Abstract
We present the first-published cochlear implant in an adolescent with sickle cell anemia (SCA) and bilateral profound sensorineural hearing loss (SNHL). A 15-year-old Saudi girl, previously diagnosed with SCA, developed gradual bilateral permanent profound SNHL over 18 months and underwent a successful cochlear implantation. SNHL associated with sickle cell crises is a well-known phenomenon. Regular hearing assessments are recommended for sickle cell patients, and early referrals for cochlear implantations are advocated for those with permanent profound SNHL.
Sensorineural hearing loss (SNHL) is a recognized complication in patients with sickle cell disease (SCD). It has been reported that patients with SCD have a much higher incidence of SNHL than the general population, with a varying degrees of severity. 1–4 There are many case reports of unilateral SSNHL in sickle cell patients5–7 and 2 previously published cases of bilateral profound SSNHL, including 1 child who received a cochlear implant.8,9 This case report describes the first cochlear implant in an adolescent with SCD and bilateral profound SNHL.
CASE
A 15-year-old Saudi girl who had been previously diagnosed with sickle cell anemia (SCA) developed gradual bilaterally decreased hearing over 18 months, which began in her left ear and spread to her right ear 3 months later. She reported occasional tinnitus in both ears and denied experiencing earache, vertigo, or ear discharge. A hearing aid trial produced no benefit for her profound hearing loss. The patient was referred to our hospital for a further hearing evaluation and for consideration for a cochlear implant.
The patient was diagnosed with SCA in early infancy after developing kernicterus in the first week of life. At that time, she was admitted to a neonatal intensive care unit for 10 days and received 2 blood transfusions. At the age of 5, she began to experience recurrent acute sickle cell crises in the form of severe back and joint pain, nausea and vomiting, low-grade fever, and acute abdominal pain, primarily in the winter and in times of stress, such as before exams. She had a history of multiple previous admissions for medical management and blood transfusions until the age of 10.
Upon examination, the patient was clinically normal apart from her deafness, and her head, neck, and cranial nerve examination was normal. She was a good lip reader, and her speech had been maintained.
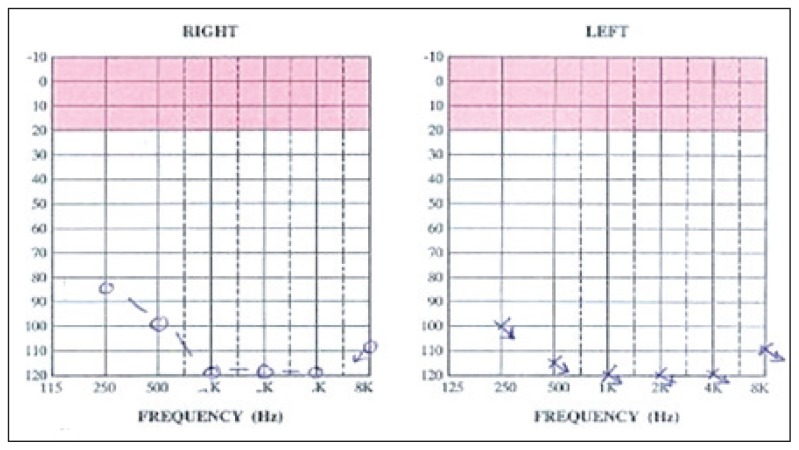
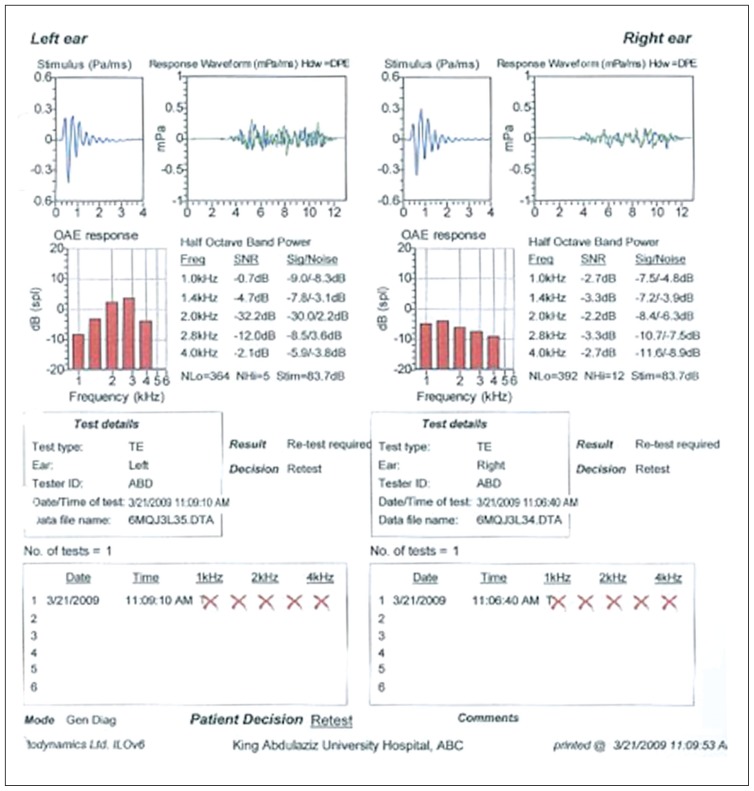
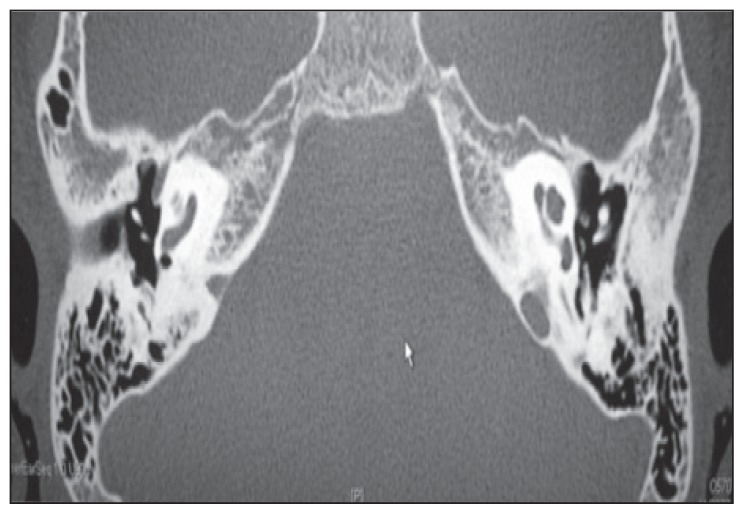
In the auditory evaluation, a pure-tone audiogram showed bilateral profound SNHL (Figure 1). Impedance audiometry revealed type A tympanograms bilaterally. Otoacoustic emissions were absent (Figure 2). Radiological investigations, including a temporal bone CT scan with thin cuts, demonstrated no abnormalities in the inner and middle ear structures other than bilateral prominent jugular bulbs (Figure 3). A laboratory investigation revealed a hemoglobin (Hb) level of 8.2, a hematocrit of 23.9, a total bilirubin of 80 (direct=11 and indirect=69), and a sickle hemoglobin (HbS) percentage of 83%.
Figure 1.
Pure-tone audiograms showing bilateral profound SNHL.
Figure 2.
Octoacoustic emissions were absent in both ears.
Figure 3.
CT scan bone window showing an axial cut of the temporal bones without contrast through the level of the promontory, revealing normal anatomy.
The patient underwent a blood exchange as preoperative preparation for a left cochlear implant (the HbS after the blood exchange reached 53.5%). The surgery went smoothly, and the patient had an uneventful recovery. The patient became a successful user of the device, with a sound-field audiogram at 30 dB. She wears the implant at all times, and she understands approximately 70% of the usual conversation with her family.
DISCUSSION
SCD is a family of Hb disorders in which a defective sickle beta-globin gene is inherited. The gene for sickle HbS contains a substitution of the amino acid valine for the glutamic acid normally present at position 6 of the beta chain of Hb. In the homozygous state (SCA), both genes are abnormal (Hb SS), whereas in the heterozygous state (sickle cell trait, Hb AS), only 1 chromosome carries the abnormal gene. The inheritance of the Hb S gene from 1 parent and the Hb C gene from the other parent gives rise to Hb SC disease, which tends to consist of a milder clinical course than SCD, albeit with more frequent thrombosis.
In Hb SS, the Hb molecule becomes deformed (insoluble) in its deoxygenated state. Rigid tubular spiral bodies are formed, which deform the red cells into a sickle shape. Irreversibly, sickled red cells have a reduced lifespan because of the premature destruction of red cells (hemolysis) and may become trapped in the microcirculation, resulting in thrombosis and distal ischemia. This phenomenon is exacerbated by low oxygen tension, dehydration, and cold. Because the production of fetal hemoglobin (HbF) is normal, the disease does not usually manifest until the HbF decreases to adult levels at approximately 6 months of age.
The reported pattern of hearing loss in SCD varies, but it is well recognized. Larger series have reported an increased incidence of high-tone sensorineural deafness by 12% to 22% in SCD than in control groups.10,11 A prospective controlled study conducted over 27 months in Qatif Central Hospital in Saudi Arabia showed an incidence of 19% of SNHL in SCD patients than in a control group. A significant relationship was observed between SNHL and the onset of the first vaso-occlusive crisis at 6 years of age or younger.12 In our case, the patient was diagnosed in early life with SCD after experiencing kernicterus, but high bilirubin levels did not cause prelingual hearing loss, even with a childhood history of multiple crises and hemolysis accompanied by high bilirubin levels.
The etiology of idiopathic SNHL is still obscure. Four popular theories posit that the cause is vascular, viral, autoimmune, or rupture of Reissner membrane. In sickle cell patients with hearing loss, the vascular cause is the main suspect, an assertion supported by Morgenstein and Manace, who examined the temporal bones of a 10-year-old boy with SCD and mild bilateral SNHL. They described degeneration of the organ of Corti consistent with diffuse ischemia.13 Studies confirming that there are changes in the inner ear structures in sickle cell patients with SNHL include the study by Whitehead, which showed labyrinthine hemorrhage by magnetic resonance imaging.5
Our patient experienced progressive hearing loss in late childhood, which could be because of the frequent SCD crises she experienced. Berry14 found that SCD patients who experienced more frequent crises exhibited poorer auditory thresholds. However, Todd et al10 noted that older subjects frequently demonstrated more significant hearing loss and suggested that the increased incidence of hearing loss with aging may be the result of cumulative sickle crises.
Friedman et al11 suggested that marrow space expansion in the petrous temporal bone could cause compression of the eighth cranial nerve in the auditory canal, resulting in deafness. This is another possible explanation for the gradual pattern of hearing loss that our patient demonstrated. In contrast, Serjeant examined the above theory and found no correlation between abnormal audiograms and the internal auditory meatus dimensions of patients with SCD.15
In conclusion, SNHL associated with sickle cell crises is a well-known complication of SCD. This is the first published case of SCD with bilateral profound hearing loss in adolescence successfully treated with a cochlear implantation. Regular hearing assessments are recommended for early detection and intervention in sickle cell patients, and cochlear implantations are advocated for those with permanent profound SNHL.
Acknowledgments
The authors would like to thank Prince Sultan Research Chair for hearing disability and implantable devices for the support.
REFERENCES
- 1.Samperi P, Bertuna G, Rossi G, Poli G, Serra A. Sensorineural hearing loss in sickle cell disease patients in Sicily. Minerva Pediatr. 2005;57:285–288. [PubMed] [Google Scholar]
- 2.Mgbor N, Emodi I. Sensorineural hearing loss in Nigerian children with sickle cell disease. Int J Pediatr Otorhinolaryngol. 2004;68:1413–1416. doi: 10.1016/j.ijporl.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Ajulo SO, Osiname AI, Myatt HM. Sensorineural hearing loss in sickle cell anaemia--a United Kingdom study. J Laryngol Otol. 1993;107:790–794. doi: 10.1017/s0022215100124442. [DOI] [PubMed] [Google Scholar]
- 4.Jovanovic-Bateman L, Hedreville R. Sensorineural hearing loss with brainstem auditory evoked responses changes in homozygote and heterozygote sickle cell patients in Guadeloupe (France) J Laryngol Otol. 2006;120:627–630. doi: 10.1017/S0022215106001678. [DOI] [PubMed] [Google Scholar]
- 5.Whitehead RE, MacDonald CB, Melhem ER, Mc-Mahon L. Spontaneous labyrinthine hemorrhage in sickle cell disease. AJNR Am J Neuroradiol. 1998;19:1437–1440. [PMC free article] [PubMed] [Google Scholar]
- 6.Tavin ME, Rubin JS, Camacho FJ. Sudden sensorineural hearing loss in haemoglobin SC disease. J Laryngol Otol. 1993;107:831–833. doi: 10.1017/s0022215100124557. [DOI] [PubMed] [Google Scholar]
- 7.Hotaling AJ, Hillstrom RP, Bazell C. Sickle cell crisis and sensorineural hearing loss: case report and discussion. Int J Pediatr Otorhinolaryngol. 1989;17:207–211. doi: 10.1016/0165-5876(89)90047-5. [DOI] [PubMed] [Google Scholar]
- 8.O’Keeffe LJ, Maw AR. Sudden total deafness in sickle cell disease. J Laryngol Otol. 1991;105:653–655. doi: 10.1017/s0022215100116949. [DOI] [PubMed] [Google Scholar]
- 9.Mace AD, Ferguson MS, Offer M, Ghufoor K, Wareing MJ. Bilateral profound sudden sensorineural hearing loss presenting a diagnostic conundrum in a child with sickle cell anaemia. J Laryngol Otol. 2009;123:811–816. doi: 10.1017/S0022215108003617. [DOI] [PubMed] [Google Scholar]
- 10.Todd GB, Serjeant GR, Larson MR. Sensorineural hearing loss in Jamaicans with SS disease. Acta Otolaryngol. 1973;76:268–272. doi: 10.3109/00016487309121507. [DOI] [PubMed] [Google Scholar]
- 11.Friedman EM, Herer GR, Luban NL, Williams I. Sickle cell anemia and hearing. Ann Otol Rhinol Laryngol. 1980;89:342–347. doi: 10.1177/000348948008900409. [DOI] [PubMed] [Google Scholar]
- 12.Al Dabbous IA, Al Jam’a AH, Obeja SK, Murugan AN, Hammad HA. Sensorineural hearing loss in homozygous sickle cell disease in Qatif, Saudi Arabia. Ann Saudi Med. 1996;16:641–644. doi: 10.5144/0256-4947.1996.641. [DOI] [PubMed] [Google Scholar]
- 13.Morgenstein KM, Manace ED. Temporal bone histopathology in sickle cell disease. Laryngoscope. 1969;79:2172–2180. doi: 10.1288/00005537-196912000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Berry RA. Sickle cell anemia: audiological findings. J Am Audiol Soc. 1975;1:61–63. [PubMed] [Google Scholar]
- 15.Serjeant GR, Norman W, Todd GB. The internal auditory canal and sensori-neural hearing loss in homozygous sickle cell disease. J Laryngol Otol. 1975;89:453–455. doi: 10.1017/s0022215100080580. [DOI] [PubMed] [Google Scholar]