Abstract
Dehydroepiandrosterone (DHEA) and its sulfated form, DHEA-S, peak in young adulthood and then decrease dramatically with age. However, there is extensive variation in this age-related hormone decline, suggesting an early decrement may be associated with lower vitality and be prognostic of poor health in old age. To determine whether DHEA-S and DHEA are correlated with physical indices of vitality, hormone levels were analyzed with respect to clinical health histories, physical functioning including grip strength, gait speed and repetitive standing, and self-reported chronic pain. The participants (N = 1,214) were 35–86 years of age from a nationally representative survey, Midlife Development in the United States. DHEA-S and DHEA below age-expected levels were associated with more chronic illness conditions and self-reported persistent pain and pain sensitivity upon manual palpation. Additionally, lower DHEA-S and DHEA correlated with poorer performance on tests of physical functioning by middle age suggesting a more precipitous decline is already indicative of reduced vigor and physical strength. When considered with respect to age- and gender-typical norms, larger decrements in DHEA-S and DHEA may be causally related to the loss of physical vitality. Conversely, when hormone secretion is sustained in older adults, it conveys reduced risk for the physical weakness and ailments that precede frailty.
Keywords: Biomarker, Endocrinology, Frailty
Dehydroepiandrosterone (DHEA) and its sulfated form, dehydroepiandrosterone sulfate (DHEA-S), are the most abundant steroid hormones in human circulation (1) and are important precursors of androgen and estrogen biosynthesis (2). Beyond functioning as a substrate for other steroids, a considerable body of evidence from both basic and clinical research indicates there are additional physiological roles for DHEA (3,4). Higher levels have been associated with psychological well-being and better physical functioning, including with muscle strength and bone density, as well as having anti-inflammatory and immunoregulatory actions (1,5). DHEA is often described as the more active hormone; however, the majority of circulating DHEA is in the sulfated form and some tissues, including the brain, are responsive to DHEA-S (6). The sulfated form is water-soluble and may be more readily excreted by the kidneys, but DHEA-S actually has a longer half-life and its levels are more stable in circulation, with less diurnal variation (7). Both DHEA-S and DHEA are much higher in young adults and decline progressively with age (5), which led to clinical studies on the benefits of supplementation. Although these trials yielded inconsistent results, the assessment of the decline in DHEA-S and DHEA levels has remained popular in studies on the biology of aging.
Decreases in DHEA-S and DHEA parallel age-related impairments in physical and mental abilities (8), but there are also marked individual differences in this age-related decline. When DHEA levels are below age-expected values, they have been linked to higher cardiovascular disease mortality and all-cause mortality (4,9). Furthermore, larger decrements in DHEA over time also appear to predict a greater likelihood of developing other chronic diseases, such as type 2 diabetes (10). Because patients with diabetes and cardiovascular disease have been found to have low DHEA levels, the directionality of these associations has yet to be fully established; however, the distinction between a typical age-related decline and an accelerated decrement in DHEA may be significant and was the specific focus of this study.
The timing and magnitude of the age-related decrement in DHEA may also provide a prognostic biomarker predictive of the risk for later physical impairment and disability. Our analyses focused on several tests of physical functioning and strength that are important dimensions of overall vitality and vigor. Vitality is typically defined as a positive mental and physical state, associated with stamina and energy, and beneficially linked to health (11). Conversely, a loss of vitality impairs the quality of life and, when significantly compromised, becomes a defining feature of the clinical condition of frailty (12). Thus, it is of importance to take an integrative life course perspective, considering earlier age-related changes in middle-aged adults that are antecedent to the geriatric declines associated with morbidity and ultimately mortality (13). The biological basis for reduced vitality is complex, but it is likely that decreased neuroendocrine activity plays a role. We were specifically interested in the relationship between DHEA and physical function. DHEA, as well as the anabolic steroid hormones derived from DHEA, can affect many processes associated with the physical aspects of vitality (14,15), including lean muscle and bone mass, which ultimately present clinically as sarcopenia and osteoporosis in older adults (16). Our aim was to determine if age-related declines in DHEA-S and DHEA are specifically associated with poorer physical functioning already in middle age, in addition to the impairments evident later in older adults.
An association between particularly low levels of DHEA-S and DHEA and frailty was reported previously in elderly patients (17–19), but tests of physical vitality are not as routinely included in nationally representative studies. We utilized the Midlife Development in the United States (MIDUS) to determine the degree to which knowledge of DHEA-S and DHEA would help to explain individual differences in physical strength and function beyond the expected influence of chronological age and gender. Physical functioning and mobility were measured with tests of gait speed, grip strength, and repetitive standing from a sitting position, providing three measures of functional capacity documented to have predictive value for major health-related outcomes. Gait speed is a key indicator of overall health status, with reduced walking speeds caused by advanced age or illness-related declines in mobility (14,20). Similarly, grip strength is a commonly used proxy of overall body strength and decreases in force parallel the increased risk of mortality in both middle-aged and elderly populations (21,22). Both slower walking speeds and decreased grip strength are accessible and sensitive indicators of vitality and have also been employed to identify the frailty phenotype because they integrate decrements in many organ systems and reflect changes in physiological functions (13,14,23). Our repetitive standing task measured balance and lower extremity strength, and it is also affected negatively by age and illness (24). A cross-sectional analysis of older males found that higher DHEA was associated with better physical performance as measured by chair-to-standing speed (25). We also incorporated an assessment of pain sensitivity because it is known that DHEA can have anti-inflammatory actions, and pain symptoms are commonly associated with age-related frailty and disabling conditions (26). Indeed, chronic pain can challenge well-being, compromise physical functioning, and accelerate the development of frailty in older adults (27). Additionally, there are well-established sex differences in the prevalence of clinical pain syndromes, with women experiencing more chronic pain and somatization disorders than men. Previous research in frail individuals of a residential care population found an inverse association between DHEA-S levels and the number of pain sites, but only in females (19).
We hypothesized that individuals with lower-than-age-expected levels of DHEA-S and DHEA would report more chronic illness conditions and perform worse on functional assessments of physical vitality. A secondary aim was to evaluate the utility of DHEA as compared to DHEA-S, because there are significant gender differences in the secretion of these two hormones. Men typically have higher levels of DHEA-S than women across the life span, but the age-related decline in DHEA-S is greater for men than women (28). DHEA levels are usually comparable, in part because premenopausal women supplement the adrenal contribution with approximately 10% of their DHEA secreted from the ovary (2,29). However, sex steroid secretion by the ovaries stops at menopause, which would end this source of DHEA in older women (29). Thus, it was possible that differential hormone levels or variation in the pace of decline could contribute to the gender differences observed in the physical functioning of older adults.
Methods
Participants
These analyses were based on 1,214 middle-aged and older adults (43.2% male; 19.1% African American) who had participated in a national survey, MIDUS (additional information about MIDUS can be found at: www.midus.wisc.edu). MIDUS is a longitudinal study, begun in 1995–1996, of a nationally representative sample of adults recruited from the 48 continental states via random telephone dialing. Participants were drawn from the Biomarker Project (30), a follow-up assessment in the second phase of MIDUS, which took place between 2004 and 2009 and included a 2-day stay at one of three Clinical and Translational Research centers (CTR, Madison, WI; Washington, DC; or Los Angeles, CA). To increase the representation of African American participants during MIDUS II, a city-specific sample was recruited from Milwaukee, WI, which resulted in a purposeful oversampling of African Americans (N = 232). Biomarker Project participants were between 35 and 86 years of age. On Day 1 of the CTR visit, participants were interviewed to obtain a detailed medical history and medication review, in addition to completing questionnaires on psychological and behavioral factors. Physical assessments were also conducted by the CTR nurses. Early on the morning of on Day 2, participants provided a fasting blood sample from which DHEA-S and DHEA were determined.
This research was approved by Institutional Review Boards at Health Sciences Center, University of Wisconsin-Madison, as well as at Georgetown University and the University of California, Los Angeles. All participants provided written informed consent. No data were included if there was incomplete information without a recorded explanation.
Measures
Self-reported health
After admittance to the CTR on Day 1, a detailed medical history was obtained. Chronic illness conditions, comorbid causes of disability, and increased risk of mortality, over the preceding 12 months, were self-reported. The presence of chronic or persistent pain was also determined via self-report during the clinical interview. In addition, the 510 participants who visited the Wisconsin CTR underwent an exam of pain sensitivity as indicated by the presence or absence of widespread muscle pain or muscle tenderness at tender points. A nurse recorded the number of localized areas of pain sensitivity after applying 4kg of pressure at the 18 body locations used traditionally for diagnosing fibromyalgia, as recommended by the American College of Rheumatology between 1990 and 2010 (31).
Physical function
Physical functioning was assessed with three tests: a timed 15.24-m walk, grip strength, and repetitive chair stands. For the timed-walk, participants walked 7.62 m to a turnaround point and then back at their usual speed. The time to complete this walk was recorded and converted into gait speed (gait speed = m/s). Eleven individuals unable to complete this task were given scores of 0 m/s. For grip strength, the subject squeezed a Dynamometer three times with their dominant hand (mean kg/force). Lower-body strength was evaluated with the Chair Stand test (24). Subjects were instructed to sit in a straight-back chair with their feet flat on the floor and arms folded; then to serially stand and sit five times as quickly as possible. Time to complete these chair stands was recorded (in seconds). Individuals unable to perform the task (N = 55) due to back or knee pain were scored at 32 seconds, 1 second longer than the highest score for those completing the task.
Hormone determinations
DHEA-S and DHEA levels were determined after an overnight fast, from blood collected by the CTR nurses on Day 2. Blood was obtained between 0600 and 0800 to control for diurnal rhythms. Plasma was separated via centrifugation and 1.0mL aliquots stored in cryovials at −65°C until DHEA-S and DHEA concentrations were quantified by immunoelectrochemiluminescence and liquid chromatography-tandem mass spectrometry, respectively. Both DHEA-S and DHEA were run at a CAP and CLIA-certified laboratory (ARUP, Salt Lake City, UT).
Covariates
Age, gender, and race were included as covariates in the analyses because they can affect DHEA levels. Age was considered both as a continuous and categorical variable (<49, 50–64, 65+ years). In addition, body mass index (BMI) was included as a fourth covariate, because of the possible influence of adiposity on hormone levels and physical function. Nurses recorded the height and weight used to calculate the BMI (weight/height2). Our aim was to examine the association between hormone levels and physical function beyond the influence of these factors. Some analyses were also run for males and females separately after determining a significant main effect for gender.
Data Analytic Strategy
Statistical tests were conducted with the Statistical Package for the Social Sciences, Version 23.0 (SPSS, Chicago, IL) and R (R Core Team, 2015). To limit the influence of extreme outliers, four DHEA-S and DHEA values (0.3% of the values) underwent winsorization and were set at the upper or lower 3 SD point (32). The three high DHEA-S values were 23769.5, 20299.5, and 17176.5 nmol/L and were set to 16482 nmol/L; the one low DHEA value of 1.39 nmol/L was set at 2.08 nmol/L. DHEA-S and DHEA values were then log transformed to reduce skew and normalize the distribution. Data were analyzed using general linear models. Analysis of covariance was used to test the association with chronic pain and pain sensitivity, after covarying for age, gender, race, and BMI. Post hoc testing was based on planned orthogonal contrasts. Linear regression was employed to analyze the relationship between DHEA-S or DHEA and both self-reported and functional assessments of health after accounting for the influence of age, gender, race, and BMI. Relationships between variables were tested with the Pearson test. Fisher R-to-Z transformation was used to calculate a Z-value, which then was used to assess the significance of a difference between two correlation coefficients. Alpha levels less than .05 were required for significance.
Results
Participants in this Biomarker Project did not differ significantly from the overall survey participants with respect to age, gender composition, or marital status (30). However, they tended to be more educated. On average, participants reported having 3 or more years of college, although 25% still had attained only a high school degree and 50% had not completed college. Table 1 provides descriptive summary statistics for the sociodemographic, health status, and biomarker variables, stratified by gender. As expected, one-way analyses of variance revealed gender differences on several measures of physical function. On average, males were faster to complete the 15.24-m walk (p < .001) and five chair stands (p < .001), and male grip strength was significantly stronger (p < .001). Females reported 0.76 more chronic and comorbid conditions, on average, than males (F(1, 1,212) = 20.50, p < .001). Females also had slightly lower mean DHEA-S (lower by 0.24 log nmol/L; t(1,212) = 152.57, p < .001), but there was not a sex difference for DHEA. Mean age and BMI were similar for male and female participants.
Table 1.
Descriptive Statistics for MIDUS Participants Including the Four Indices of Function
| Male | Female | |
|---|---|---|
| No. of subjects | 524 | 690 |
| No. reporting chronic pain | 175 | 254 |
| Mean (SD) | Mean (SD) | |
| Age (y) | 57.61 (11.65) | 56.85 (11.37) |
| BMI (kg/m2) | 29.67 (5.42) | 29.68 (7.42) |
| DHEA-S (log nmol/L) | 3.48 (0.30) | 3.24 (0.35)* |
| DHEA (log nmol/L) | 1.22 (0.26) | 1.21 (0.32) |
| Chronic conditions | 3.63 (2.55) | 4.39 (3.13)* |
| Gait speed (m/s) | 1.07 (0.24) | 1.00 (0.26)* |
| Chair stand (s) | 11.00 (8.03) | 13.14 (10.06)* |
| Grip strength (kg/force) | ||
| Left hand | 43.36 (10.29) | 25.57 (7.37)* |
| Right hand | 44.27 (10.31) | 27.16 (7.65)* |
| Dominant hand | 44.37 (10.16) | 27.29 (7.57)* |
Notes: Mean values for men and women. DHEA-S and DHEA were log transformed for analysis. BMI = body mass index; DHEA = dehydroepiandrosterone; DHEA-S = dehydroepiandrosterone sulfate; MIDUS = Midlife Development in the United States.
*p < .05 indicates significant effect of gender.
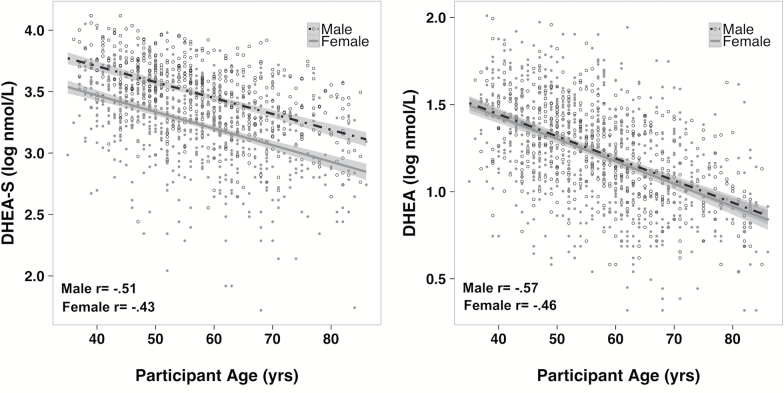
DHEA-S and DHEA declined significantly with age and were highly correlated with chronological age (r = −.60, p < .001 and r = −.56, p < .001, respectively) (Figure 1). Therefore, the subsequent analyses focused on the degree to which each participant’s DHEA-S or DHEA value was below or above the age-typical decrement. Because physical functioning is also affected by age, all analyses first controlled for the effect of age and the influence of three other factors: gender, race, and BMI.
Figure 1.
Plasma levels of DHEA-S and DHEA decreased significantly with age for both males (p < .001 and p < .001, respectively) and females (p < .001 and p < .001, respectively). The correlations with chronological age from the nationally representative participants in the MIDUS project are shown in each graph. Men had higher levels of DHEA-S than women, but DHEA levels were similar. The DHEA-S and DHEA residuals used in our analyses and in following figures were created with respect to the regression line and thus, were generated with respect to the average value for each year of age. DHEA = dehydroepiandrosterone; DHEA-S = dehydroepiandrosterone sulfate; MIDUS = Midlife Development in the United States.
Assessments of Vitality
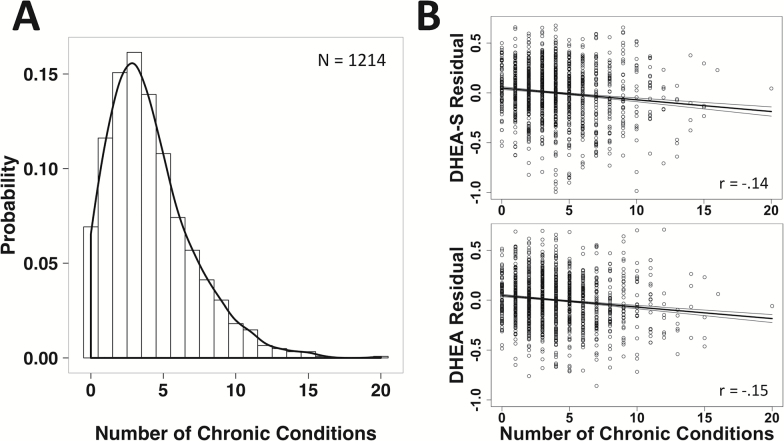
On average, participants reported four chronic conditions (95% CI = 3.89–4.22), with the number of comorbid conditions increasing with participant age (b = .09, t(1,212) = 13.54, p < .001). Levels of DHEA-S (b = −.02, t(1,208) = −5.68, p < .001) and DHEA (b = −.02, t(1,208) = −5.94, p < .001) beyond the expected age-related decline co-occurred with a higher number of chronic illness conditions after taking into account the participant’s age, gender, race, and adiposity (Figure 2).
Figure 2.
Probability distribution of chronic health conditions reported by participants (A), and the association between number of symptoms and the extent of the decrement in DHEA-S and DHEA (B). For illustrative purposes, the unstandardized residuals of DHEA-S and DHEA are plotted, after accounting for variance attributed to participant age, gender, race, and body mass index. As the number of ailments and illnesses increased, the residual DHEA-S and DHEA values decreased, indicating that hormone levels were below the level expected for the participant’s age after taking gender, race, and adiposity into account. Symptom number was inversely correlated with both the residuals for DHEA-S (r = −.14, p < .001) and DHEA (r = −.15, p < .001). DHEA = dehydroepiandrosterone; DHEA-S = dehydroepiandrosterone sulfate.
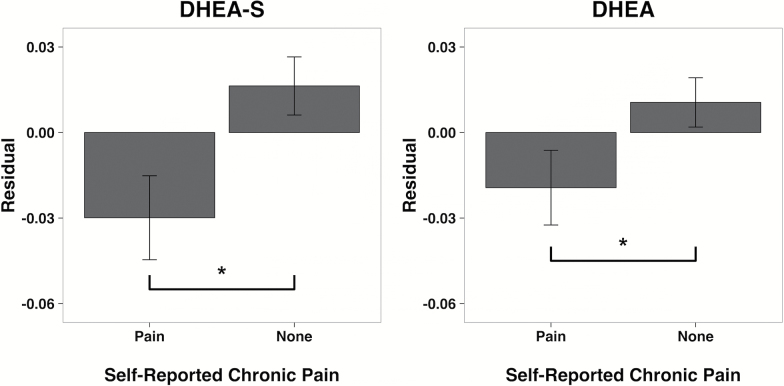
Self-reported chronic pain also was more common in participants with lower DHEA-S and DHEA after taking into account the influence of age and hormone differences predicted by gender, race, and adiposity (Figure 3). The DHEA-S and DHEA residuals were significantly lower in those reporting pain (F(1, 1,208) = 6.97, p = .008; F(1, 1,208) = 3.91, p = .048, respectively). As compared to participants without pain, the 426 individuals who self-reported experiencing chronic or severe pain had both significantly lower DHEA-S (3.30 vs 3.37 log nmol/L, respectively) and DHEA (1.19 vs 1.23 log nmol/L, respectively).
Figure 3.
Association between self-reported presence of chronic pain and DHEA-S and DHEA. For illustrative purposes, unstandardized residuals of DHEA-S and DHEA are shown, after taking into account the participant’s age, gender, race, and body mass index. The 429 individuals reporting persistent and chronic pain had DHEA-S and DHEA below the levels expected for their age, significantly lower than the 785 participants not experiencing chronic pain. This effect was more prominent for DHEA-S; participants reporting pain had larger, negative residuals than those with no pain (below the predicted level for their age; p < .01). Mean ± SE values are portrayed. *p < .05, significantly below residuals for those with no chronic pain. DHEA = dehydroepiandrosterone; DHEA-S = dehydroepiandrosterone sulfate.
A similar relationship was found with the quantitative assessment of pain sensitivity conducted on a subset of the participants by the CTR nursing staff. Of the 510 participants who completed this exam at the Wisconsin CTR, 384 reported no pain, 73 reported one to two sensitive locations, and 53 reported experiencing pain at three or more locations. After taking into account the influence of age, gender, race, and adiposity, there was a significant difference in plasma DHEA-S and DHEA between these three groups (F(2, 503) = 7.81, p < .001 and F(2, 503) = 3.42, p = .033, respectively). Post hoc tests indicated individuals who experienced pain at three or more body locations were more likely to have lower-than-expected DHEA-S and DHEA, levels significantly below those with no pain sensitivity who had hormone levels appropriate for their age.
Lower scores on performance-based measures of physical functioning were also significantly associated with decrements in DHEA-S and DHEA below the age-predicted values, after adjusting for the influence of gender, race, and adiposity. For each decline in gait speed by 1 m/s, the mean level of DHEA-S decreased by 0.096 log nmol/L (t(1,208) = 2.64, p = .008) and the mean DHEA decreased by 0.086 log nmol/L (t(1,208) = 2.73, p = .006). Similarly, DHEA-S and DHEA decrements were larger and more negative as hand grip strength weakened, after taking age, gender, and race into account (t(1,208) = 2.69, p = .007; t(1,208) = 2.67, p = .008, respectively). The capacity to engage in repetitive standing from a sitting position was also impaired when DHEA-S and DHEA were below the age- and gender-predicted levels. On average, for each additional second required to complete five chair stands, DHEA-S decreased by 0.004 log nmol/L (t(1,208) = −2.91, p = .004) and DHEA decreased by 0.004 log nmol/L (t(1,208) = −3.44, p = .001).
Gender Considerations
The magnitude of these relationships with the functional indices of vitality varied by gender in a differential way with respect to age. For females, the correlations between chronic illness conditions, gait speed, grip strength, and the time taken to perform five chair stands were more strongly associated with DHEA-S in younger individuals below 49 years than in women 65 years or older (p = .048, p = .026, p = .048, p = .050, respectively; Table 2). Hand grip strength in women younger than 49 years was also a better predictor of variation in DHEA-S than in women 50–64 years of age (r = .23 vs r = .05, respectively, p = .016). Similarly, DHEA was also more strongly correlated with chronic illness and self-reported pain in younger women. For grip strength, the extent of weakness was more strongly linked with DHEA in women below 49 years of age as compared to those 50–64 years of age. To convey the magnitude of this relationship with grip strength among females aged 49 and under, each 1 log nmol/L decrease in the age-adjusted DHEA-S residual would be expected to predict a 2.23kg/force weaker grip strength, after accounting for the participant’s race and BMI. The lowest age-adjusted residual within the younger age group was −2.66, which would predict a decrease in grip strength of 5.93kg/force, similar to the functional capability of a 71-year-old woman.
Table 2.
Age Analysis of DHEA-S/DHEA Levels and Chronic Illness Conditions, as well as Three Objective Indicators of Physical Functioning, in Female Participants
| DHEA-S (log nmol/L) | DHEA (log nmol/L) | |||||
|---|---|---|---|---|---|---|
| Vitality Measure | N | Mean (SD) | r | p | r | p |
| Chronic conditions | ||||||
| ≤49 | 271 | 3.29 (2.86) | −.23† | <.001* | −.22 | <.001* |
| 50–64 | 277 | 4.65 (2.86) | −.15 | .011* | −.25§ | <.001* |
| ≥65 | 142 | 5.96 (3.36) | −.06 | .43 | −.08 | .30 |
| Gait speed | ||||||
| ≤49 | 271 | 1.03 (0.23) | .19† | .001* | .14 | .019* |
| 50–64 | 277 | 1.00 (0.27) | .09 | .140 | .12 | .048* |
| ≥65 | 142 | 0.93 (0.25) | −.01 | .88 | .02 | .79 |
| Grip strength | ||||||
| ≤49 | 271 | 30.06 (7.16) | .23†,‡ | <.001* | .19‡ | .002* |
| 50–64 | 277 | 26.70 (7.57) | .05 | .38 | .04 | .53 |
| ≥65 | 142 | 23.37 (6.20) | .06 | .40 | .14 | .107 |
| Chair stand | ||||||
| ≤49 | 271 | 10.64 (5.75) | −.19† | .001* | −.09 | .126 |
| 50–64 | 277 | 12.54 (6.77) | −.14 | .024* | −.15 | .013* |
| ≥65 | 142 | 14.15 (6.78) | −.02 | .79 | −.08 | .37 |
Notes: All analyses controlled for the influence of body mass index and race. DHEA-S and DHEA values were log transformed. DHEA = dehydroepiandrosterone; DHEA-S = dehydroepiandrosterone sulfate.
*p < .05. Fisher’s r to z test was used to determine whether r values differed significantly (p < .05) between the three age groups.
†Significantly larger correlation than in women ≥65 y.
‡Significantly larger correlation than in women 50–64 y.
§Stronger correlation in women 50–64 years than in those ≥65 y (p = .047).
Among men, the association between physical function and hormone levels was most evident with respect to the test of Chair Stand speed. The pace of repetitive standing was slower if either DHEA-S or DHEA were below predicted levels, more so for men below 49 years than among men 50–64 year of age (r = −.19 vs r = −.03, p = .059; r = −.28 vs r = −.04, p = .008, respectively). To convey the extent of this association among males aged 49 and under, each 1 log nmol/L decrease in the age-adjusted DHEA-S residual would predict a 1.72 second increase in time needed to complete the chair standing task, after accounting for the participant’s race and BMI. Within the group of male participants between 35 and 49 years of age, the mean age-adjusted DHEA-S residual for those in the bottom quartile was −0.03. This decrement in DHEA-S would predict a slowing of the standing pace by 0.05 second, more comparable to the functional capacity of a 52-year-old male.
Discussion
These analyses have confirmed that age-associated decrements in DHEA-S and DHEA are correlated with age-related decreases in physical indices of vitality. Our models accounted for the typical age-related decline in both physical performance and endocrine function, and still found that lower DHEA-S and DHEA levels were associated with poorer physical functioning, both in middle-aged and older adults. Conversely, sustained hormone secretion in the elderly appears to be indicative of a decreased susceptibility to a loss of physical vitality. These results are consistent with the extant literature and support the use of DHEA as a bioindicator of vitality. It may also convey prognostic risk for the decrements in physical function characteristic of the clinical condition of frailty, which may further lower DHEA levels beyond the age-expected level in the frail elderly (17–19).
Furthermore, this relationship with physical functioning was evident even after taking into account the combined influence of age, gender, race, and BMI, suggesting that hormone decreases may also be causally related to reduced physical vitality. Both DHEA-S and DHEA have long been known to decline with age, and those decreases across the life span were clearly evident in our dataset. Lower DHEA-S would contribute to decreases in other anabolic steroids, including androstenedione, testosterone, and estrogen (3,4). Previous research has documented that these declines are associated with age-related reductions in muscle and bone mass and strength (5). However, our analytical strategy was also based on a more specific consideration of hormone decreases that went beyond the normal age-related decrement. Participants with DHEA-S and DHEA below age- and gender-expected levels performed worse on several tests of physical functioning as measured by gait speed, grip strength, and repetitive chair stands. These results likely reflect a more direct relationship between steroid hormones and muscle strength, bone strength, and mobility. When DHEA-S or DHEA levels are high, other studies have found better self-reported physical functioning (19) and demonstrated linkages with objective tests of physical function (25,33). Furthermore, DHEA supplementation trials indicate that while it does not enable rejuvenation, DHEA administration can increase muscle strength in aged and frail individuals (18,34). The protective effect of DHEA on muscle and bone may be largely mediated by conversion of DHEA to androgens and estrogens, but it is known that DHEA promotes the proliferation of osteoblasts and inhibit apoptosis via a mitogen-activated protein kinase signaling pathway independent of androgen and estrogen receptors (35).
DHEA values below age-expected levels have been found by others to be indicative of more cardiovascular disease mortality and all-cause mortality (4). In our study, men and women with DHEA-S and DHEA levels below age-expected norms reported significantly more chronic illness conditions. This finding concurs with the basic science findings on the immunomodulatory, antioxidant, and anti-inflammatory actions of DHEA (4). Additionally, DHEA is thought to have a protective influence on cardiovascular health through increasing endothelial cell proliferation and function, including via pathways independent of androgen and estrogen receptors (36). Alternatively, the association between DHEA and health could partially reflect the impact of illness or even subclinical inflammation on the neuroendocrine axis, which might shift adrenal steroid biosynthesis to favor the production of cortisol over DHEA (37). Thus, especially in older adults, lower DHEA levels may be a consequence of chronic illness and a correlate of the overall pathophysiology.
We also assessed the possible association with persistent pain, as many chronic health conditions include pain sensitization and symptoms, both of which increase with age (27). As predicted, both men and women with larger-than-normal decrements in DHEA-S and DHEA were more likely to report chronic pain conditions and evinced more tenderness when pressure was manually applied to sensitive body locations. These findings concur with a prior paper reporting that low DHEA-S was correlated with a higher number of pain sites in frail elderly individuals (19). Even in younger adults, persistent pain may tax physiologic reserves, impacting endocrine activity and predisposing for reduced physical and mental vigor (26).
The associations were more evident in younger adults. Specifically, lower-than-expected DHEA-S was the strongest predictor of reduced physical functioning among middle-aged females. This finding concurs with a prior study reporting that DHEA-S was associated with subjective vitality, as measured with the Psychological General Wellbeing Index, in premenopausal but not in postmenopausal women (38). This interaction with age suggests that an early and more precipitous decline in endocrine activity in young adulthood is of greater significance. Alternatively, in all elderly participants, there is a common statistical convergence toward lower DHEA-S and DHEA; thus, the overt impact of low values may be less salient.
The literature on gender differences in the association between DHEA and physical functioning in older adults has been inconsistent. One study reported finding an association between DHEA-S levels and physical performance in older men, but not women (39). Additionally, while age-related declines in DHEA-S are well documented in both men and women, the hormone decrements are more consistently found in older men than in older women (8). More research is still needed on changes in DHEA-S and DHEA secretion in older women after menopause, which brings the important ovarian source of DHEA to an end (29,40). One limitation of the current cross-sectional analysis was that we could not clearly delineate the menopausal transition.
Several other caveats should be acknowledged. Our tests of physical functioning focused primarily on physiological indices of vitality, rather than on frailty, and few participants met the clinical criteria of a frail patient in a care setting. MIDUS does not include hospitalized or nursing home patients, and the participants had to be able to travel to the study sites for an overnight stay. A previous paper on renal function in these MIDUS participants found a significant age-related decline in glomerular filtration rate, but there were no individuals with Stage 4 or 5 Chronic Kidney Disease (41). Similarly, a recent study exploring executive function and indicators of frailty in 690 MIDUS participants, who were 50 years of age or older, found that only 17 met the frailty criteria posited by the Fried Frailty measure (42). Thus, despite multiple tests of physical functioning confirming age-related declines in performance (21,22,24), future research is needed to demonstrate that DHEA would be a robust correlate among already frail individuals. MIDUS is a longitudinal project, however, and thus will afford the opportunities to verify the predictive value of these biomarkers in the future. Finally, while the associations were statistically significant, some effect sizes were small. But our statistical testing strategy was also very conservative and required that the relationship be evident and significant after taking into account age, gender, race, and adiposity, all of which account for a substantial portion of variance in both physical function and DHEA-S/DHEA.
In summary, even after taking the typical age-related decline in DHEA-S and DHEA into consideration, it was possible to discern significant links to physical functioning and health. The findings highlight the value of evaluating the extent that hormone levels deviate from age- and gender-typical norms. A striking result was that when DHEA-S and DHEA were already low in a middle-aged adult, both were associated with reduced vitality and vigor well before the onset of degenerative changes that occur later in old age. We believe that the age and gender-adjusted residual of DHEA-S and DHEA, rather than the absolute concentration in blood, provides a more useful and sensitive biological correlate of vitality. Our participants were drawn from a large, national cohort derived from the 48 continental states; thus, the findings are indicative of hormone levels and physical functioning in middle-aged and older American adults.
Funding
This research was supported by a grant from the National Institute on Aging (P01-AG020166). Blood collection and nurse assessments were facilitated by the General Clinical Research Centers program (M01-RR023942 [Georgetown], M01-RR00865 [UCLA]), and at UW from the Clinical and Translational Science Award program (1UL1RR025011). D.N.R. received support from the Training Program in Emotion Research (5T32MH018931).
References
- 1. Ohlsson C, Vandenput L, Tivesten A. DHEA and mortality: What is the nature of the association? J Steroid Biochem Mol Biol. 2015;145:248–253. doi:10.1016/j.jsbmb.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 2. Arlt W. Dehydroepiandrosterone and ageing. Best Pract Res Clin Endocrinol Metab. 2004;18(3):363–380. doi:10.1016/j.beem.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 3. Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)–a precursor steroid or an active hormone in human physiology. J Sex Med. 2011;8(11):2960–2982; quiz 2983. doi:10.1111/j.1743-6109.2011.02523.x [DOI] [PubMed] [Google Scholar]
- 4. Rutkowski K, Sowa P, Rutkowska-Talipska J, Kuryliszyn-Moskal A, Rutkowski R. Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs. 2014;74(11):1195–1207. doi:10.1007/s40265-014-0259-8 [DOI] [PubMed] [Google Scholar]
- 5. Leowattana W. DHEAS as a new diagnostic tool. Clin Chim Acta. 2004;341(1–2):1–15. doi:10.1016/j.cccn.2003.10.031 [DOI] [PubMed] [Google Scholar]
- 6. Dong Y, Zheng P. Dehydroepiandrosterone sulphate: Action and mechanism in the brain. J Neuroendocrinol. 2012;24(1):215–224. doi:10.1111/j.1365-2826.2011.02256.x [DOI] [PubMed] [Google Scholar]
- 7. Longcope C. Metabolism of dehydroepiandrosterone. Ann NY Acad Sci. 1995;774:143–148. doi:10.1111/j.1749-6632.1995.tb17378.x [DOI] [PubMed] [Google Scholar]
- 8. Lasley BL, Santoro N, Randolf JF, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87(8):3760–3767. doi:10.1210/jcem.87.8.8741 [DOI] [PubMed] [Google Scholar]
- 9. Tivesten Å, Vandenput L, Carlzon D, et al. Dehydroepiandrosterone and its sulfate predict the 5-year risk of coronary heart disease events in elderly men. J Am Coll Cardiol. 2014;64(17):1801–1810. doi:10.1016/j.jacc.2014.05.076 [DOI] [PubMed] [Google Scholar]
- 10. Kameda W, Daimon M, Oizumi T, et al. Association of decrease in serum dehydroepiandrosterone sulfate levels with the progression to type 2 diabetes in men of a Japanese population: The Funagata Study. Metabolism. 2005;54(5):669–676. doi:10.1016/j.metabol.2004.12.011 [DOI] [PubMed] [Google Scholar]
- 11. Hirsch JK, Molnar D, Chang EC, Sirois FM. Future orientation and health quality of life in primary care: Vitality as a mediator. Qual Life Res. 2015;24:1653–1659. doi:10.1007/s11136-014-0901-7 [DOI] [PubMed] [Google Scholar]
- 12. Bortz WM., 2nd The physics of frailty. J Am Geriatr Soc. 1993;41:1004–1008. [PubMed] [Google Scholar]
- 13. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 14. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011(1);305:50–58. doi:10.1001/jama.2010.1923.Gait [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riechman SE, Fabian TJ, Kroboth PD, Ferrell RE. Steroid sulfatase gene variation and DHEA responsiveness to resistance exercise in MERET. Physiol Genomics. 2004;17(3):300–306. doi:10.1152/physiolgenomics.00097.2003 [DOI] [PubMed] [Google Scholar]
- 16. Xue QL. The frailty syndrome: Definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi:10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM. The association between dehydroepiandrosterone and frailty in older men and women. Age Ageing. 2009;38(4):401–406. doi:10.1093/ageing/afp015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58(9):1707–1714. doi:10.1111/j.1532-5415.2010.03019.x [DOI] [PubMed] [Google Scholar]
- 19. Morrison MF, Katz IR, Parmelee P, Boyce AA, TenHave T. Dehydroepiandrosterone sulfate (DHEA-S) and psychiatric and laboratory measures of frailty in a residential care population. Am J Geriatr Psychiatry. 1998;6(4):277–284. doi:10.1097/00019442-199811000-00002 [PubMed] [Google Scholar]
- 20. Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J Gerontol A Biol Sci Med Sci. 2013;68(1):39–46. doi:10.1093/gerona/gls174 [DOI] [PubMed] [Google Scholar]
- 21. Ling CH, de Craen AJ, Slagboom PE, Westendorp RG, Maier AB. Handgrip strength at midlife and familial longevity: The Leiden Longevity Study. Age (Dordr). 2012;34(5):1261–1268. doi:10.1007/s11357-011-9295-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bohannon RW. Dynamometer measurements of hand-grip strength predict multiple outcomes. Percept Mot Skills. 2001;93(2):323–328. doi:10.2466/pms.2001.93.2.323 [DOI] [PubMed] [Google Scholar]
- 23. Sternäng O, Reynolds CA, Finkel D, Ernsth-Bravell M, Pedersen NL, Dahl Aslan AK. Grip strength and cognitive abilities: Associations in old age. J Gerontol B Psychol Sci Soc Sci. 2015:1–7. doi:10.1093/geronb/gbv017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Macfarlane DJ, Chou KL, Cheng YH, Chi I. Validity and normative data for thirty-second chair stand test in elderly community-dwelling Hong Kong Chinese. Am J Hum Biol. 2006;18(2):418–421. doi:10.1002/ajhb [DOI] [PubMed] [Google Scholar]
- 25. O’Donnell AB, Travison TG, Harris SS, Tenover JL, McKinlay JB. Testosterone, dehydroepiandrosterone, and physical performance in older men: Results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2006;91(2):425–431. doi:10.1210/jc.2005-1227 [DOI] [PubMed] [Google Scholar]
- 26. Rastogi R, Meek BD. Management of chronic pain in elderly, frail patients: Finding a suitable, personalized method of control. Clin Interv Aging. 2013;8:37–46. doi:10.2147/CIA.S30165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: A case for homeostenosis. J Am Geriatr Soc. 2012;60(1):113–117. doi:10.1111/j.1532-5415.2011.03769.x.Persistent [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tannenbaum C, Barrett-Connor E, Laughlin GA, Platt RW. A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: The Rancho Bernardo Study. Eur J Endocrinol. 2004;151(6):717–725. doi:10.1530/eje.0.1510717 [DOI] [PubMed] [Google Scholar]
- 29. Labrie F. DHEA, important source of sex steroids in men and even more in women. Prog Brain Res. 2010;182:97–148. doi:10.1016/S0079-6123(10)82004-7 [DOI] [PubMed] [Google Scholar]
- 30. Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. J Aging Health. 2010;22(8):1059–1080. doi:10.1177/0898264310374355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62(5):600–610. doi:10.1002/acr.20140 [DOI] [PubMed] [Google Scholar]
- 32. Hastings C, Mosteller F, Tuckey JW, Winsor CP. Low moments for small samples: A comparative study of order statistics. Ann Math Stat. 1947;18(3):413–426. [Google Scholar]
- 33. Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. [DOI] [PubMed] [Google Scholar]
- 34. Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf). 1998;49:421–432. doi:10.1046/j.1365-2265.1998.00507.x [DOI] [PubMed] [Google Scholar]
- 35. Wang L, Wang YD, Wang WJ, Zhu Y, Li DJ. Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. J Mol Endocrinol. 2007;38(4):467–479. doi:10.1677/jme.1.02173 [DOI] [PubMed] [Google Scholar]
- 36. Williams MR, Dawood T, Ling S, et al. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J Clin Endocrinol Metab. 2004;89(9):4708–4715. doi:10.1210/jc.2003-031560 [DOI] [PubMed] [Google Scholar]
- 37. Allolio B, Arlt W. DHEA treatment: myth or reality? Trends Endocrinol Metab. 2002;13(7):288–294. [DOI] [PubMed] [Google Scholar]
- 38. Bell RJ, Donath S, Davison SL, Davis SR. Endogenous androgen levels and well-being: Differences between premenopausal and postmenopausal women. Menopause. 2006;13(1):65–71. doi:10.1097/01.gme.0000191212.58856.96 [DOI] [PubMed] [Google Scholar]
- 39. Abbasi A, Duthie EH, Jr, Sheldahl L, et al. Association of dehydroepiandrosterone sulfate, body composition, and physical fitness in independent community-dwelling older men and women. J Am Geriatr Soc. 1998;46(3):263–273. [DOI] [PubMed] [Google Scholar]
- 40. Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: Relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–1522. doi:10.1210/jc.2002-020777 [DOI] [PubMed] [Google Scholar]
- 41. Costello-White R, Ryff CD, Coe CL. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age (Omaha). 2015;37(4):75. doi:10.1007/s11357-015-9808-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roiland RA, Lin F, Phelan C, Chapman BP. Stress regulation as a link between executive function and pre-frailty in older adults. J Nutr Health Aging. 2015;19(8):828–838. doi:10.1007/s12603-015-0476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]