Abstract
BACKGROUND AND OBJECTIVES
β-thalassemia major is one of the most frequent hematological genetic disorders, worldwide. Chemokines are the main components of the immune system and play fundamental roles in pathogenesis of inflammatory disorders. Therefore, the present study aimed to examine whether serum CXC chemokines are altered in β-thalassemia major patients.
DESIGN AND SETTINGS
We enrolled 63 β-thalassemia patients and 80 controls in this cross-sectional study, which was performed during 2012–2013 in Kerman, Iran.
METHODS
We enrolled 63 β-thalassemia patients and 80 controls in the present study. Patients were selected from referrals to Samenolhojaj clinic for thalassemia, Kerman, Iran. The circulating levels of CXCL1, CXCL9, CXCL10, and CXCL12 were detected by enzyme-linked immunosorbent assay in thalassemia patients and healthy controls immediately after blood collection. Data were analyzed by χ2, t-test, and analysis of variance statistical methods and using SPSS, version 13 (SPSS Inc., Chicago, IL).
RESULTS
The results of the study demonstrated a significant elevation of CXCL1, CXCL9, CXCL10, and CXCL12 in thalassemia patients than in control. These results also demonstrated that serum chemokine levels are related to transfusion duration and post-transfusion viral infections.
CONCLUSION
According to the results obtained, it can probably be concluded that chemokines are also involved in the pathogenesis of β-thalassemia major and its clinical complications in addition to several other parameters.
Thalassemia is described as a heterogeneous group of autosomal recessive inherited anemia resulting from either reduced level or absence of the globin chains of hemoglobin (Hb) that form the heterotetrameric structure. Thalassemia is classified into the following 2 main subclasses on the basis of Hb structure: (a) β-thalassemia, which result from functional mutations in genes that code for β-globin genes and (b) β-thalassemia, which result from mutations in genes that code for β-globin genes.1,2
Several parameters and statuses such as carrier identification, genetic counseling programs, and prenatal diagnosis are required for thalassemia prevention.3 The incidence of symptomatic individuals is estimated at 1 in 100 000 and 1 in 10 000 worldwide and in the European Union countries, respectively.4
Thalassemia is prevalent in different geographical regions and ethnic groups. The disorder is mostly prevalent in the Eastern Mediterranean, South Asia, and South East Asian countries.5
According to the latest reports from the Prenatal Diagnosis Team for Prevention of Thalassemia program of Iran, approximately 4% to 5% of Iranians are the carriers of β-thalassemia. This rate is more in some provinces, for example, in Mazandaran—a northern province of Iran, where about 10% of the population are carriers.6
Infection and immune system abnormalities are most often considered as the main causes of morbidity and mortality in β-thalassemia. A wide range of immune abnormalities are associated with β-thalassemia in patients with a history of multiple transfusions.7–12 Multiple transfusions are responsible for immune abnormalities. In addition to causing iron overload, repetitive transfusions lead to continuous alloantigenic stimulation. Additionally, transfusion increases the risk of transmission of viruses with immuneosuppressive properties, such as cytomegalovirus, human immunodeficiency virus, Ebstein–Barr virus, hepatitis B virus (HBV), and hepatitis C viruses (HCV).13 Chemokines play key roles in pathogenesis of inflammatory disorders of the immune system.14,15 They are functionally active in several important biological activities, which vary from recruitment of immune and hematological cells to the injured and infected tissues, stem cell homing, and mobilization to making balance between angiogenesis and angiostasis. Chemokines are classified into the following four subclasses according to the position of cystein motifs in their structure: C, CC, CX3C, and CXC.16–20 Although, elevated serum levels of TNF-α and IFN-α (critical molecules involved in inflammatory responses) are well evidenced in thalassemia,21,22 sufficient information regarding the role of chemokines is not available in pathogenesis of thalassemia. Therefore, we hypothesized that a relationship between serum chemokines and inflammatory responses in thalassemia patients may be established because of the presence of active inflammation in these patients. Thus, the present study aimed to determine the serum levels of chemokines CXCL1, CXCL9, CXCL10, and CXCL12 in β-thalassemia major patients.
METHODS
Study subjects
In the current study we enrolled 63 β-thalassemia major patients and 80 controls. The patients were selected from referrals to Samenolhojaj especial clinic for thalassemia, Kerman, Iran. All patients suffered from the homozygous form of β-thalassemia major, and regularly received transfusion to maintain their Hb at a balanced level. Peripheral blood specimens were collected approximately 24 hours before transfusion with washed cells to be sure that the circulating blood belongs to the patient and is not the transfused blood. Patients and controls with any acute illness or pathological injuries were not enrolled in the study. This project was approved by the regional ethical committee of Rafsanjan University of Medical Sciences. Written consent forms were also filled out by the parents of all patients and controls prior to sample collection.
Chemokine assay
The circulating levels of CXCL1, CXCL9, CXCL10, and CXCL12 were detected by enzyme-linked immunosorbent assay (R&D systems, UK) in thalassemia patients and healthy controls immediately after blood collection. Ninety-six–well EIA/RIA (enzyme immunoassay/radio Immunoassay) plates were coated with a capturing monoclonal antibody against CXCL1, CXCL9, CXCL10, and CXCL12 chemokines and were then blocked with a mixture of 1% bovine serum albumin (BSA). The supernatants were diluted in reagent diluents (1% of BSA) and incubated for 24 hours at room temperature. The detection antibody was then diluted in reagent diluents and incubated for 2 hours at room temperature. Antibody binding was detected with streptavidin-conjugated horseradish peroxides and developed with a substrate solution. A standard curve was generated for each set of samples assayed and was made from 7 points of a twofold dilution series. Each standard or sample was assayed in duplicate. The sensitivity of kits was 2 pg/mL, and inter- and intra-assay assessments of reliability of the kits were conducted.
Statistical analysis
The statistical analysis of the differences between groups was determined by χ2, t test, and analysis of variance using SPSS, version 13 (SPSS Inc., Chicago, IL) to achieve a power of 90%. A P value of less than .05 was considered significant.
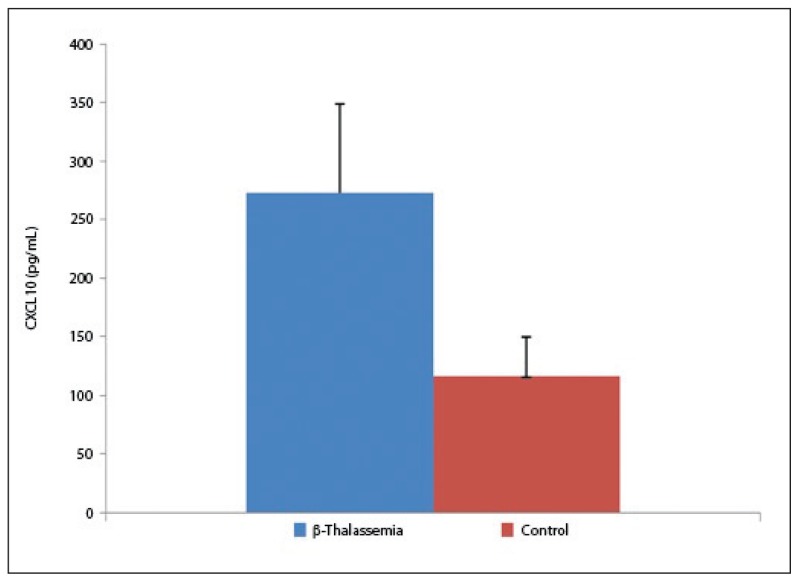
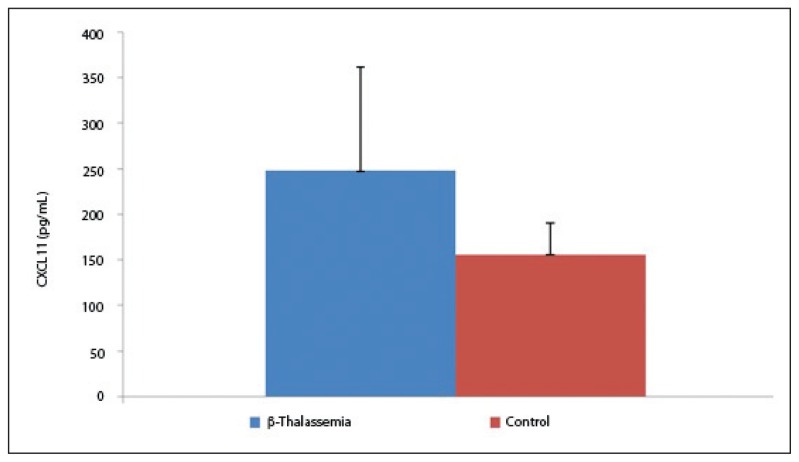
The statistical analysis of the demographic characteristics demonstrated no marked difference between the mean age and gender of the participants. The average age of the patients and controls was 16.1 (7.1) and 16.1 (6.9) years, respectively (P=.85). Of the patients, 37 were female and 26 were male. In the control group, 36 cases were female and 44 were male (P=.9), (Table 1). No significant difference was observed in age and gender between patients and controls. The results of the present study demonstrated that all of the studied CXC chemokines (CXCL1, CXCL9, CXCL10, and CXCL12) were significantly elevated in thalassemia patients than in control. Data analysis revealed statistically significant differences between β-thalassemia patients and controls in all of the studied chemokines. The mean serum level of CXCL10 was 273.4 (76.3) pg/mL and 116.79 (33.53) pg/mL in β-thalassemia patients and controls, respectively. A significant difference was observed between patients and controls (P<.05; Figure 1). The results of our study also indicated that the mean serum level of CXCL1 was 248 (113.1) pg/mL and 156.3 (34.5) pg/mL in β-thalassemia patients and controls, respectively (P<.03, Figure 2).
Table 1.
Demonstrates some of clinical characteristics of study participants, either patient or control.
| Variable | Thalassemia patients | Control | |
|---|---|---|---|
|
| |||
| Age (y) | 16.1 (7.1) | 16.1 (6.9) | |
| Gender | Male | 37 | 44 |
| Female | 26 | 36 | |
| Familial history | 17.3 (2.0) | - | |
| Alcohol abuse | 5 | - | |
| Hb (g/dL) | 9.5 (0.9) | - | |
| Desferrioxamine treatment | All | - | |
| Transfusion interval (d) | 29 (6) | - | |
| Transfusion volume (mL) | 450 (60) | - | |
| Iron | 130.5 (37.2) | - | |
| TIBC | 237.6 (102) | - | |
| Ferritin | 3129.6 (1973.5) | - | |
Figure 1.
Circulating levels of CXCL10 in β-thalassemia major patients and controls. Significant difference with control (P<.05).
Figure 2.
Circulating levels of CXCL1 β-thalassemia major patients and control. Significant difference with control (P<.03).
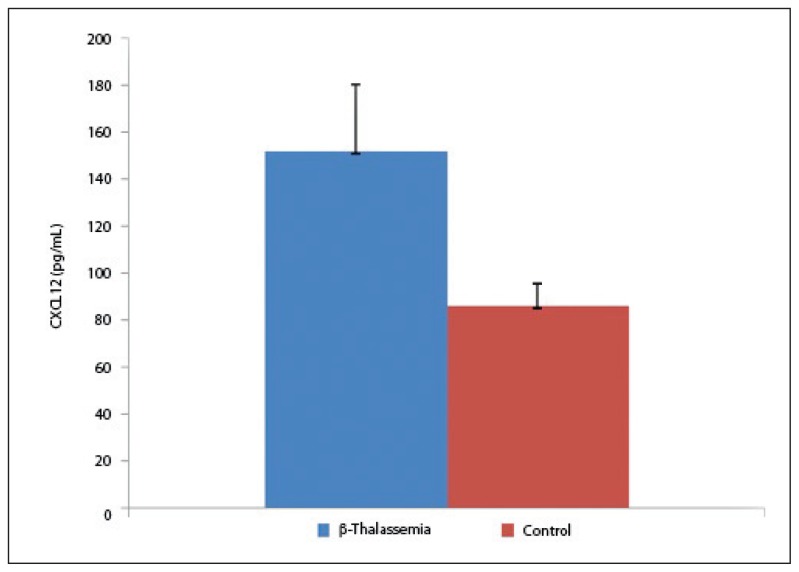
The mean serum level of CXCL12 was 152.1 (28.2) pg/mL and 86.2 (9.4) pg/mL in β-thalassemia patients and controls, respectively (P<.05, Figure 3), exhibiting a significant difference between the two.
Figure 3.
Circulating levels of CXCL9 in β-thalassemia major patients and control. Significant difference with (P<.05).
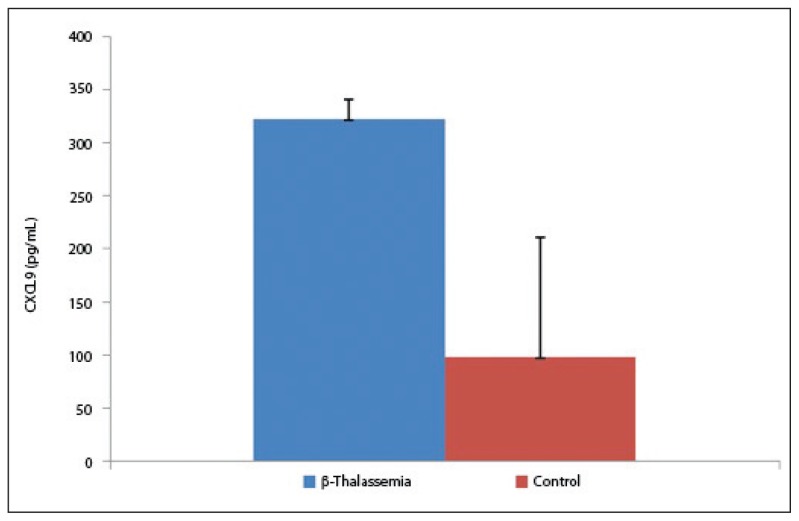
The results of our study demonstrated a significant increase of CXCL9 in β-thalassemia patients than in controls. The mean serum level of CXCL9 was 322.4 (18.7) pg/mL and 98.5 (111.8) pg/mL in β-thalassemia patients and controls, respectively (P=.05, Figure 4).
Figure 4.
Circulating levels of CXCL12 in β-thalassemia major patients and control. Significant difference with control (P=.05).
Our findings indicated that transfusion intervals affected the production of CXC chemokines. Table 2 shows elevated chemokines in patients who were being transfused for more than 100 months than in patients who were being transfused for a smaller duration (Table 2). Surprisingly, our results demonstrated that although the circulating levels of assessed CXC chemokines elevated in β-thalassemia patients than in controls, the levels of chemokines in patients suffering from viral infections were not different (HCV and HBV) (Table 2).
Table 2.
Demonstrates the chemokines serum levels in accordance to the transfusion intervals and post-transfusion infections. HBV: Hepatitis B virus, HCV: Hepatitis C virus.
| Variable | CXCL1 (pg/mL) | CXCL9 (pg/mL) | CXCL10 (pg/mL) | CXCL12 (pg/mL) | |
|---|---|---|---|---|---|
|
| |||||
| Duration of transfusion (mo) | Less than 100 | a212 (13.1) | a147.3 (17.9) | a146.8 (33.5) | a106.2 (49.1) |
| More than 100 | 292 (11.1) | 276.3 (15.2) | 253 (18.1) | 162 (112.1) | |
| Type of infection | HCV | 293 (18.2) | 348 (16.2) | 278.2 (18.5) | 276 (48) |
| HBV | 303 (28.3) | 329.4 (31.2) | 261.3 (63.2) | 251.3 (51.6) | |
Significant difference with patients received less than 100 mo transfusion.
Our finding indicated that the serum levels of CXCL1, CXCL9, CXCL10, and CXCL12 were 212 (13.1) pg/mL, 147.3 (17.9) pg/mL, 146.8 (33.5) pg/mL, and 106.2 (49.1) pg/mL, respectively, in thalassemia patients who were transfused for less than 100 months. We also observed that the serum levels of CXCL1, CXCL9, CXCL10, and CXCL12 were 292 (11.1) pg/mL, 276.3 (15.2) pg/mL, 253 (18.1) pg/mL, and 162 (112.1) pg/mL, respectively, in thalassemia children who were transfused for more than 100 months. The statistical analysis of data showed a significant difference between chemokine serum levels in patients transfused for less and more than 100 months’ duration (Table 2). No difference was observed when the patients were compared for the post-transfusion HCV and HBV infections (Table 2).
DISCUSSION
The present study was undertaken to evaluate the circulatory levels of some CXC chemokines in β-thalassemia patients and age- and gender-matched controls. The study showed elevated serum concentrations of CXCL1, CXCL9, CXCL10, and CXCL12 in thalassemia patients than in age- and gender-matched controls. This supports previous reports showing elevated serum levels of TNF-α, IL-1a, and IFN-α in thalassemia patients. A correlation was also reported between the severity of clinical symptoms and the cytokines level in β-thalassemia patients.23 In Iran, thalassemia patients were previously transfused with packed red blood cells, which sometimes is contaminated with other biological and cellular fractions including, leukocytes, plasma protein deposits, fragmented cells, and several other unknown materials. These are all antigenic and are able to stimulate the cellular immune system, which may lead to an elevated chemokine expression by tissue and cellular sources.24–28 Some of the previous studies showed that reactive T-cells are an enriched source of CXC chemokines,29,30 and some studies revealed an increased number of these cells in β-thalassemia patients. Elevated absolute counts and percentages of cells with CD3+/DR+, CD3+/CD25+, and CD3+/CD71+ phenotypes were observed in these patients, supporting our finding that the levels of chemokines. This study established a relationship between the transfusion intervals and the level of chemokines, revealing that chemokines increased if the patients had received more transfusions. This probably could be because of sensitization and further chemokine production by immune cells, bone marrow, and reticuloendothelial system (macrophages, denderitic cells) because these cells are known as the most important sources of cytokines such as TNF-α, IFN-α, and chemokines. 31–34 This study confirms our results that showed elevated levels of pro-inflammatory CXC chemokines (CXCL1 and CXCL10) in response to TNF-α in in vitro cultures. The increased concentrations of these chemokines in thalassemia patients may also be attributed to the regulatory role played by TNF-α in up-regulation of chemokine.35–37 Moreover, Kori et al. claimed a significant increase in both absolute numbers and percentages of activated T and NKT cells in thalassemia patients (especially elderly patients).38 Thus, the simultaneous elevation of these cell phenotypes and levels of CXC chemokine might possibly be related to each other.39 Therefore, massive transfusion, especially in elderly patients, probably increases the risk of multiple exposures to different antigens—in fact, an elevated chemokine level. Again, Salasa and co-workers showed that peripheral blood mononuclear cells from thalassemia patients secrete more IFN-α than the control group following stimulation. Having chronic HCV infection or treatment with IFN-α could be another explanation for increased IFN-α production by stimulated lymphocytes.40 IFN-α, in turn, probably up-regulates the IFN-α–inducible chemokines in these patients. Although, IFN-α is known as the most potent inducer of CXCL9 and CXCL10 synthesis, the production of these chemokines is costimulated by TNF-α and lipopolysaccharides.41,42
It is widely evidenced that in anemia, hematopoietic stem/progenitor cells express a subset of surface receptors for inflammatory cytokines, and several studies reported that the direct action of cytokines on hematopoietic cell lines in vitro can impair erythroid lineage development and further decrease erythroid progenitor cells. This hypothesis could probably be extended to proinflammatory chemokines, which need to be analyzed in futher investigations. Hence, it is necessary to analysis more precisely the expression of specific receptors for these chemokines, e.g., CXCR1, CXCLR3, and CXCR4, on progenitor cells in thalassemia patients.43
Acknowledgments
Authors of the present study take this opportunity to warmly appreciate all thalassemia patients and their parents for donating blood for this study, although they needed their blood for life. We also thank all of the control subjects and their parents. The project was financially supported by Rafsanjan University of Medical Sciences.
Footnotes
Conflict of interest
None of authors of this study claimed conflict of interest.
REFERENCES
- 1.Alan RGC, Pennell DJ, Cunning MJVE. Thalassemia. Hematology (Am Soc Hematol Educ Program) 2004:14–34. doi: 10.1182/asheducation-2004.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: emerging roles in immunity and disease. Nature reviews Immunology. 2003 Jun;3(6):454–62. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 3.Kuliev AM, Modell B. Problems in the control of genetic disorders. Biomedical science. 1990 Jan;1(1):3–17. [PubMed] [Google Scholar]
- 4.Rund D, Rachmilewitz E. Beta-thalassemia. The New England journal of medicine. 2005 Sep 15;353(11):1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 5.Joshi DD. Organisation of veterinary public health in the south Asia region. Revue scientifique et technique (International Office of Epizootics) 1991 Dec;10(4):1101–29. doi: 10.20506/rst.10.4.587. [DOI] [PubMed] [Google Scholar]
- 6.Jameela S, Sabirah SO, Babam J, Phan CL, Visalachy P, Chang KM, et al. Thalassaemia screening among students in a secondary school in Ampang, Malaysia. The Medical journal of Malaysia. 2011 Dec;66(5):522–4. [PubMed] [Google Scholar]
- 7.Martin S, Wolf-Eichbaum D, Duinkerken G, Scherbaum WA, Kolb H, Noordzij JG, et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. The New England journal of medicine. 2001 Oct 4;345(14):1036–40. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001 Jul 21;358(9277):221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 9.Multicentre study on prevalence of endocrine complications in thalassaemia major. Italian Working Group on Endocrine Complications in Nonendocrine Diseases. Clinical endocrinology. 1995 Jun;42(6):581–6. doi: 10.1111/j.1365-2265.1995.tb02683.x. [DOI] [PubMed] [Google Scholar]
- 10.Ciesielski CJ, Andreakos E, Foxwell BM, Feldmann M. TNFalpha-induced macrophage chemokine secretion is more dependent on NF-kappaB expression than lipopolysaccharides-induced macrophage chemokine secretion. European journal of immunology. 2002 Jul;32(7):2037–45. doi: 10.1002/1521-4141(200207)32:7<2037::AID-IMMU2037>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Ezer U, Gulderen F, Culha VK, Akgul N, Gurbuz O. Immunological status of thalassemia syndrome. Pediatric hematology and oncology. 2002 Jan-Feb;19(1):51–8. doi: 10.1080/088800102753356194. [DOI] [PubMed] [Google Scholar]
- 12.Alidoost F, Gharagozloo M, Bagherpour B, Jafarian A, Sajjadi SE, Hourfar H, et al. Effects of silymarin on the proliferation and glutathione levels of peripheral blood mononuclear cells from beta-thalassemia major patients. International immunopharmacology. 2006 Aug;6(8):1305–10. doi: 10.1016/j.intimp.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Dillman JF, 3rd, McGary KL, Schlager JJ. An inhibitor of p38 MAP kinase downregulates cytokine release induced by sulfur mustard exposure in human epidermal keratinocytes. Toxicology in vitro: an international journal published in association with BIBRA. 2004 Oct;18(5):593–9. doi: 10.1016/j.tiv.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Arababadi MK, Hassanshahi G, Yousefi H, Zarandi ER, Moradi M, Mahmoodi M. No detected hepatitis B virus-DNA in thalassemic patients infected by hepatitis C virus in Kerman province of Iran. Pakistan journal of biological sciences: PJBS. 2008 Jul 1;11(13):1738–41. doi: 10.3923/pjbs.2008.1738.1741. [DOI] [PubMed] [Google Scholar]
- 15.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nature medicine. 2012 Jul;18(7):1060–8. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aminzadeh F, Ghorashi Z, Nabati S, Ghasemshirazi M, Arababadi MK, Shamsizadeh A, et al. Differential expression of CXC chemokines CXCL10 and CXCL12 in term and pre-term neonates and their mothers. American journal of reproductive immunology (New York, NY: 1989) 2012 Oct;68(4):338–44. doi: 10.1111/j.1600-0897.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 17.Khandany BK, Hassanshahi G, Khorramdelazad H, Balali Z, Shamsizadeh A, Arababadi MK, et al. Evaluation of circulating concentrations of CXCL1 (Gro-alpha), CXCL10 (IP-10) and CXCL12 (SDF-1) in ALL patients prior and post bone marrow transplantation. Pathology, research and practice. 2012 Oct 15;208(10):615–9. doi: 10.1016/j.prp.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Azin H, Vazirinejad R, Ahmadabadi BN, Khorramdelazad H, Zarandi ER, Arababadi MK, et al. The SDF-1 3’a genetic variation of the chemokine SDF-1alpha (CXCL12) in parallel with its increased circulating levels is associated with susceptibility to MS: a study on Iranian multiple sclerosis patients. Journal of molecular neuroscience: MN. 2012 Jul;47(3):431–6. doi: 10.1007/s12031-011-9672-6. [DOI] [PubMed] [Google Scholar]
- 19.Arababadi MK, Hassanshahi G, Pourfathollah AA, Zarandi ER, Kennedy D. Post-transfusion occult hepatitis B (OBI): a global challenge for blood recipients and health authorities. Hepatitis monthly. 2011 Sep;11(9):714–8. doi: 10.5812/kowsar.1735143X.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arababadi MK, Pourfathollah AA, Jafarzadeh A, Hassanshahi G. Serum Levels of IL-10 and IL-17A in Occult HBV-Infected South-East Iranian Patients. Hepatitis monthly. 2010 Winter;10(1):31–5. [PMC free article] [PubMed] [Google Scholar]
- 21.Hassanshahi G, Patel SS, Jafarzadeh AA, Dickson AJ. Expression of CXC chemokine IP-10/Mob-1 by primary hepatocytes following heat shock. Saudi medical journal. 2007 Apr;28(4):514–8. [PubMed] [Google Scholar]
- 22.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. The Journal of biological chemistry. 1995 Nov 10;270(45):27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 23.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. The Journal of experimental medicine. 1993 Jun 1;177(6):1809–14. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker C, Chang L, Elsegood KA, Bishop AJ, Gannon DH, Narendran P, et al. Activated T cell subsets in human type 1 diabetes: evidence for expansion of the DR+ CD30+ subpopulation in new-onset disease. Clinical and experimental immunology. 2007 Mar;147(3):472–82. doi: 10.1111/j.1365-2249.2006.03307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinquan T, Jing C, Jacobi HH, Reimert CM, Millner A, Quan S, et al. CXCR3 expression and activation of eosinophils: role of IFN-gamma-inducible protein-10 and monokine induced by IFN-gamma. Journal of immunology (Baltimore, Md: 1950) 2000 Aug 1;165(3):1548–56. doi: 10.4049/jimmunol.165.3.1548. [DOI] [PubMed] [Google Scholar]
- 26.Hanaoka R, Kasama T, Muramatsu M, Yajima N, Shiozawa F, Miwa Y, et al. A novel mechanism for the regulation of IFN-gamma inducible protein-10 expression in rheumatoid arthritis. Arthritis research & therapy. 2003;5(2):R74–81. doi: 10.1186/ar616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewing CA, Rumsey DH, Langberg AF, Sandler SG. Immunoprophylaxis using intravenous Rh immune globulin should be standard practice when selected D-negative patients are transfused with D-positive random donor platelets. Immunohematology/American Red Cross. 1998;14(4):133–7. [PubMed] [Google Scholar]
- 28.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocrine reviews. 2007 Aug;28(5):492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- 29.Touraine JL. In utero fetal liver cell transplantation in the treatment of immunodeficient or thalassemic human fetuses. Transfusion science. 1993 Jul;14(3):299–304. doi: 10.1016/0955-3886(93)90013-K. [DOI] [PubMed] [Google Scholar]
- 30.Keens TG, O’Neal MH, Ortega JA, Hyman CB, Platzker AC. Pulmonary function abnormalities in thalassemia patients on a hypertransfusion program. Pediatrics. 1980 May;65(5):1013–7. [PubMed] [Google Scholar]
- 31.Kaplan G, Luster AD, Hancock G, Cohn ZA. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. The Journal of experimental medicine. 1987 Oct 1;166(4):1098–108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horuk R. Chemokine receptors. Cytokine & growth factor reviews. 2001 Dec;12(4):313–35. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 33.Murphy PM. Chemokine receptors: structure, function and role in microbial pathogenesis. Cytokine & growth factor reviews. 1996 Jun;7(1):47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 34.Vanguri P, Farber JM. Identification of CRG-2. An interferon-inducible mRNA predicted to encode a murine monokine. The Journal of biological chemistry. 1990 Sep 5;265(25):15049–57. [PubMed] [Google Scholar]
- 35.Bauer C, Loher F, Dauer M, Mayer C, Lehr HA, Schonharting M, et al. The ICE inhibitor pralnacasan prevents DSS-induced colitis in C57BL/6 mice and suppresses IP-10 mRNA but not TNFalpha mRNA expression. Digestive diseases and sciences. 2007 Jul;52(7):1642–52. doi: 10.1007/s10620-007-9802-8. [DOI] [PubMed] [Google Scholar]
- 36.Hassanshahi G, Arababadi MK, Khoramdelazad H, Yaghini N, Zarandi ER. Assessment of CXCL12 (SDF-1alpha) polymorphisms and its serum level in posttransfusion occult HBV-infected patients in Southeastern Iran. Archives of medical research. 2010 Jul;41(5):338–42. doi: 10.1016/j.arcmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Hassanshahi G, Jafarzadeh A, James Dickson A. Expression of stromal derived factor alpha (SDF-1 alpha) by primary hepatocytes following isolation and heat shock stimulation. Iranian journal of allergy, asthma, and immunology. 2008 Jun;7(2):61–8. [PubMed] [Google Scholar]
- 38.Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, et al. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood. 2009 Jul 16;114(3):667–76. doi: 10.1182/blood-2009-02-205492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gombart AF, Krug U, O’Kelly J, An E, Vegesna V, Koeffler HP. Aberrant expression of neutrophil and macrophage-related genes in a murine model for human neutrophil-specific granule deficiency. Journal of leukocyte biology. 2005 Nov;78(5):1153–65. doi: 10.1189/jlb.0504286. [DOI] [PubMed] [Google Scholar]
- 40.Towfighi F, Gharagozlou S, Kardar GA, Sharifian RA, Karimi K, Lak M, et al. Assessment of in vitro cytokine response in hemophilia A patients with or without factor VIII inhibitory antibody. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2007 Aug;27(8):665–74. doi: 10.1089/jir.2006.0184. [DOI] [PubMed] [Google Scholar]
- 41.Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents--time for a reevaluation. The New England journal of medicine. 2010 Jan 21;362(3):189–92. doi: 10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]
- 42.Park JW, Gruys ME, McCormick K, Lee JK, Subleski J, Wigginton JM, et al. Primary hepatocytes from mice treated with IL-2/IL-12 produce T cell chemoattractant activity that is dependent on monokine induced by IFN-gamma (Mig) and chemokine responsive to gamma-2 (Crg-2) Journal of immunology (Baltimore, Md: 1950) 2001 Mar 15;166(6):3763–70. doi: 10.4049/jimmunol.166.6.3763. [DOI] [PubMed] [Google Scholar]
- 43.Neville LF, Abdullah F, McDonnell PM, Young PR, Feuerstein GZ, Rabinovici R. Mob-1 expression in IL-2-induced ARDS: regulation by TNF-alpha. The American journal of physiology. 1995 Dec;269(6 Pt 1):L884–90. doi: 10.1152/ajplung.1995.269.6.L884. [DOI] [PubMed] [Google Scholar]