Abstract
This study characterizes global and hemispheric brain growth in healthy human fetuses during the second half of pregnancy using three-dimensional MRI techniques. We studied 166 healthy fetuses that underwent MRI between 18 and 39 completed weeks gestation. We created three-dimensional high-resolution reconstructions of the brain and calculated volumes for left and right cortical gray matter (CGM), fetal white matter (FWM), deep subcortical structures (DSS), and the cerebellum. We calculated the rate of growth for each tissue class according to gestational age and described patterns of hemispheric growth. Each brain region demonstrated major increases in volume during the second half of gestation, the most pronounced being the cerebellum (34-fold), followed by FWM (22-fold), CGM (21-fold), and DSS (10-fold). The left cerebellar hemisphere, CGM, and DSS had larger volumes early in gestation, but these equalized by term. It has been increasingly recognized that brain asymmetry evolves throughout the human life span. Advanced quantitative MRI provides noninvasive measurements of early structural asymmetry between the left and right fetal brain that may inform functional and behavioral laterality differences seen in children and young adulthood.
Keywords: asymmetry, brain volume, cortical development, fetus, growth, quantitative MRI
Introduction
Lateralization of brain function after infancy has been recognized for centuries. Structural differences in right–left symmetry as detected by volumetric changes on quantitative MRI or connectivity differences detected by functional MRI are increasingly recognized (Chance 2014; Ribolsi et al. 2014). Even subtle deviations in brain lateralization have been associated with various neuropsychiatric disorders (Chance 2014; Ribolsi et al. 2014). While the onset and mechanisms of these disorders are still poorly understood, the “fetal-onset of adult disease” hypothesis suggests that alterations in the intrauterine environment may be associated with lifelong neurologic sequealae (Lane 2014; Miller et al. 2015). Increasingly, fetal MRI is allowing for the early detection of aberrant neurodevelopment, including abnormalities in neuronal proliferation, migration, and organization (Denis et al. 2001; Righini et al. 2004; Rubod et al. 2005; Blondiaux et al. 2013; Lang et al. 2014). However, the more subtle deviations from normal development during critical periods of brain growth and differentiation in the fetus that may result in neuropsychiatric disorders presenting later in life remain more difficult to identify (Volpe 2008; Bale et al. 2010). For example, placental insufficiency and resulting fetal growth restriction has been associated with schizophrenia, autism, and cerebral palsy (Haglund and Kallen 2011; Hsiao and Patterson 2012; Eide et al. 2013; Grissom and Reyes 2013; Nielsen et al. 2013; Nelson and Blair 2015), but nonetheless remain difficult to diagnose until after the onset of clinical symptoms. To date, gross lateralization in the fetal period has been described in the development and emergence of major sulci and gyri (Habas et al. 2012). Quantification of cortical thickness and gyrification have demonstrated diffuse areas of structural asymmetry in the adult cortex (Chiarello et al. 2016). In the healthy fetus, cerebral development has been characterized using cortical surface analyses and gyrification indices (Clouchoux et al. 2012b; Clouchoux et al. 2013). However, quantitative evaluation of structural lateralization in the fetal cerebrum has not been well studied. Structural and functional asymmetry of the cerebellum has also been demonstrated in adults, and is associated with cerebral asymmetry, suggesting functional lateralization of cerebro-cerebellar networks (Wang et al. 2013). Cerebral and cerebellar volumes are also highly correlated in the fetus; however, volumetric hemispheric analysis do not demonstrate significant differences in volume (Scott et al. 2012). The extent to which aberrant fetal development of structural brain lateralization may be associated with neuropsychiatric disorders in later life remains poorly understood. Before we can begin to explore the relationship between deviations in structural fetal brain lateralization and neuropsychiatric conditions, a better understanding of normal trajectories of fetal brain lateralization is essential. To overcome these gaps in our knowledge, the objective of the current study was to quantify global and hemispheric brain growth in healthy human fetuses during the second half of pregnancy using three-dimensional MRI techniques.
Materials and Methods
Subjects
We prospectively enrolled healthy volunteers with singleton pregnancies and a normal pregnancy history, with term deliveries and normal postnatal imaging (Clouchoux et al. 2012a) from Boston Children's Hospital and Children's National Health Systems. Excluded were multiple gestations, known or suspected genetic or chromosomal abnormalities, as well as any maternal contraindication for MRI, including physical (e.g., metal implants) or psychosocial (e.g., claustrophobia) contraindications. We studied 166 healthy women volunteers with uncomplicated pregnancies between 18.3 and 39.1 (median 30.2) weeks gestation; 60 subjects were enrolled in the Boston Children's cohort and 106 in the Children's National cohort. Fifty-two percent were male (n = 87). Approximately 24% of MRI studies were performed in the second trimester (18–27 completed weeks) and 76% in the third trimester (28–39 completed weeks). The study was approved by the Institutional Review Boards of both Boston Children's Hospital and Children's National Health Systems, and written informed consent was obtained by all study participants as part of an ongoing prospective observational study.
MRI Acquisition
Images were acquired on a 1.5 Tesla MR scanner (Philips Healthcare, Best, the Netherlands) with a 5-channel phased array coil for the Boston Children's cohort or a 1.5 Tesla MRI scanner (GE Healthcare, Waukesha, WI) using an 8-channel receiver coil (USAI, Aurora, OH) for the Children's National Health Systems cohort. The pulse sequences used to obtain anatomic images were T2-weighted multi-planar single shot fast spin echo sequences performed as follows: on the Philips scanner (TR/TE = 971/120 ms) and on the GE scanner (TR/TE = 1100/160 ms), voxel size 1.25 × 1.66 × 2 mm3 to 1.32 × 1.4 × 2 mm3 in all three axial, coronal, and sagittal planes with an acquisition time of 2–3 min per plane.
Preprocessing
For each subject, a single three-dimensional motion-corrected high-resolution brain volume was reconstructed using a slice-to-volume reconstruction method (Rousseau et al. 2013). The fetal brain was extracted from surrounding fetal and maternal tissue using an atlas-based approach. Each image was aligned to the closest age-matched template from a publicly available spatiotemporal fetal brain atlas (Serag et al., 2012a), and the resulting transformation was used to propagate the brain mask from the atlas’ space to the subject's native space. Then, images were processed using the N4 algorithm (Tustison et al. 2010) to correct for intensity in homogeneity in order to achieve a consistent, spatially invariant, signal intensity distribution for each tissue. All MR imaging studies were reviewed by one of the center's expert pediatric neuroradiologists (R.R. at Boston Children's Hospital or G.V. at Children's National Health Systems).
Image Segmentation and Volumetric Analysis
The reconstructed fetal brain volumes were segmented using an automatic atlas-based images segmentation approach (Serag et al. 2012a, 2012b). Each MR image was affinely (9 degrees of freedom) aligned to each template of the spatiotemporal atlas, and a similarity metric (i.e., normalized mutual information) was calculated between the image and the registered template. Then, n-nearest templates (n = 5) with the highest similarity were chosen to guide the segmentation using its spatial priors. The chosen templates were registered to the MR image using nonrigid B-spline image registration (Rueckert et al. 1999). The resulting transformations were used to propagate the probability maps from the templates’ coordinate system to the MR image coordinates. Probabilities were then represented using barycentric coordinates. Finally, Expectation-Maximization classification was used to classify each voxel (based on its intensity and its probability of belonging to a specific class into one of four tissue classes: cortical gray matter (CGM), deep subcortical structures (DSS), fetal white matter (FWM), and cerebellum (Fig. 1). Each automatic segmentation was then manually inspected by a single reader (N.N.A.) to review and correct any misclassifications.
Figure 1.
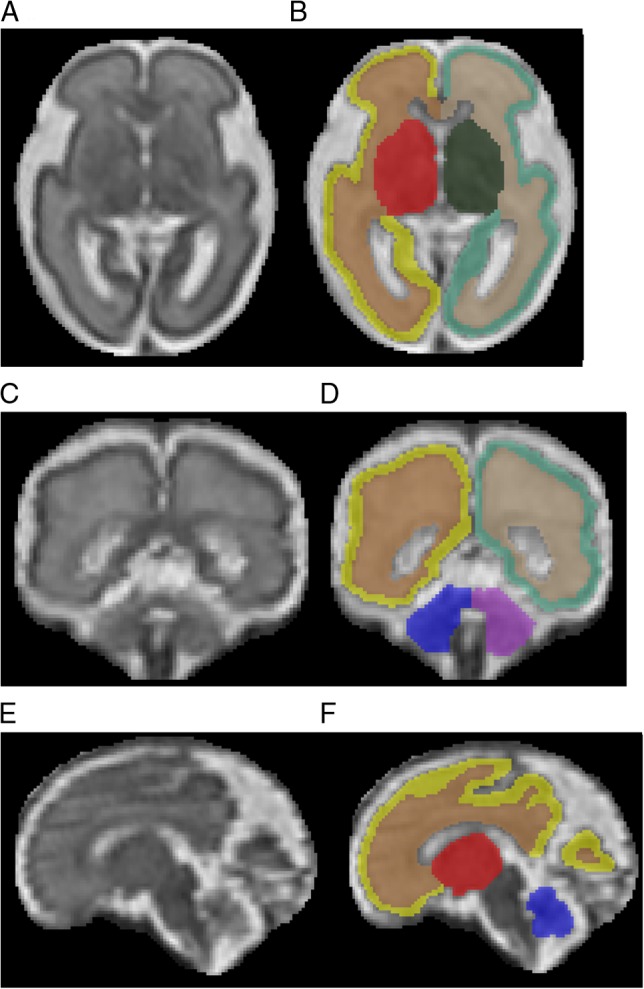
T2-Weighted of the high-resolution fetal brain reconstruction at 27 weeks gestation in the axial (A and B), coronal (C and D), and sagittal (E and F) planes, with anatomical images on the left (A, C, E) and corresponding segmentations on the right (B, D, F). Color key: yellow = left CGM; brown = left FWM; red = left DSS; blue = left cerebellum; light green = right CGM; tan = right FWM; dark green = right DSS; purple = right cerebellum.
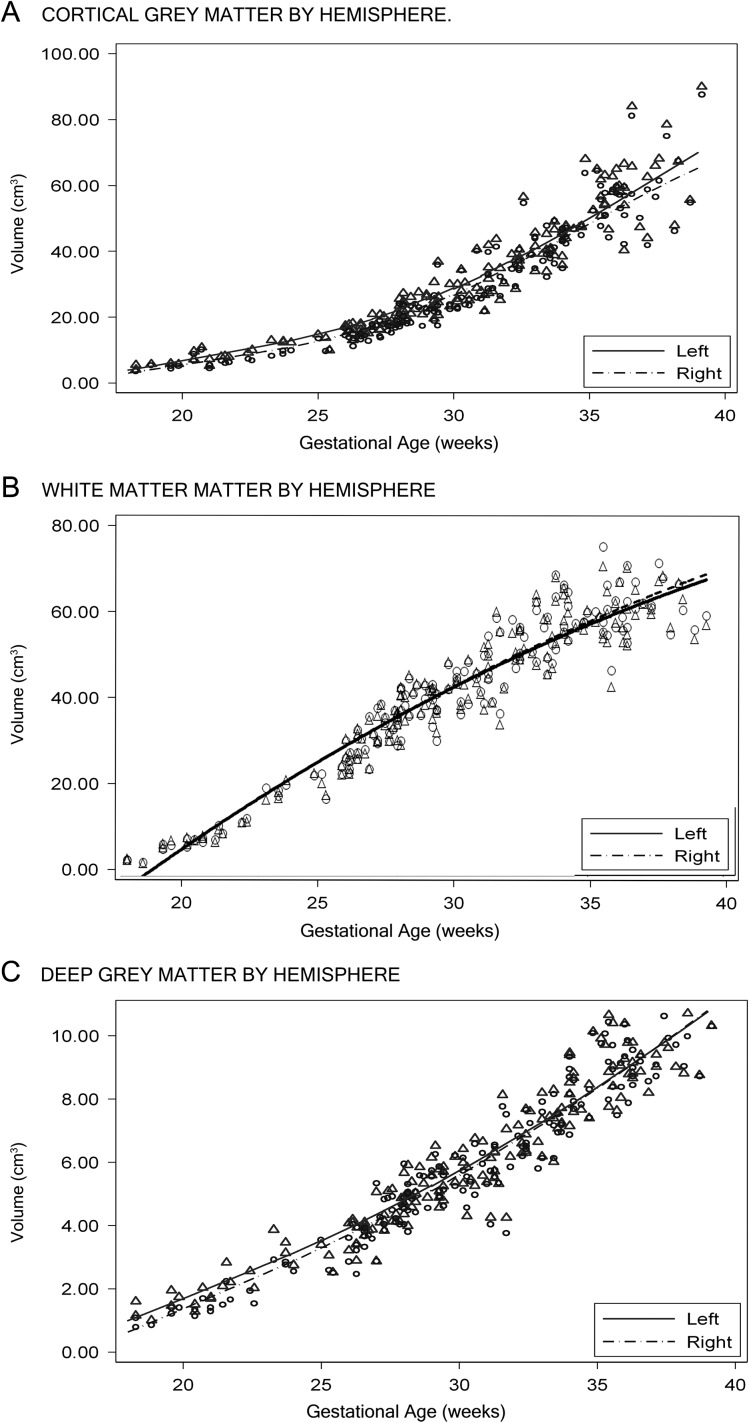
CGM was delineated at the surface of the cerebrum, primarily representing neurons of the cortical plate; FWM was identified as moderate to high signal intensity, representing developing white matter (WM), corpus callosum, as well as the transient ventricular, subplate, and intermediate zones; DSS was identified by lower signal intensities adjacent to the lateral ventricles, primarily representing deep gray structures, but also the emerging internal capsule (Kostovic et al. 2002; Kostovic et al. 2014; Vasung et al. 2016). Volumes of each region described above were calculated and measured independently for left and right hemispheres using ITK-SNAP software by multiplying the voxel count by the voxel unitary volume and converting to cubic centimeters (Yushkevich et al. 2006). Total cerebral volume was calculated as the sum of the voxels of CGM, FWM, and DSS.
Statistical Analysis
Descriptive statistics were used to characterize the cohort. We used quantile regression (Stata 13) to evaluate change in regional brain volume across gestational age in typical term delivered infants. We chose this approach because the distribution of volume estimates within most regions violated the normality assumption and was resistant to typical normalizing transformations. This approach allowed us to plot predicted regional volume change, including accounting for laterality, by gestational age for selected growth percentiles, including the 5th, 25th, 50th (median), 75th, and 95th percentiles.
Results
Regional and Tissue-Specific Volumetric Growth Measures
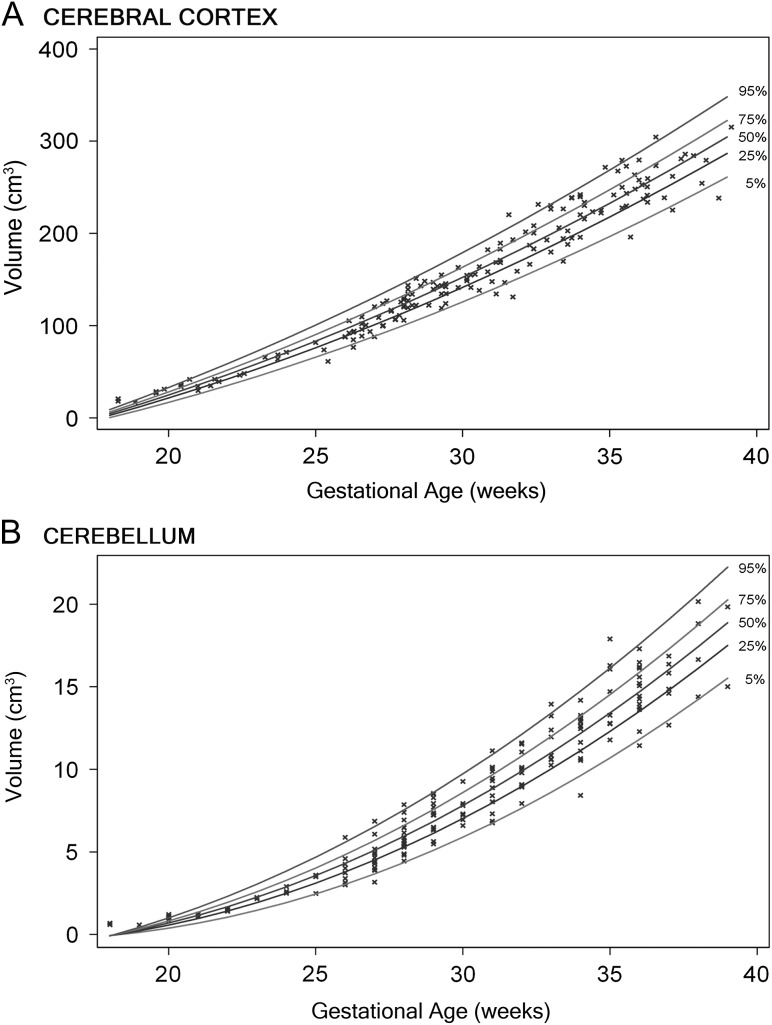
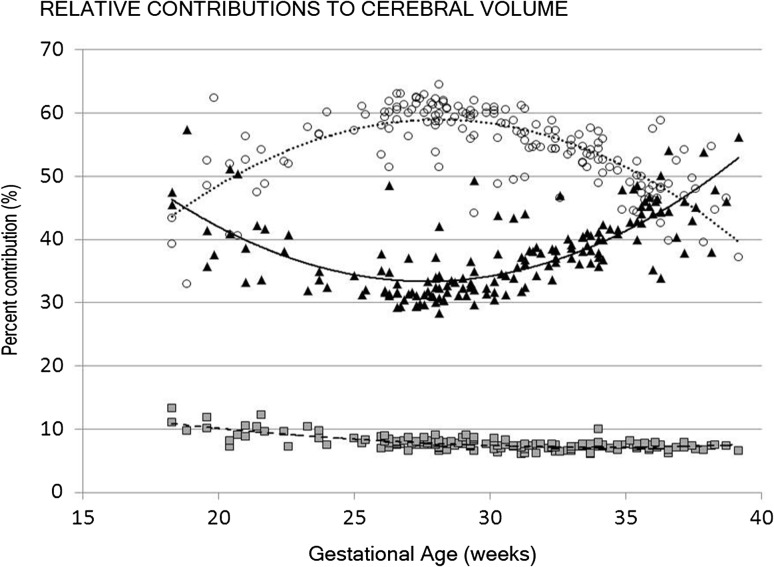
All measured brain regions and tissues demonstrated pronounced growth over a 21-week study period in the second and third trimester. Figures 2 and 3 illustrate the growth curves for the regional and tissue-specific classes that were segmented from 166 healthy fetuses. The cerebellar volume increased the most strikingly, from 0.58 to 20.16 cm3, or 34-fold, over the 21-week gestational age study window. This was followed by cortical volume, increasing 20-fold, an increase in FWM of 22-fold, an increase of 21-fold in CGM, and a 10-fold increase in DSS (Table 1). There were no gender differences in any of the measured volumes. The relative contribution of each cerebral class to overall cerebral volume also changed throughout gestation (Fig. 4).
Figure 2.
Growth curves of the cortex (A) and cerebellum (B) in the second half of gestation, with regression lines for the 5th, 25th, 50th, 75th, and 95th percentiles.
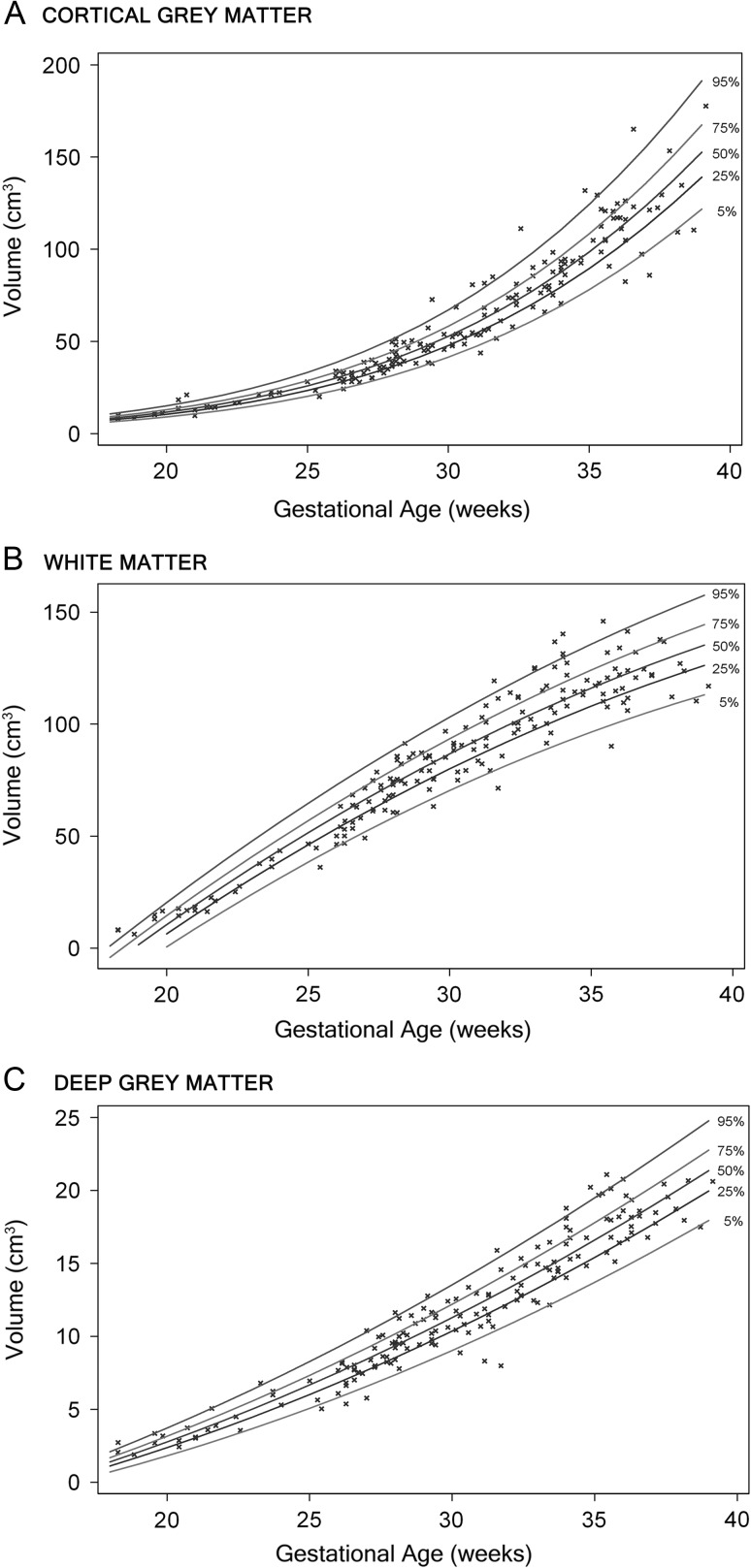
Figure 3.
Growth curves of CGM (A), WM (B), and deep GM (C) volumes with advancing gestational age, with regression curves for the 5th, 25th, 50th, 75th, and 95th percentiles.
Table 1:
Increase in regional and global volumes between 18 and 39 weeks gestation
| Minimum volume (cm3) | Maximum volume (cm3) | Total increase (fold) | |
|---|---|---|---|
| CGM | 8.01 | 177.67 | 21 |
| Developing WM | 6.24 | 145.95 | 22 |
| Deep cerebral structures | 1.89 | 21.09 | 10 |
| Total cerebrum | 16.14 | 344.71 | 20 |
| Cerebellum | 0.58 | 20.16 | 34 |
Figure 4.
The relative contribution of CGM (black triangles, solid line), FWM (open circles, dotted line), and deep subcortical structures (gray squares, dashed line) to cerebral cortical volume with advancing gestational age.
Fetal Brain Growth in the Second Versus Third Trimester of Pregnancy
We compared growth trajectories in the late second and third trimesters. FWM, DSS, and cerebellum had greater growth trajectories in the second trimester compared with the third trimester. For CGM, the third trimester growth was notable for a 4-fold increase in volume, compared with a 2-fold increase in the late second trimester.
Hemispheric Differences in Volumetric Growth Measures
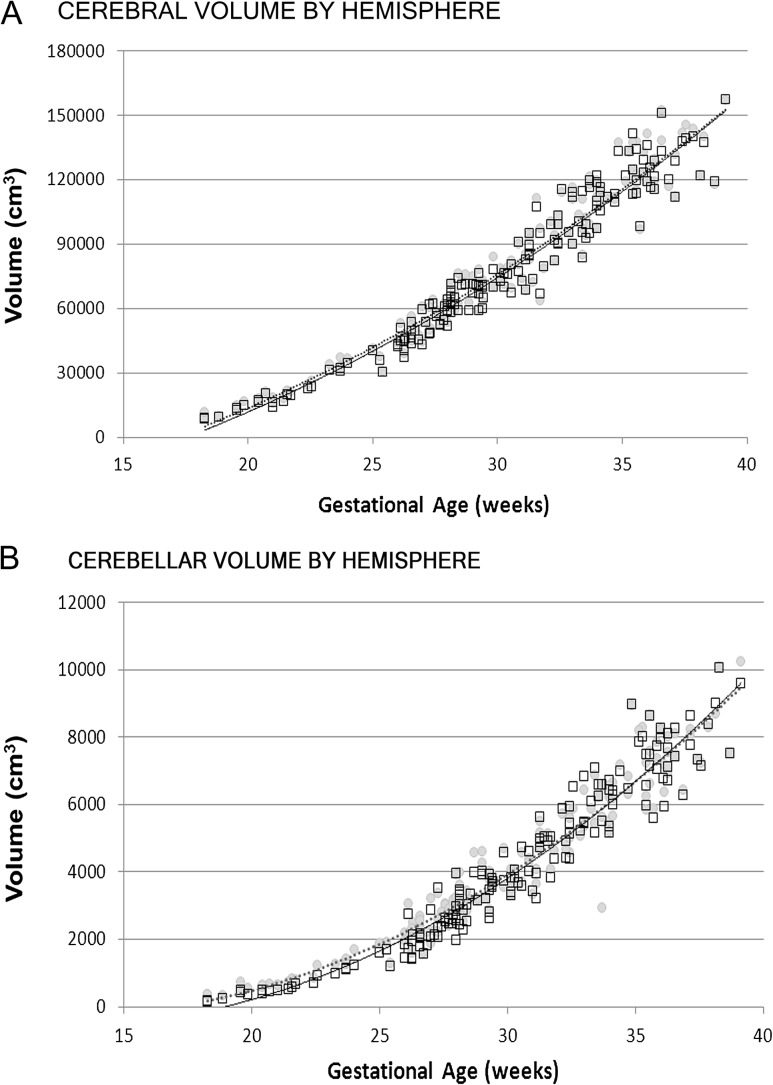
The left and right cerebral hemispheres showed overall similar growth trajectories; however, there was a small but significant difference earlier in gestation with the left hemisphere demonstrating a larger volume compared with the right hemisphere. Similarly, the left cerebellar hemisphere was also larger than the right (Fig. 5). Interestingly, the left and right hemispheric trajectories for both the cerebrum and cerebellum equalized by term (P < 0.05).
Figure 5.
Volume of left and right cerebral (A) and cerebellar (B) hemispheres with advancing gestational age (left depicted by gray circles and dashed line, right by open squares and solid line).
We then examined cerebral growth by tissue class and found that CGM demonstrated greater volumes in the left hemisphere compared with the right throughout gestation (P < 0.01). In the second trimester, DSS demonstrated greater volumes in the left hemisphere compared with the right before 28 weeks gestation, but were no longer evident by term gestation (P < 0.05) (Fig. 6). FWM tended towards greater volumes in the right hemisphere compared with the left; however, these differences did not reach statistical significance (P = 0.08) (Fig. 6).
Figure 6.
Volume of left and right CGM (A), FWM (B), and subcortical structures (C) with advancing gestational age (left depicted by gray circles and dashed line, right by open squares and solid line).
Discussion
In this report, we present normative data for volumetric growth in the left and right sides of the fetal brain across the second half of gestation in a large sample of healthy fetuses. We include measures of cerebral CGM, FWM and DSS, as well as the cerebellum and report for the first time important left–right volumetric growth differences in the second versus third trimester of pregnancy. Specifically, we use advanced quantitative MRI techniques to measure brain development between 18 and 39 completed weeks in a cohort of normal fetuses from 166 healthy pregnant volunteers. We demonstrate exponential growth of cerebellum followed by the cerebral cortex. We also demonstrate differential and evolving left–right interhemispheric asymmetries of the cerebrum and cerebellum that change throughout gestation.
Development of Cerebral Tissue Classes In Utero
The period between the late second trimester and term is one of profuse cortical maturation, including dendritic and synaptic development, axonal outgrowth, glial proliferation, and neuronal connectivity and circuit formation (Volpe 2008). Previous work has demonstrated a linear increase in cerebral volume of 2.3-fold between 25 and 36 weeks (i.e., an 11-week period) (Clouchoux et al. 2012a). In this report, the third trimester increase in cerebral volume between 28 and 39 weeks gestation reveals a very similar growth trajectory of 2.6-fold increase in volume, a similar growth trajectory to what has also been demonstrated in autopsy data (Orasanu et al. 2014). Noteworthy, the inclusion of late second trimester growth and the expanded study window over 21 gestational weeks (18 through 39 weeks) demonstrates a much more significant increase of 20-fold. Furthermore, we demonstrate that cerebral growth is exponential throughout the second half of pregnancy, rather than linear.
A detailed evaluation of the cerebral tissue classes reveals that the increases in FWM account for the greatest contribution to overall cerebral growth in the second half of pregnancy. Specifically we report a 22-fold increase in FWM volume between 18 and 40 weeks gestation (6.24–145 cm3). This is followed closely by increases in CGM, demonstrating a 21-fold increase in volume during the same gestational period. Despite similar overall growth rates, the trajectories and relative contribution to the cerebral volume between 18 and 39 weeks differs for the two tissue classes, likely reflecting the dramatic evolution of cerebral development during this period. As the cerebral CGM and WM mature, along with the deep gray matter (GM) structures, fetal brain development includes numerous transient fetal compartments that transform during our period of study. These transient structures, namely the intermediate and subplate zones, are histologically complex, containing migrating neurons and support cells along with developing axons (Bystron et al. 2008; Vasung et al. 2016). We show that the FWM is the dominant contributor to cerebral development and peaks between 29 and 30 weeks gestation (Fig. 4). As migrating neurons reach their final destination at the cortical plate, our data show that the relative contribution of CGM increases by term gestation (Fig. 4). These data are consistent with postmortem studies that demonstrate maximal volume of the subplate zone at approximately 30 weeks (Vasung et al. 2016). In the preterm (ex utero) infant, there is a relative increase in CGM volume but the relative volumes of WM and deep gray structures decrease between 27 and 45 weeks (Makropoulos et al. 2015). Given that alterations in regional and global contributions of GM and WM growth associated with preterm birth persist through adolescence and are associated with cognitive delays (Nosarti et al. 2008), it is plausible that similar disruptions in fetal life may contribute to various neurodevelopmental disorders. This intriguing question awaits further study.
Age-related changes in WM have been described throughout the life span, with increases in volume noted through young adulthood (Ostby et al. 2009; Groeschel et al. 2010), which begin to decrease after middle adult hood (Ge et al. 2002). In infants, WM increases by approximately 11% the first year of life and 19% in the second (Dubois et al. 2014). In older children, WM continue to increase by 6.49 cm3 per year between 4 and 18 years of age (2012). In premature infants, WM volume increases linearly from 50 cm3 at 29 weeks to 170 cm3 at 44 weeks (Dubois et al. 2014). Our measures of in utero FWM volume during a similar gestational period of 29 weeks to term show similar rates of increase. However, compared with the premature infants at 29 weeks, our normal fetuses had an average WM volume of 80 cm3. This difference may relate in part to stresses and challenges of maintaining adequate postnatal growth in the ex utero environment and the hazards of early extrauterine exposure of the immature and vulnerable preterm brain.
Cerebral gray volume has been reported to increase more rapidly in the third trimester compared with the WM in the third trimester, particularly in the cortical rather than subcortical regions (Corbett-Detig et al. 2011; Scott et al. 2011; Vasung et al. 2016). GM volume changes throughout the life span follows a different trajectory to that of WM. Total cortical GM volume peaks earlier in childhood (Groeschel et al. 2010), and between 4 and 18 years, whole brain GM (including cortical and deep GM) decreases by approximately 6.56 cm3 per year (2012). During this same time period, deep GM structures appear relatively constant (2012) but begin to demonstrate variable rates of volume loss in young adults (Ostby et al. 2009), more constant volume loss compared with WM in middle adulthood. In infancy, GM is the primary contribution to hemispheric cerebral growth, increasing by 149% in the first year of life (Knickmeyer et al. 2008). Between ages 1 and 2, the deep gray structures increase an additional 13–19% (Knickmeyer et al. 2008). Previous reports of GM structures in the fetus demonstrate a linear weekly increase of 18% of the cortical plate, and 15.56% increase of deep gray structures between 20 and 31 weeks (Scott et al. 2011) with similar absolute values to our measures during the same period. Notably, extending the study period by an additional 10 weeks in this study suggests that the trajectory of growth throughout the second half of pregnancy is exponential rather than linear, particularly for cortical GM in the third trimester. As described with WM volume, in utero volumes of deep gray and cortical gray volumes are greater than measures of ex vivo infants born prematurely (Kuklisova-Murgasova et al. 2011).
Development of the Cerebellum
Our findings demonstrate that the region of most rapid brain growth over the second and third trimesters is the cerebellum, which is in line with previous reports of fetal brain development (Grossman et al. 2006; Clouchoux et al. 2012a; Scott et al. 2012). During the second and third trimesters, the cerebellum undergoes profuse proliferation and migration of the external granular cells, formation of the internal granular layer, enlargement of the cerebellar WM and Purkinje cells (Griffiths et al. 2004; Lavezzi et al. 2006; Liu et al. 2011), all of which underlie the remarkable volumetric growth of the cerebellum. This continues through early infancy, with a volumetric increase of 240% in the first year of life (Knickmeyer et al. 2008). Thereafter, cerebellar GM decreases nonlinearly during childhood and adolescence, while cerebellar WM increases (Ostby et al. 2009). Decreases in total and regional cerebellar volume in young children with cerebellar malformations are associated with delays in global development, cognition, and motor function, as well as social–affective disturbances (Bolduc et al. 2012). In premature infants, cerebellar volume also increases at a much faster rate than intracranial or total brain volumes (Limperopoulos et al. 2005). Despite this rapid increase, by term corrected age, mean cerebellar volume in premature infants is significantly smaller than the volumes in healthy term infants (Limperopoulos et al. 2005). In premature infants with and without direct cerebellar injury, the presence of brain injury is also highly correlated with cerebellar growth impairment (Limperopoulos et al. 2005).
Cerebral Hemispheric Asymmetry
Cerebral lateralization is implicated in language development, handedness, as well as higher-order reasoning and processing (Steinmetz et al. 1991; Turner et al. 2015). Abnormalities in structural and functional lateralization are associated with numerous neuropsychiatric disease states, including Alzheimer's, autism, and schizophrenia (Thompson et al. 1998; Bakalar et al. 2009; Kim et al. 2012; Szeszko et al. 2012; Tullett et al. 2012; Lindell and Hudry 2013; Floris et al. 2015; Peng et al. 2015). Despite gross similarities in size and weight between the left and right hemispheres of the adult brain, morphological asymmetries between the two hemispheres have been well described (Toga and Thompson 2003). Upon more detailed evaluation of the adult brain, the volume of the right cerebral hemisphere is larger than the left, with the most prominent regional differences noted in the prefrontal and occipital cortex (Raz et al. 2004). It has been proposed that lateralization of gene expression may explain subtle changes in structure, neuronal circuit formation, and subsequent cognitive and behavioral outcomes (Francks 2015). In fact, molecular mechanisms that determine early structural asymmetry throughout the body have also been implicated in the development of dyslexia (Brandler et al. 2013).
Increasingly, the dynamic changes of hemispheric asymmetries throughout the life span are being recognized. Between 1 and 10 years of age, hemispheric asymmetries are more subtle but also demonstrate larger right hemispheres, particularly for WM (Matsuzawa et al. 2001). Regional analysis in an older cohort of children between 4 and 18 years demonstrates a predominantly leftward asymmetry of WM for all lobes with the exception of the temporal lobe (2012). In infants, the left hemisphere is nearly 5% larger the right—and this difference is more pronounced in GM than WM (Gilmore et al. 2007). More evident than the volume differences between hemispheres is the emergence of asymmetries in morphological development of the temporal lobes. Interhemispheric differences in the size of the Sylvian fissure are noted both in the newborn and young infant, again left larger than right (Seidenwurm et al. 1985), and continue to change dynamically through adulthood (Sowell et al. 2002). The most prominent asymmetries in the temporal lobe involve the peri-Sylvian region and superior temporal sulcus, with earlier and greater growth of the left-sided peri-Sylvian structures and right-sided superior temporal structures in term and preterm infants (Dubois et al. 2008; 2010; Li et al. 2014). This level of morphological asymmetry has also been noted in the fetus as early as 23 weeks gestation (Wada et al. 1975; Kasprian et al. 2011; Habas et al. 2012). An earlier report by Scott et al. (2011) suggested no hemispheric difference in cerebral volume of the fetus; we demonstrate dynamic changes in overall and relative growth of the left and right cerebral hemispheres throughout the second half of pregnancy. These differences between the two studies likely reflect the larger sample size (168 vs. 38) as well as the extended study period (21 vs. 11 gestational weeks) in the current study. We show that the left and right hemisphere increase at different rates throughout gestation. Similar to reports of hemispheric GM and WM volume in neonates (Gilmore et al. 2007), our data demonstrate greater volumes of fetal GM on the left compared with right. Hemispheric WM volume differences also change throughout gestation and into the neonatal period; by adulthood, these differences disappear (Gilmore et al. 2007).
Cerebellar Hemispheric Asymmetry
Hemispheric asymmetry of the cerebellum has also been noted in infancy and later adulthood, with regional differences in cerebellar GM between males and females (Fan et al. 2010). In both adults and infants, the right cerebellar hemisphere is larger than the left (Bernard and Seidler 2013; Holland et al. 2014). Similar to variations in cerebral hemispheric asymmetry, cerebellar hemispheric asymmetries have also been associated with neuropsychiatric disorders, including developmental dyslexia, autism, expressive language, and cognitive disorders (Cherbuin et al. 2010; Hodge et al. 2010; Bolduc et al. 2012; Elnakib et al. 2014). In the fetal period, we show for the first time that the left cerebellar hemisphere is larger in mid-gestation, but that the right hemisphere grows at a faster pace.
Limitations and Future Directions
This study has several limitations. Our algorithm segmented the developing brain into 4 regions of interest, 3 cerebral tissues and the cerebellum. We did not isolate transient structures of the developing brain, such as the cortical subplate and intermediate zone, given their inconsistent appearance in the gestational period studied. Rather, to account for the evolving nature and resolution of these neuroanatomic structures, they were included in the broader tissue classes of the “FWM.” In DSS, the primary contributor is from the development of deep GM structures, however, does also include the emerging internal capsule and subventricular zone. This type of “contamination” of GM and WM throughout the fetal zones does limit the ability to compare GM versus WM development, however, does allow for the consistent measures of evolving tissue classes as they develop into the mature cerebrum. Improvements in in vivo fetal imaging will hopefully improve image resolution and allow for the more accurate delineation of these small but important structures. Furthermore, important ex vivo studies correlating high-field MRI with neurohistopathology will provide more precise understanding of how these tissue classes develop and evolve in vivo (Kostovic et al. 2014; Vasung et al. 2016).
The evaluation of cerebral lateralization is incomplete without the additional analysis of regional cortical surface development. There is a paucity of automated methods to accurately distinguish the cortical–WM interface, which is needed to quantify cortical thickness and gyral formation, and manual isolation remains subjective and time-consuming. Technical improvements in automated methods of surface analysis for the rapidly evolving fetal cerebrum, however, will allow for future studies to quantify volumetric growth as well as regional and lobar cortical surface development. Ongoing studies to determine the impact of fetal lateralization on functional outcome, such as language development and handedness, are also warranted.
Our study was also limited in that images were acquired on two different platforms (Philips and GE Healthcare) including variations in voxel size. Eighty-nine percent of subject images were acquired with voxel size of 1.25 × 1.66 × 2 mm3 using 32 cm field of view (FOV); the remaining 11% of subject images were acquired with a larger FOV and subsequent voxel dimension based on fetal brain size. However, in comparing growth trajectories between the 2 study sites, we did not detect any significant differences, although the differences in voxel size may account for some of the variability seen at the higher gestational ages. In addition, overall more subjects were studied in the third trimester compared with the second trimester where brain volumes are already quite small. Future studies are warranted to confirm second trimester brain development, although the exponential increase in volume we demonstrate is in line with previous reports of neuropathology specimens. Similarly, there is a relative decrease in study subjects beyond 37 weeks gestational age, although our results are comparable with existing data for the same gestational window.
Conclusion
This study provides a novel insight into the complex trajectories of in utero brain development at the global hemispheric, regional, and tissue levels in healthy control fetuses. Recognizing the in utero influences on hemispheric development, including cerebro-cerebellar development, may allow for the in utero detection of cognitive and neuropsychiatric diseases that otherwise would present later in childhood or young adulthood. Moreover, to date, third trimester cerebral cortical development in premature infants has been compared with MRI measures of full-term healthy newborns at term equivalent age. The availability of in utero comparable gestational age fetal MRI studies will offer a novel way to establish and compare ex utero brain development in premature infants from true, in utero normal development. This will provide the opportunity to study the onset and progression of cerebral cortical development following early extrauterine life exposure and provide a more accurate reference for neurodevelopmental outcomes.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Supplementary Material
Notes
We thank our families who participated in this study. Conflict of Interest: None declared.
Funding
The Canadian Institutes of Health Research (MOP-81116); the Intellectual and Developmental Disabilities Award (NICHD-2P30HD040677-11) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
- Brain Development Cooperative Group. 2012. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb Cortex. 22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalar JL, Greenstein DK, Clasen L, Tossell JW, Miller R, Evans AC, Mattai AA, Rapoport JL, Gogtay N. 2009. General absence of abnormal cortical asymmetry in childhood-onset schizophrenia: a longitudinal study. Schizophr Res. 115:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, et al. . 2010. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 68:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD. 2013. Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum. 12:721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondiaux E, Sileo C, Nahama-Allouche C, Moutard ML, Gelot A, Jouannic JM, Ducou le Pointe H, Garel C. 2013. Periventricular nodular heterotopia on prenatal ultrasound and magnetic resonance imaging. Ultrasound Obstet Gynecol. 42:149–155. [DOI] [PubMed] [Google Scholar]
- Bolduc ME, du Plessis AJ, Sullivan N, Guizard N, Zhang X, Robertson RL, Limperopoulos C. 2012. Regional cerebellar volumes predict functional outcome in children with cerebellar malformations. Cerebellum. 11:531–542. [DOI] [PubMed] [Google Scholar]
- Brandler WM, Morris AP, Evans DM, Scerri TS, Kemp JP, Timpson NJ, St Pourcain B, Smith GD, Ring SM, Stein J, et al. . 2013. Common variants in left/right asymmetry genes and pathways are associated with relative hand skill. PLoS Genet. 9:e1003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 9:110–122. [DOI] [PubMed] [Google Scholar]
- Chance SA. 2014. The cortical microstructural basis of lateralized cognition: a review. Front Psychol. 5:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Reglade-Meslin C, Kumar R, Sachdev P, Anstey KJ. 2010. Mild cognitive disorders are associated with different patterns of brain asymmetry than normal aging: The PATH through Life Study. Front Psychiatry. 1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Vazquez D, Felton A, McDowell A. 2016. Structural asymmetry of the human cerebral cortex: regional and between-subject variability of surface area, cortical thickness, and local gyrification. Neuropsychologia. 300012–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, Tworetzky W, McElhinney DB, Brown DW, Gholipour A, Kudelski D, Warfield SK, McCarter RJ Jr, et al. . 2013. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 23:2932–2943. [DOI] [PubMed] [Google Scholar]
- Clouchoux C, Guizard N, Evans AC, du Plessis AJ, Limperopoulos C. 2012. a. Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am J Obstet Gynecol. 206 (173):e171–178. [DOI] [PubMed] [Google Scholar]
- Clouchoux C, Kudelski D, Gholipour A, Warfield SK, Viseur S, Bouyssi-Kobar M, Mari JL, Evans AC, du Plessis AJ, Limperopoulos C. 2012. b. Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct Funct. 217:127–139. [DOI] [PubMed] [Google Scholar]
- Corbett-Detig J, Habas PA, Scott JA, Kim K, Rajagopalan V, McQuillen PS, Barkovich AJ, Glenn OA, Studholme C. 2011. 3D global and regional patterns of human fetal subplate growth determined in utero. Brain Struct Funct. 215:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis D, Maugey-Laulom B, Carles D, Pedespan JM, Brun M, Chateil JF. 2001. Prenatal diagnosis of schizencephaly by fetal magnetic resonance imaging. Fetal Diagn Ther. 16:354–359. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Borradori-Tolsa C, Mangin JF, Huppi PS. 2008. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 18:1444–1454. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Lazeyras F, Borradori-Tolsa C, Leuchter RH, Mangin JF, Huppi PS. 2010. Structural asymmetries of perisylvian regions in the preterm newborn. Neuroimage. 52:32–42. [DOI] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Huppi PS, Hertz-Pannier L. 2014. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 276:48–71. [DOI] [PubMed] [Google Scholar]
- Eide MG, Moster D, Irgens LM, Reichborn-Kjennerud T, Stoltenberg C, Skjaerven R, Susser E, Abel K. 2013. Degree of fetal growth restriction associated with schizophrenia risk in a national cohort. Psychol Med. 43:2057–2066. [DOI] [PubMed] [Google Scholar]
- Elnakib A, Soliman A, Nitzken M, Casanova MF, Gimel'farb G, El-Baz A. 2014. Magnetic resonance imaging findings for dyslexia: a review. J Biomed Nanotechnol. 10:2778–2805. [DOI] [PubMed] [Google Scholar]
- Fan L, Tang Y, Sun B, Gong G, Chen ZJ, Lin X, Yu T, Li Z, Evans AC, Liu S. 2010. Sexual dimorphism and asymmetry in human cerebellum: an MRI-based morphometric study. Brain Res. 1353:60–73. [DOI] [PubMed] [Google Scholar]
- Floris DL, Lai MC, Auer T, Lombardo MV, Ecker C, Chakrabarti B, Wheelwright SJ, Bullmore ET, Murphy DG, Baron-Cohen S, et al. . 2015. Atypically rightward cerebral asymmetry in male adults with autism stratifies individuals with and without language delay. Hum Brain Mapp 230–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francks C. 2015. Exploring human brain lateralization with molecular genetics and genomics. Ann N Y Acad Sci. 1359:1–13. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. 2002. Age-related total gray matter and white matter changes in normal adult brain. Part II: quantitative magnetization transfer ratio histogram analysis. AJNR Am J Neuroradiol. 23:1334–1341. [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, et al. . 2007. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 27:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths PD, Wilkinson ID, Variend S, Jones A, Paley MN, Whitby E. 2004. Differential growth rates of the cerebellum and posterior fossa assessed by post mortem magnetic resonance imaging of the fetus: implications for the pathogenesis of the Chiari 2 deformity. Acta Radiol. 45:236–242. [DOI] [PubMed] [Google Scholar]
- Grissom NM, Reyes TM. 2013. Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int J Dev Neurosci. 31:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeschel S, Vollmer B, King MD, Connelly A. 2010. Developmental changes in cerebral grey and white matter volume from infancy to adulthood. Int J Dev Neurosci. 28:481–489. [DOI] [PubMed] [Google Scholar]
- Grossman R, Hoffman C, Mardor Y, Biegon A. 2006. Quantitative MRI measurements of human fetal brain development in utero. Neuroimage. 33:463–470. [DOI] [PubMed] [Google Scholar]
- Habas PA, Scott JA, Roosta A, Rajagopalan V, Kim K, Rousseau F, Barkovich AJ, Glenn OA, Studholme C. 2012. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb Cortex. 22:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund NG, Kallen KB. 2011. Risk factors for autism and Asperger syndrome. Perinatal factors and migration. Autism. 15:163–183. [DOI] [PubMed] [Google Scholar]
- Hodge SM, Makris N, Kennedy DN, Caviness VS Jr, Howard J, McGrath L, Steele S, Frazier JA, Tager-Flusberg H, Harris GJ. 2010. Cerebellum, language, and cognition in autism and specific language impairment. J Autism Dev Disord. 40:300–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Chang L, Ernst TM, Curran M, Buchthal SD, Alicata D, Skranes J, Johansen H, Hernandez A, Yamakawa R, et al. . 2014. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol. 71:1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. 2012. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 72:1317–1326. [DOI] [PubMed] [Google Scholar]
- Kasprian G, Langs G, Brugger PC, Bittner M, Weber M, Arantes M, Prayer D. 2011. The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cereb Cortex. 21:1076–1083. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JW, Kim GH, Roh JH, Kim MJ, Seo SW, Kim ST, Jeon S, Lee JM, Heilman KM, et al. . 2012. Cortical asymmetries in normal, mild cognitive impairment, and Alzheimer's disease. Neurobiol Aging. 33:1959–1966. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. 2008. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 28:12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Jovanov-Milosevic N, Rados M, Sedmak G, Benjak V, Kostovic-Srzentic M, Vasung L, Culjat M, Huppi P, Judas M. 2014. Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct. 219:231–253. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M, Rados M, Hrabac P. 2002. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 12:536–544. [DOI] [PubMed] [Google Scholar]
- Kuklisova-Murgasova M, Aljabar P, Srinivasan L, Counsell SJ, Doria V, Serag A, Gousias IS, Boardman JP, Rutherford MA, Edwards AD, et al. . 2011. A dynamic 4D probabilistic atlas of the developing brain. Neuroimage. 54:2750–2763. [DOI] [PubMed] [Google Scholar]
- Lane RH. 2014. Fetal programming, epigenetics, and adult onset disease. Clin Perinatol. 41:815–831. [DOI] [PubMed] [Google Scholar]
- Lang SS, Goldberg E, Zarnow D, Johnson MP, Storm PB, Heuer GG. 2014. Prenatal diagnosis of hemimegalencephaly. World Neurosurg. 82 (241):e245–248. [DOI] [PubMed] [Google Scholar]
- Lavezzi AM, Ottaviani G, Terni L, Matturri L. 2006. Histological and biological developmental characterization of the human cerebellar cortex. Int J Dev Neurosci. 24:365–371. [DOI] [PubMed] [Google Scholar]
- Li G, Nie J, Wang L, Shi F, Lyall AE, Lin W, Gilmore JH, Shen D. 2014. Mapping longitudinal hemispheric structural asymmetries of the human cerebral cortex from birth to 2 years of age. Cereb Cortex. 24:1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ. 2005. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 115:688–695. [DOI] [PubMed] [Google Scholar]
- Lindell AK, Hudry K. 2013. Atypicalities in cortical structure, handedness, and functional lateralization for language in autism spectrum disorders. Neuropsychol Rev. 23:257–270. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhang Z, Lin X, Teng G, Meng H, Yu T, Fang F, Zang F, Li Z, Liu S. 2011. Development of the human fetal cerebellum in the second trimester: a post mortem magnetic resonance imaging evaluation. J Anat. 219:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makropoulos A, Aljabar P, Wright R, Huning B, Merchant N, Arichi T, Tusor N, Hajnal JV, Edwards AD, Counsell SJ, et al. . 2015. Regional growth and atlasing of the developing human brain. Neuroimage. 125:456–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, Miyawaki T. 2001. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb Cortex. 11:335–342. [DOI] [PubMed] [Google Scholar]
- Miller SL, Huppi PS, Mallard C. 2015. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol. 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KB, Blair E. 2015. Prenatal factors in singletons with cerebral palsy born at or near term. N Engl J Med. 373:946–953. [DOI] [PubMed] [Google Scholar]
- Nielsen PR, Mortensen PB, Dalman C, Henriksen TB, Pedersen MG, Pedersen CB, Agerbo E. 2013. Fetal growth and schizophrenia: a nested case-control and case-sibling study. Schizophr Bull. 39:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, Chitnis X, Williams SC, Murray RM. 2008. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 131:205–217. [DOI] [PubMed] [Google Scholar]
- Orasanu E, Melbourne A, Cardoso MJ, Modat M, Taylor AM, Thayyil S, Ourselin S. 2014. Brain volume estimation from post-mortem newborn and fetal MRI. Neuroimage Clin. 6:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. 2009. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci. 29:11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Li G, Shi F, Shi C, Yang Q, Chan RC, Shen D. 2015. Cortical asymmetries in unaffected siblings of patients with obsessive-compulsive disorder. Psychiatry Res. 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. 2004. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 25:377–396. [DOI] [PubMed] [Google Scholar]
- Ribolsi M, Daskalakis ZJ, Siracusano A, Koch G. 2014. Abnormal asymmetry of brain connectivity in schizophrenia. Front Hum Neurosci. 8:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righini A, Zirpoli S, Mrakic F, Parazzini C, Pogliani L, Triulzi F. 2004. Early prenatal MR imaging diagnosis of polymicrogyria.. AJNR Am J Neuroradiol. 25:343–346. [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Oubel E, Pontabry J, Schweitzer M, Studholme C, Koob M, Dietemann JL. 2013. BTK: an open-source toolkit for fetal brain MR image processing. Comput Methods Programs Biomed. 109:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubod C, Robert Y, Tillouche N, Devisme L, Houfflin-Debarge V, Puech F. 2005. Role of fetal ultrasound and magnetic resonance imaging in the prenatal diagnosis of migration disorders. Prenat Diagn. 25:1181–1187. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill D, Leach MO, Hawkes DJ. 1999. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 18:712–721. [DOI] [PubMed] [Google Scholar]
- Scott JA, Habas PA, Kim K, Rajagopalan V, Hamzelou KS, Corbett-Detig JM, Barkovich AJ, Glenn OA, Studholme C. 2011. Growth trajectories of the human fetal brain tissues estimated from 3D reconstructed in utero MRI. Int J Dev Neurosci. 29:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Hamzelou KS, Rajagopalan V, Habas PA, Kim K, Barkovich AJ, Glenn OA, Studholme C. 2012. 3D morphometric analysis of human fetal cerebellar development. Cerebellum. 11:761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenwurm D, Bird CR, Enzmann DR, Marshall WH. 1985. Left-right temporal region asymmetry in infants and children. AJNR Am J Neuroradiol. 6:777–779. [PMC free article] [PubMed] [Google Scholar]
- Serag A, Aljabar P, Ball G, Counsell SJ, Boardman JP, Rutherford MA, Edwards AD, Hajnal JV, Rueckert D. 2012. a. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage. 59:2255–2265. [DOI] [PubMed] [Google Scholar]
- Serag AK, Kyriakopoulou V, Rutherford MA, Edwards AD, Hajnal JV, Aljabar P, Counsel SJ, Boardmen JP, Rueckert D. 2012. b. A multi-channel 4D probabilistic atlas of the developing brain: application to fetuses and neonates. Annals of the BMVA. 2012:1–14. [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, Toga AW. 2002. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex. 12:17–26. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Volkmann J, Jancke L, Freund HJ. 1991. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol. 29:315–319. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Narr KL, Phillips OR, McCormack J, Sevy S, Gunduz-Bruce H, Kane JM, Bilder RM, Robinson DG. 2012. Magnetic resonance imaging predictors of treatment response in first-episode schizophrenia. Schizophr Bull. 38:569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Moussai J, Zohoori S, Goldkorn A, Khan AA, Mega MS, Small GW, Cummings JL, Toga AW. 1998. Cortical variability and asymmetry in normal aging and Alzheimer's disease. Cereb Cortex. 8:492–509. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. 2003. Mapping brain asymmetry. Nat Rev Neurosci. 4:37–48. [DOI] [PubMed] [Google Scholar]
- Tullett AM, Harmon-Jones E, Inzlicht M. 2012. Right frontal cortical asymmetry predicts empathic reactions: support for a link between withdrawal motivation and empathy. Psychophysiology. 49:1145–1153. [DOI] [PubMed] [Google Scholar]
- Turner BO, Marinsek N, Ryhal E, Miller MB. 2015. Hemispheric lateralization in reasoning. Ann N Y Acad Sci. 1359:47–64. [DOI] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. 2010. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Lepage C, Rados M, Pletikos M, Goldman JS, Richiardi J, Raguz M, Fischi-Gomez E, Karama S, Huppi PS, et al. . 2016. Quantitative and qualitative analysis of transient fetal compartments during prenatal human brain development. Front Neuroanat. 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. 2008Neurology of the newborn. Philadelphia: Saunders/Elsevier. [Google Scholar]
- Wada JA, Clarke R, Hamm A. 1975. Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch Neurol. 32:239–246. [DOI] [PubMed] [Google Scholar]
- Wang D, Buckner RL, Liu H. 2013. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J Neurophysiol. 109:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. 2006. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 31:1116–1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.