated CDI increases treatment complexity. Lack of enteral access for vancomycin may have contributed to some under-treatment of severe-complicated CDI cases, in addition to the lack of knowledge regarding the need for intravenous metronidazole in these cases. Delayed clinical recognition of severe-complicated CDI may also have been a contributing factor. Additionally, clinicians may be unaware of the SHEA-IDSA guidelines and their treatment recommendations.
There has been a long-standing debate since the 1980s as to the use of metronidazole versus vancomycin for the treatment of CDI. Current IDSA guidelines recommend metronidazole for mild-moderate CDI and vancomycin or vancomycin and metronidazole for severe and severe-complicated CDI.10 Our study revealed four patient deaths in the under-treated group who received metronidazole alone, representing 20% of all-cause deaths in the under-treated subset of patients.
Failure of metronidazole has been noted in two CDI-related studies. Researchers studied disease severity in the CDI strain NAP-1, a highly virulent strain of CDI found to produce up to 10 times more toxin than other strains. They noted that in 251 patients initially treated with metronidazole, 21.1% required switching to or adding vancomycin to their treatment regimen.18 Additionally, in a recent publication, metronidazole was found to be inferior to vancomycin in the treatment of CDI based on combined data from two large multicentre Phase III clinical trials that have provided the largest dataset thus far for randomized, controlled and double-blinded comparison of the two agents.19
Our results complement the findings from the above two studies. In our cohort, over-treated patients had the best outcomes including the lowest percentage of CDI and all-cause 90 day mortality and the lowest percentage of CDI-associated ICU transfer. In patients with mild-moderate CDI, over-treatment consisted of oral vancomycin rather than metronidazole. Thus, changing treatment guidelines to advocate vancomycin as a first-line agent may simplify treatment decisions and improve patient outcomes. CDI leads to increased VRE colonization and/or VRE-related complications.20 Data also suggest that the prevalence of VRE is the same in both vancomycin- and metronidazole-treated CDI patients.21 Newer treatments including fidaxomicin and faecal transplantation can serve as alternative agents. Additionally, liquid formulations of vancomycin are cost-effective and unlikely to be cost-prohibitive.
More recently, fidaxomicin and faecal transplantation have emerged as treatment options for CDI. These treatment options have focused mostly on CDI recurrence, while our study analysed initial episodes of CDI. More data are warranted to suggest the benefit of these newer therapeutic modalities as first-line treatment for initial episodes of CDI.
CDI treatment guidelines at our institution (BIDMC) incorporate criteria not included in the IDSA guidelines. BIDMC criteria include stratification into severe CDI for immunocompromised patients, patients with known inflammatory bowel disease, hypoalbuminaemia and a serum creatinine increase of 1.5× the patient's baseline. Determination of severe-complicated CDI includes ICU-level care for CDI, lactate >2.5 mmol/L, pressor requirement and confluent pseudomembranous colitis seen on colonoscopy.15 These additional CDI severity criteria lead to increased vancomycin use and underlie many of the ‘over-treated’ patients in this study.
Our internal CDI treatment guidelines are frequently presented to and discussed with trainees and faculty at educational events that include morning report, firm conference, grand rounds and attending rounds. It is relevant to note that our institutional CDI management guidelines now advocate the use of vancomycin as a first-line agent for CDI therapy in a broader patient population than that recommended in the 2010 SHEA-IDSA guidelines.
Our study has several limitations. As demonstrated in the Results section, patient outcomes based on disease severity and treatment appropriateness were not statistically significant. The lack of statistical significance may be attributed to insufficient power. Because under-treatment is different for each severity group based on IDSA guidelines, analysing each group separately is ideal. However, based on our study population of 230 patients, a separate analysis of each severity group would result in insufficient power.
Furthermore, based on SHEA-IDSA treatment guidelines, mild-moderate disease CDI is highly unlikely to be under-treated given their recommendations for 500 mg of metronidazole three times daily. Additionally, over-treatment for severe-complicated CDI is unlikely due to SHEA-IDSA recommendations for high-dose vancomycin in addition to intravenous metronidazole in these patients. Our results again support this observation.10 This inability to over- or under-treat is not apparent in patients with severe disease, based on SHEA-IDSA guidelines. In patients with severe disease, despite the lack of statistical significance and power, a trend towards increased CDI-related complications could be appreciated in under-treated patients. Patients with severe CDI who were under-treated were more likely to have a CDI-related ICU transfer (20%) compared with patients who were appropriately treated (7.4%, P = 0.33) or over-treated (0%). These results complement our additional findings that CDI-related ICU transfers were more common in under-treated patients (17.4%), regardless of disease severity, as compared with appropriately treated (4.8%) or over-treated (1.7%) patients and were 4.6 times more common overall in under-treated patients (17.4% versus 3.8%, P = 0.003).
Our findings point towards the possibility of larger studies specifically targeting patients with severe and severe-complicated CDI and analysing patient outcomes based on treatment appropriateness within each patient subgroup. We calculate that 269 patients with severe disease would be needed to demonstrate a 15% difference in all-cause mortality; this would require a cohort of 1287 CDI patients in total (20.9% with severe disease). Similarly, there was a trend towards increased mortality in under-treated severe-complicated CDI patients, at 56.7%, compared with appropriately treated patients, at 37.5%. Again, the lack of statistical significance may be due to insufficient power. Thus, future studies including a greater number of patients are necessary to investigate this question.
Another limitation of our study is that identifying CDI-related outcomes and deaths is a subjective determination. Additionally, during the process of stratifying disease severity, other causes not related to CDI may have attributed to changes in severity markers (white blood cell count, serum creatinine and hypotension). Similarly, the increased 90 day mortality in under-treated patients may not be secondary to CDI alone and other causes including non-aggressive care and comorbidities may have contributed to increased mortality in this subset of patients. There was also a significant difference in treatment appropriateness between different age groups (Table S1, available as Supplementary data at JAC Online). There was a tendency to under-treat older patients and a trend to over-treat younger patients. Greater disease severity in the older patients may also have contributed to this finding. Our study did collect data regarding CDI recurrence; however, because this endpoint was not one of the main outcomes of the study, statistical analysis was not conducted due to missing data. Additionally, data regarding readmission rates were not collected.
According to the CDC, CDI is a common and deadly nosocomial disease with nearly half a million infections in 2011 and 29 000 deaths within 30 days of initial CDI diagnosis.22 Thus, optimizing treatment for CDI is of central importance. Our study found that adherence to treatment guidelines was higher in mild-moderate cases of CDI and decreased as disease severity increased. We also found lower all-cause mortality in patients who were treated in adherence to SHEA-IDSA treatment guidelines. Under-treated patients had a higher rate of CDI-related ICU transfers and higher all-cause 90 day mortality. Modifications to the guidelines to simplify treatment choices and to encourage earlier use of vancomycin may be beneficial.
Funding
This work was supported by the National Institutes of Health (RO1 AI 095256, U19 AI 109776, R01 AI 116596 and R21 AI 103612) to C. P. K.
C. D. A. reports research funding from Merck.
Transparency declarations
C. D. A. is on the advisory board for Merck and Sanofi Pasteur. D. A. L. has been a consultant and/or provided research support for Alba Therapeutics, Alvine Pharmaceuticals, INOVA Diagnostics, Genzyme, Coronado Biosciences, Sidney Frank Foundation, Pfizer and GI Supply. All other authors: none to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Supplementary Material
References
- 1. Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. New Engl J Med 2008; 359: 1932–40. [DOI] [PubMed] [Google Scholar]
- 2. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. New Engl J Med 1994; 330: 257–62. [DOI] [PubMed] [Google Scholar]
- 3. Bartlett JG. Antibiotic-associated diarrhea. Clin Infect Dis 1992; 15: 573–81. [DOI] [PubMed] [Google Scholar]
- 4. O'Brien JA, Lahue BJ, Caro JJ et al. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol 2007; 28: 1219–27. [DOI] [PubMed] [Google Scholar]
- 5. Miller BA, Chen LF, Sexton DJ et al. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 2011; 32: 387–90. [DOI] [PubMed] [Google Scholar]
- 6. Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect 2012; 18Suppl 6: 5–12. [DOI] [PubMed] [Google Scholar]
- 7. Laffan AM, Bellantoni MF, Greenough WB III et al. Burden of Clostridium difficile-associated diarrhea in a long-term care facility. J Am Geriatr Soc 2006; 54: 1068–73. [DOI] [PubMed] [Google Scholar]
- 8. Minino AM, Murphy SL, Xu J et al. Deaths: final data for 2008. Nat Vital Stat Rep 2011; 59: 1–126. [PubMed] [Google Scholar]
- 9. Lessa FC, Mu Y, Bamberg WM et al. Burden of Clostridium difficile infection in the United States. New Engl J Med 2015; 372: 825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen SH, Gerding DN, Johnson S et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31: 431–55. [DOI] [PubMed] [Google Scholar]
- 11. Surawicz CM, Brandt LJ, Binion DG et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108: 478–98; quiz 499. [DOI] [PubMed] [Google Scholar]
- 12. Bauer MP, Kuijper EJ, van Dissel JT et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect 2009; 15: 1067–79. [DOI] [PubMed] [Google Scholar]
- 13. Aroori S, Blencowe N, Pye G et al. Clostridium difficile: how much do hospital staff know about it? Ann R Coll Surg Engl 2009; 91: 464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curtin BF, Zarbalian Y, Flasar MH et al. Clostridium difficile-associated disease: adherence with current guidelines at a tertiary medical center. World J Gastroenterol 2013; 19: 8647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Interdisciplinary Practice Guidelines for the Diagnosis and Treatment of Clostridium difficile Infection (CDI). https://portal.bidmc.org.
- 16. Brown AT, Seifert CF. Effect of treatment variation on outcomes in patients with Clostridium difficile. Am J Med 2014; 127: 865–70. [DOI] [PubMed] [Google Scholar]
- 17. Jury LA, Tomas M, Kundrapu S et al. A Clostridium difficile infection (CDI) stewardship initiative improves adherence to practice guidelines for management of CDI. Infect Control Hosp Epidemiol 2013; 34: 1222–4. [DOI] [PubMed] [Google Scholar]
- 18. Cloud J, Noddin L, Pressman A et al. Clostridium difficile strain NAP-1 is not associated with severe disease in a nonepidemic setting. Clin Gastroenterol Hepatol 2009; 7: 868–73.e2. [DOI] [PubMed] [Google Scholar]
- 19. Johnson S, Louie TJ, Gerding DN et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59: 345–54. [DOI] [PubMed] [Google Scholar]
- 20. Poduval RD, Kamath RP, Corpuz M et al. Clostridium difficile and vancomycin-resistant Enterococcus: the new nosocomial alliance. Am J Gastroenterol 2000; 95: 3513–5. [DOI] [PubMed] [Google Scholar]
- 21. Al-Nassir WN, Sethi AK, Li Y et al. Both oral metronidazole and oral vancomycin promote persistent overgrowth of vancomycin-resistant enterococci during treatment of Clostridium difficile-associated disease. Antimicrob Agents Chemother 2008; 52: 2403–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. CDC. Healthcare-Associated Infections (HAIs). http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_clinicians.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.