Abstract
Acroangiodermatitis (AAD) (synonym, pseudo-Kaposi sarcoma) is a term that encompasses 2 different conditions: (1) AAD of Mali, which refers to skin lesions that mainly develop bilaterally on the lower extremities of patients with chronic venous insufficiency and is an extreme form of stasis dermatitis and (2) Stewart–Bluefarb syndrome, which consists of an arteriovenous malformation that mainly affects the limbs of young patients unilaterally. We present a case of a 68-year-old lady with progressive skin lesions on both lower limbs (right > left) as a result of chronic venous insufficiency that became worse after the leg-vein harvest for coronary artery bypass grafting was taken from the right leg. Up to our knowledge this is the first case of its kind to be reported.
Acroangiodermatitis (AAD), often known as pseudo-Kaposi sarcoma, is a vaso-proliferative disease characterized by a reactive proliferation of small blood vessels in response to congenital or acquired vascular alterations. The lesions initially appear as circumscribed, slowly-evolving, red violaceous or dusky macules, papules, or plaques and sometimes become verrucous or ulcerated.1–3
There are two main clinical variants of AAD—the Mali-type, associated with venous hypertension, and the Stewart-Bluefarb type, associated with arteriovenous malformation (AVM) or acquired arteriovenous fistula (iatrogenic as in patients with chronic renal failure or traumatic). It is also described as being linked with paralysis of the affected limb, congenital myopathy,4 amputation stumps,5 the use of poorly fitting suction-type prosthesis,6 20210A mutation in the prothrombin gene,7 activated protein c resistance,8 and syndromes such as the Prader-Labhart-Willi and Klippel-Trenaunay syndrome. 9 A case of idiopathic pseudo–Kaposi sarcoma is recently reported.10 AAD is also reported in pregnancy and appears as gravity purpura (Dermite ocre of Favre) on lower legs over site of venous varicosities extending to the dorsa of feet and toes.11
We present a case of AAD of Mali in a patient who presented with chronic venous insufficiency that became worse after leg-vein harvest for coronary artery bypass grafting (CABG) taken from the right leg.
CASE
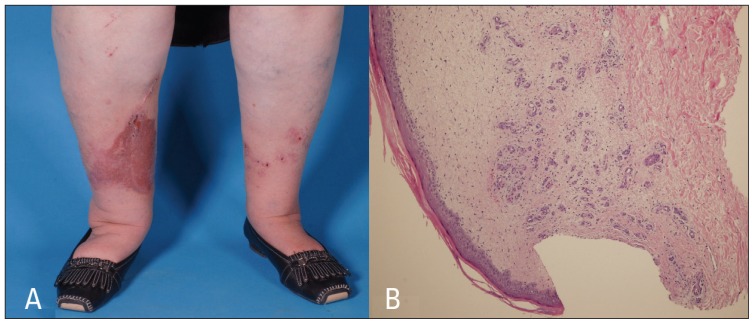
A 68-year-old lady, known to have insulin-dependent diabetes mellitus with nephropathy, retinopathy, hypertension, dyslipidemia, and left carotid stenosis status after left carotid endarterectomy presented to our facility with itchy skin lesions over both legs of few weeks duration. Prior to the second follow-up visit, the patient had undergone right leg-vein harvest for CABG and the skin lesions in the right leg progressively increased in size (Figure 1A).
Figure 1.
A) Multiple well-defined erythematous to violaceous indurated scaly papules and plaques with some excoriated papules on both lower legs (more in right leg, at the site of the surgical scar). B) Lobular proliferation of small capillaries in a loose stroma with extravasated erythrocytes and a sparse mononuclear cell infiltrate throughout the upper dermis. Interstitial fibroblast cells are slightly increased in number. Hyperkeratosis and mild spongiosis of the overlying epidermis. (Hematoxylin eosin stain 1×10).
On physical examination, she is an obese lady with multiple well-defined erythematous to violaceous indurated scaly papules and plaques (of variable sizes) with some excoriated papules on both lower legs (more in right leg, at the site of the surgical scar) with varicose veins and bilateral lower limb pitting edema. Mucous membranes, nails, and hair were normal.
Histologic examination from both the legs showed lobular proliferation of small capillaries in a loose stroma with extravasated erythrocytes and a sparse mononuclear cell infiltrate throughout the upper dermis. Interstitial fibroblast cells were slightly increased in number, mainly along thickened and sclerotic collagen bundles that were seen adjacent to an area of more pronounced vascular proliferation with hyperkeratosis and mild spongiosis of the overlying epidermis (Figure 1B)
All routine laboratory results were normal. Protein S, protein C, and antithrombin III were normal. Venous Doppler examination of legs showed reflux in the right greater saphenous vein junction and popliteal junction.
The patient was treated with oral erythromycin, which was planned to be increased up to 2 g/d. As the patient could not tolerate high dose of erythromycin, she was put on 1 g/d in the first 6 weeks, then decreased to 500 mg/d for another 6 weeks without improvement. She also received azithromycin 500 mg daily for 3 months with little improvement and subsequent worsening of the lesions. The patient was referred to the physiotherapist for compression therapy. After 5 months of compression therapy, an improvement in the condition was observed, although complete regression was not achieved.
DISCUSSION
In 1964, Kopf and Gonzalez12 first described a condition that they termed as congenital dysplastic angiopathy. One year later, Mali et al13 introduced the term AAD to describe peculiar mauve-colored macules and plaques developing on the extensor surface of the feet in 18 patients with chronic venous insufficiency. In 1967, Stewart14 and Bluefarb and Adams15 independently reported the same condition described by Mali et al,13 but this time it was associated with congenital AVM. In 1974, Earhart et al.16 reported a case associated with congenital AVM and suggested the name pseudo-Kaposi sarcoma because of the clinical and histopathological similarity with early Kaposi’ sarcoma.
Although the etiology is still unknown, some authors proposed that proliferation of fibroblasts and small vessels is secondary to high perfusion rate in tissues. Additionally, prostaglandin E1 or a heparin-like factor is responsible for the lesion development.1 A possible role of microtrauma has been suggested.17 Other reports showed local increases of vascular endothelial growth factors in hypoxic conditions, which play an important part in angiogenesis. Products released during mast cell degranulation, such as heparin, histamine, and TNF-α, are also responsible for neoangiogenesis by stimulation of the stem cell factor.18
Histologic examination usually shows a slight proliferation of endothelial cells with the formation of new thick-walled vessels invested by pericytes, often in a lobular arrangement, in the papillary dermis. Extravasation of red blood cells; hemosiderin pigment deposition; dermal fibrosis; and a superficial perivascular infiltrate consisting of lymphocytes, histiocytes, and eosinophils are additional findings. Small luminal thrombi may be seen.1,2
We considered diffuse dermal angiomatosis in our differential diagnosis because our patient had violaceous plaques mainly on the lower extremities with a history of peripheral vascular atherosclerotic disease. But it was excluded histologically as the process was limited to the papillary and upper reticular dermis, there was some lobular nature to it, and there was associated marked edema—all pointing to the diagnosis of AAD.
The absence of proliferation of spindle-shaped CD34+ cells and of vascular slits, which are typical of Kaposi sarcoma, can help in the differential diagnosis with this condition.16 In pseudo-Kaposi sarcoma, unlike classic Kaposi sarcoma, human herpes virus type 8 is not detected and the endothelial cells are positive for factor VIII.19,20
Color Doppler scan, MRI angiography, and arteriography are useful in detecting chronic venous insufficiency or an arteriovenous shunt. Laboratory testing must investigate for alterations of antithrombin III, protein C, protein S, and for the presence of mutations in the prothrombin G20210A and methylene tetrahydrofolate reductase C677T genes.7,21
Treatment of AAD tends to be conservative. Most of the treatment reports are of anecdotal nature. Mainstays of therapy include compression stockings or a compression pump for venous stasis and local wound care for ulcers. In the case of AVMs, embolization, sclerotherapy, or surgery can be employed1 and anticoagulants for thrombophilic conditions lead to the resolution of the cutaneous disease.7,8 Medical therapy options are limited. There is one case report of regression of lesions with treatment with oral dapsone 50 mg bid for 3 months in combination with leg elevation and compression.22 Stanozolol and erythromycin have also been used successfully.23,24 Their exact mode of action is still unknown, but erythromycin appears to have anti-inflammatory effects, and has shown to inhibit the chemotaxis of leukocytes, monocytes, and eosinophils.1 However erythromycin was not effective in our patient.
Acknowledgment
We acknowledge the help of our dermatopathologist, Dr. Abdulmonem Almutawa for his valuable histological description and photomicrographs.
REFERENCES
- 1.Samad A, Dodds S. Acroangiodermatitis: review of the literature and report of a case associated with symmetrical foot ulcers. Eur J Vasc Endovasc Surg. 2002;24(6):558–560. doi: 10.1053/ejvs.2002.1772. [DOI] [PubMed] [Google Scholar]
- 2.Rongioletti F, Rebora A. Cutaneous reactive angiomatosis: patterns and classification of reactive vascular proliferation. J Am Acad Dermatol. 2003;49(5):887–896. doi: 10.1016/s0190-9622(03)02100-5. [DOI] [PubMed] [Google Scholar]
- 3.Hung NA, Strack M, Van Rij A, North CJ, Blennerhassett JB. Spontaneous acroangiodermatitis in a young woman. Dermatol Online J. 2004;10:8. [PubMed] [Google Scholar]
- 4.Jindal R, Dipankar D, Dogra S, Saikia UN, Kanwar AJ. Acroangiodermatitis of Mali in a patient with congenital myopathy. Dermatol Online J. 2010;16:4. [PubMed] [Google Scholar]
- 5.Güçlüer H, Gürbüz O, Kotiloglu E. Kaposi-like acroangiodermatitis in an amputee. Br J Dermatol. 1999;141:380–91. doi: 10.1046/j.1365-2133.1999.03017.x. [DOI] [PubMed] [Google Scholar]
- 6.Sbano P, Miracco C, Risulo M, Fimiani M. Acroangiodermatitis (pseudo-Kaposi sarcoma) associated with verrucous hyperplasia induced by suction-socket lower limb prosthesis. J Cutan Pathol. 2005;32(6):429–432. doi: 10.1111/j.0303-6987.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin L, MacHet L, Michalak S, et al. Acroangiodermatitis in a carrier of the thrombophilic 20210A mutation in the prothrombin gene. Br J Dermatol. 1999;141(4):752. doi: 10.1046/j.1365-2133.1999.03127.x. [DOI] [PubMed] [Google Scholar]
- 8.Scholz S, Schuller-Petrovic S, Kerl H. Mali acroangiodermatitis in homozygous activated protein C resistance. Arch Dermatol. 2005;141(3):396–397. doi: 10.1001/archderm.141.3.396. [DOI] [PubMed] [Google Scholar]
- 9.Lyle WG, Given KS. Acroangiodermatitis (pseudo-Kaposi’s sarcoma) associated with Klippel-Trénaunay syndrome. Ann Plast Surg. 1996;37:654–6. doi: 10.1097/00000637-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Sang HC, Eujin C, Jeong DL, Sang HC, Young BL. A case of unusual idiopathic pseudo-kaposi sarcoma. Journal of the American Academy of Dermatology. 2010;62(3) Supplement 1:AB33. [Google Scholar]
- 11.Lugovic L, Pusic J, Situm M, Buljan M, Vedrana B, Klaudija S, et al. Acroangiodermatitis (Pseudo-Kaposi sarcoma): Three case reports. Acta Dermatovenerol Croat. 2007;15:152–7. [PubMed] [Google Scholar]
- 12.Kopf AW, Gonzalez V. Congenital dysplastic angiopathy of the skin and underlying tissues. Arch Dermatol. 1964;90:360–362. [Google Scholar]
- 13.Mali JWH, Kuiper JP, Hamers AA. Acroangiodermatitis of the foot. Arch Dermatol. 1965;92:515–518. [PubMed] [Google Scholar]
- 14.Stewart WM. Fausse angiosarcomatose de Kaposi par fistules arterioveneuses multiples. Bull Soc Fr Derm Syph. 1967;74:664–665. [PubMed] [Google Scholar]
- 15.Bluefarb SM, Adams LA. Arteriovenous malformation with angiodermatitis. Stasis dermatitis simulating Kaposi’s disease. Arch Dermatol. 1967;96(2):176–181. [PubMed] [Google Scholar]
- 16.Earhart RN, Aeling JA, Nuss DD, Mellette JR. Pseudo-Kaposi Sarcoma. Arch Dermatol. 1974;110:907–910. doi: 10.1001/archderm.110.6.907. [DOI] [PubMed] [Google Scholar]
- 17.Pfleger L. Zur pathogenes unklarer purpuraformen. Arch f Dermatologie u Syphilis. 1954;197:187–208. [PubMed] [Google Scholar]
- 18.Ikeda E, Kano E, Baba S, Suzuki H. Mast cells in pseudo-Kaposi’s sarcoma lesions. J Eur Acad Dermatol Venereol. 2001;15(5):487–489. doi: 10.1046/j.1468-3083.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- 19.Kanitakis J, Narvaez D, Claudy A. Expression of the CD34 antigen distinguishes Kaposi’s sarcoma from pseudo-Kaposi’s sarcoma (acroangiodermatitis) Br J Dermatol. 1996;134(1):44–46. [PubMed] [Google Scholar]
- 20.Agrawal S, Rizal A, Agrawal CS, Anshu A. Pseudo-Kaposi’s sarcoma (Bluefarb-Stewart type) Int J Dermatol. 2005;44(2):136–138. doi: 10.1111/j.1365-4632.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- 21.Goldblum OM, Kraus E, Bronner AK. Pseudo-Kaposi’s sarcoma of the hand associated with an acquired, iatrogenic arteriovenous fistula. Arch Dermatol. 1985;121(8):1038–1040. [PubMed] [Google Scholar]
- 22.Rashkovsky I, Gilead L, Schamroth J, Leibovici V. Acroangiodermatitis: review of the literature and report of a case. Acta Derm Venereol. 1995;75(6):475–478. doi: 10.2340/0001555575475478. [DOI] [PubMed] [Google Scholar]
- 23.Boyle J, Burton JL. Pseudo-Kaposi sarcoma. Lancet. 1986;2(8512):921. doi: 10.1016/s0140-6736(86)90442-3. [DOI] [PubMed] [Google Scholar]
- 24.Kim TH, Kim KH, Kang JS, Kim JH, Hwang IY. Pseudo-Kaposi’s sarcoma associated with acquired arteriovenous fistula. J Dermatol. 1997;24(1):28–33. doi: 10.1111/j.1346-8138.1997.tb02734.x. [DOI] [PubMed] [Google Scholar]