This editorial refers to ‘Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in human induced pluripotent stem cell-derived cardiomyocytes’†, by Z. Chen et al., on page 292.
Cellular modelling of human heart disease has been hampered by several limitations, including difficult accessibility of human cardiac tissues and low proliferative capacity of freshly dissociated human cardiomyocytes (CMs). The advent of human pluripotent stem cells (hPSCs), which include both human embryonic stem cells (ESCs) and human induced pluripotent stem cells (iPSCs), has provided an unprecedented opportunity to generate potentially limitless quantities of hPSC-derived somatic cell types (including human CMs) for studying disease mechanisms, identifying novel drug targets, and accelerating drug screening.1 However, two major limitations must be overcome before such wide-ranging application of iPSC-CM technology is possible. The first problem is that current cardiac differentiation methods produce a heterogeneous population of ventricular-, atrial-, and nodal-like cells.2–4 Despite efforts to produce a more enriched population of a specific cardiac phenotype to model specific cardiac diseases (e.g. atrial fibrillation, ventricular arrhythmias, etc.), no effective and validated strategies are yet available. A second limitation is that iPSC-CMs are assessed by low-throughput functional assays, primarily the labour-intensive patch-clamp electrophysiology, which considerably slows the pace of identification and characterization of the disease and/or drug-induced cellular phenotypes. Therefore, there is an urgent need to develop sensitive, scalable, and high-throughput functional assays to capitalize fully on our ability to generate iPSC-CMs at an industrial scale.5,6
Over the past two decades, membrane voltage-sensitive dyes (VSDs) have been used as an alternative to patch-clamp electrophysiology to record both cardiac action potential (AP) and calcium transients. However, the use of VSDs in AP recordings is limited by the indiscriminate cell staining and dye-mediated acute phototoxicity, especially in the setting of repeated single-cell measurement.7,8 The introduction of genetically encoded voltage-sensitive fluorescent protein (VSFP) in 1997 has overcome some of the limitations associated with VSDs. Recently, the successful application of VSFP to record optical APs has been demonstrated in both ESC-derived CMs8 and iPSC-derived CMs.9,10 Notwithstanding the use of a non-ratiometric VSFP (i.e. ArcLight) readout, these studies clearly demonstrated the proof-of-principle underlying the potential use of VSFP-based all-optical AP recording as a faster and high-throughput alternative to patch-clamp electrophysiology.
Building on the work that showed the feasibility of an all-optical AP recording in iPSC-CMs, Chen et al. in this issue of the journal now report the development of a novel genetically encoded, FRET (Förster resonance energy transfer)-based optical membrane potential sensor under the control of a cardiac lineage-specific promoter to record all-optical APs in a cardiac subtype-specific manner.11 Chen et al. first designed a membrane VSFP by fusing the voltage-sensing transmembrane domain of two tandem fluorescent proteins [the green fluorescent protein (GFP) variant clover VSFP-CR and the red fluorescent protein (RFP) variant mRuby2], which shows a significant increase in membrane depolarization-induced FRET signal. Using a lentiviral gene transfer strategy, the authors successfully recorded trains of optical APs by measuring the membrane depolarization-induced stable increase in the RFP/GFP emission ratio. The AP waveform recorded in this fashion showed AP durations similar to those recorded with the patch-clamp method. After demonstrating proof of principle, the investigators tested the possibility of using subtype-specific markers in conjunction with the VSFP-based optical AP recording to identify and discriminate a specific cardiac cell subtype in a heterogeneous population of iPSC-CMs. A systematic single cell-based functional and molecular screen identified MLC2v, SLN, and SHOX2 as specific markers for ventricular-, atrial-, and nodal-like cells, respectively, which were then used to drive the VSFP expression. The authors found a high degree of concordance between AP parameters [e.g. AP duration at 90% (APD90) and APD50] obtained by optical AP recordings vs. patch-clamp electrophysiology.
Because the two major applications of iPSC-CMs are in disease modelling and cardiotoxicity drug testing,1 Chen et al. tested if the optical AP recording could identify and discern the APD prolongation and arrhythmogenicity associated with patient-specific LQT1 iPSCs (caused by a heterozygous missense c.569G>A p.R190Q mutation in the potassium channel gene KCNQ1) or those induced by drugs known to cause QT/QTc prolongation. Consistent with patch-clamp results, the optical AP recordings in MLC2v–VSFP- and SLN–VSFP- but not in SHOX2–VSFP-positive LQT1R190Q iPSC-CMs showed a significant APD prolongation when compared with CTR iPSC-CMs or the isogenic LQT1corr iPSC-CMs. Commensurate with the magnitude of APD prolongation, the optical AP recording revealed a very high incidence of early afterdepolarizations (EADs) in LQT1 iPSC-CMs, a relatively lower incidence in LQT1corr iPSC-CMs, and an insignificant rate of incidence in CTR iPSC-CMs.
Despite being the gold standard, the invasive and destructive nature of the patch-clamp technique makes it incompatible for long-term measurement and quantification of APs. In a next series of experiments, the authors demonstrated superior adoptability and capability of the optical AP approach to measure dynamic changes in AP in a single cell over a period lasting up to several days. Assessment of the optical AP recordings, before and after overexpression of the (wt) KCNQ1 ion channel subunit in patient-specific LQT1 iPSCs to rescue the disease phenotype, showed a significant APD shortening in the LQT1R190Q iPSC-CMs, with APD values similar to those measured in LQT1corr iPSC-CMs. Finally, the authors went on to demonstrate that the optical AP recording approach could faithfully capture the AP phenotype induced by cisapride, a drug known to cause QT prolongation and life-threatening cardiac arrhythmia (i.e. torsades de pointes; TdP) that has resulted in Food and Drug Administration (FDA)-initiated withdrawal from the market.1,12 The optical AP recordings showed a significant APD prolongation and increased the prevalence of EADs in single CTR iPSC-CMs treated with 100 nM cisapride. Similarly, Ivabradine, a ‘funny’ current (If) inhibitor used clinically to reduce heart rate, showed a cardiac subtype-specific pharmacological pattern. Ivabradine caused a complete inhibition of spontaneous beating only in the If current-expressing, SHOX2–VSFP-positive nodal cells, with minimal or no effects on other iPSC-CM subtypes, underscoring a functional contribution of If current in automaticity. These findings are further corroborated by the observation that the automaticity observed in ventricular-like hiPSC-CMs is independent of If current.13
The study by Chen et al. provides important and novel insights into the potential application of optical AP recording as a high-throughput alternative to patch-clamp electrophysiology, which is hampered by issues such as low throughput, labour-intensive, short recording time, and destructive nature.11 Importantly, this study paves the way for the high-hroughput quantification of electrical phenotypes in patient-specific iPSC-CMs, and subtype-specific investigation of diseases and precise drug effects. Notably, a further validation of the all-optical approach is provided by a recent study demonstrating the pairing of both monitor (i.e. co-expression of a genetically encoded, calcium and voltage reporter, CaViar) and control (e.g. channelrhodopsin variant, CheRiff) tools to record simultaneously optogenetically paced all-optical AP, and the use of calcium signals to assess the cardiotoxic effects of drugs.7 However, some concerns must be addressed still. First, although the VSFP-induced FRET signal captures three discernible cardiac AP subtypes, important differences exist with respect to both the AP shape and quantitative absolute values of AP parameters (e.g. MDP, dV/dt, and amplitude) that are essential for cellular phenotyping and assessing the disease/drug-induced effects.5,6,12 As noted by the authors, the optical AP values appear more triangular than those recorded with the patch-clamp method, which can be attributed to the slow intrinsic conformational kinetics of VSFP with a time constant of several milliseconds in response to the much faster changes in the transmembrane potential. Secondly, the VSFP-induced FRET signal does not provide the absolute values of the AP parameters and the current practice of ratiometric calibration of the voltage sensors could produce confounding results due to temporal differences in photobleaching rates of the protein sensor.14 Thirdly, the use of expression patterns of MLC2a and MLC2v as bona fide markers to define iPSC-CM subtype identity may be imprecise given that studies have demonstrated the prevalence of iPSC-CMs expressing both MLC2v/MLC2a.15 Finally, the potential unaccounted for effects of the lentiviral gene transfer and/or genetic overexpression, and technical challenges associated with scale-up of this strategy to be applied to ever-increasing numbers of iPSC lines, still need to be addressed.
In summary, the work of Chen et al. shows that genetically encoded VSFP, which optically reports the electrical APs in distinct iPSC-CM populations, can in principle be harnessed as a useful high-throughput tool in stem cell-based disease modelling and cardiotoxicity drug testing (Figure 1).11 Although the all-optical AP recording is not functionally equivalent to those recorded using ‘the gold standard’ patch-clamp electrophysiology, the fact that genetically encoded VSFPs are sensitive enough to provide a cardiac cell type-specific dynamic AP signature in a time-dependent, high-throughput, and non-destructive manner is extremely encouraging. Future work focusing on developing VSFP with a greater quantum yield, high dynamic spatiotemporal resolution, increased brightness with faster kinetics, and a high signal-to-noise ratio and low phototoxicity14 will undoubtedly pave the way for an accelerated and widespread adoption of all optical AP recording.
Figure 1.
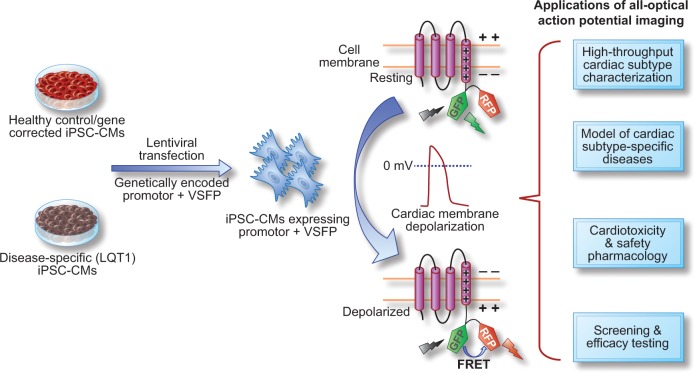
Overview of the application of all-optical action potential (AP) imaging approach as a novel high-throughput tool in cardiac subtype characterization, modeling cardiac subtype-specific disease, and cardiotoxicity and safety pharmacology. By using the genetically-encoded voltage-sensitive fluorescent protein (VSFP)-based ratiometric fluorescence resonance energy transfer (FRET) signal under the control of cardiac lineage-specific markers, the unique action potential signature representative of three major cardiac-subtype can be recorded from both healthy control and disease-specific (LQT1) iPSC-CMs. The all-optical AP waveform is recorded as a function of the cardiac membrane depolarization-induced stable increase in the RFP/GFP emission ratio (FRET signal) and exhibit the AP durations similar to those recorded with patch clamp method.
Funding
This work is supported by research grants from the National Institute of Health R01 HL123968, NIH HL130020, NIH R01 HL128170, NIH R01 HL126527, and NIH R01 HL133272 to (J.C.W.).
Conflict of interest: none declared.
References
- 1. Shukla P, Garg P, Wu JC. Human pluripotent stem cell-derived cardiomyocytes as a predictive pharmacological and toxicological platform in drug discovery and development In: Will Y, McDuffie JE, Olaharski AJ, Jeffy BD, eds. Drug Discovery Toxicology: From Target Assessment to Translational Biomarkers. John Wiley & Sons, Inc; 2016, p346–364. [Google Scholar]
- 2. Burridge PW, Sharma A, Wu JC. Genetic and epigenetic regulation of human cardiac reprogramming and differentiation in regenerative medicine. Annu Rev Genet 2015;49:461–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat Rev Cardiol 2016;13:333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karakikes I, Ameen M, Termglinchan V, Wu JC. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ Res 2015;117:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods 2014;11:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsa E, Ahrens JH, Wu JC. Human induced pluripotent stem cells as a platform for personalized and precision cardiovascular medicine. Physiol Rev 2016;96:1093–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dempsey GT, Chaudhary KW, Atwater N, Nguyen C, Brown BS, McNeish JD, Cohen AE, Kralj JM. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J Pharmacol Toxicol Methods 2016:in press. [DOI] [PubMed] [Google Scholar]
- 8. Leyton-Mange JS, Mills RW, Macri VS, Jang MY, Butte FN, Ellinor PT, Milan DJ. Rapid cellular phenotyping of human pluripotent stem cell-derived cardiomyocytes using a genetically encoded fluorescent voltage sensor. Stem Cell Reports 2014;2:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shinnawi R, Huber I, Maizels L, Shaheen N, Gepstein A, Arbel G, Tijsen AJ, Gepstein L. Monitoring human-induced pluripotent stem cell-derived cardiomyocytes with genetically encoded calcium and voltage fluorescent reporters. Stem Cell Reports 2015;5:582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song L, Awari DW, Han EY, Uche-Anya E, Park SH, Yabe YA, Chung WK, Yazawa M. Dual optical recordings for action potentials and calcium handling in induced pluripotent stem cell models of cardiac arrhythmias using genetically encoded fluorescent indicators. Stem Cells Transl Med 2015;4:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Z, Xian W, Bellin M, Dorn T, Tian Q, Goedel A, Dreizehnter L, Schneider CM, Oostwaard DW-v, Ng JKM, Hinkel R, Pane LS, Mummery CL, Lipp P, Moretti A, Laugwitz K-L, Sinnecker D. Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in hiPSC-derived cardiomyocytes. Eur Heart J 2017;38:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, Diecke S, Sallam K, Knowles JW, Wang PJ, Nguyen PK, Bers DM, Robbins RC, Wu JC. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation 2013;127:1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JJ, Yang L, Lin B, Zhu X, Sun B, Kaplan AD, Bett GC, Rasmusson RL, London B, Salama G. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J Mol Cell Cardiol 2015;81:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Storace D, Sepehri Rad M, Kang B, Cohen LB, Hughes T, Baker BJ. Toward better genetically encoded sensors of membrane potential. Trends Neurosci 2016;39:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 2012;111:344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]