Abstract
BACKGROUND AND OBJECTIVES
To evaluate differences in erythropoietin requirements between diabetic and non-diabetic patients on hemodialysis and peritoneal dialysis.
DESIGN AND SETTINGS
This was a retrospective, cross-sectional study conducted between January 2010 and December 2011, at King Khalid University Hospital Riyadh, Saudi Arabia, with 47 peritoneal and 57 hemodialysis patients.
METHODS
A total of 24 (51%) peritoneal dialysis and 30 (52.6%) hemodialysis patients were suffering from diabetes. We compared demographics, hemoglobin, ferritin, transferrin saturation, C-reactive protein, parathyroid hormone, and weekly erythropoietin dose.
RESULTS
The mean weekly dose of erythropoietin was 5391.3 (4692.7) units in peritoneal dialysis (diabetic and non-diabetic) patients compared to 9869.7 (5631.7) units in hemodialysis (diabetic and non-diabetic) patients, with a difference of 4478.3 (6615) units (P=.001). The mean weekly dose in diabetic peritoneal dialysis patients was 3818.2 (4489.5) units, compared to 8814.8 (5121.9) units in hemodialysis (P=.001) patients. The mean weekly dose in non-diabetic peritoneal dialysis patients was 6545.4 (3863.5) units compared to 12 222 (6210) units in non-diabetic hemodialysis patients (P=.02). Diabetic peritoneal dialysis patients required a lower dose of erythropoietin compared to non-diabetic peritoneal dialysis patients (3818.2 [4489.5] units vs 6545.4 [3863.5] units per week) (P=.036). In hemodialysis patients, the mean erythropoietin dose was lower in diabetic patients compared to non-diabetic patients (8814.8 [5121.9] units vs 12 222 [6210] units per week) (P=.043).
CONCLUSION
The diabetic patients in both groups (hemodialysis and peritoneal dialysis) required less erythropoietin than non-diabetic patients. Diabetic patients on peritoneal dialysis required less erythropoietin diabetic patients on hemodialysis.
The population of patients with chronic kidney disease (CKD) and its associated complications is rising globally. This factor calls for the importance of conducting an in-depth study of the complications related to end-stage renal disease (ESRD), including anemia and malnutrition, to improve patient survival and quality of life (QOL). Anemia is an independent risk factor for cardiac disease and mortality in CKD patients. 1,2 Hemoglobin (Hb) concentration also correlates with QOL.3 Both European and American guidelines recommend correcting anemia to an Hb level of 11×12 g/dL (European Best Practice Guidelines [EBPG] 2004, National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative [NKF-K/DOQI] Guidelines 2006).4,5
Diabetes mellitus is a leading cause of ESRD world-wide. The diabetic patients who receive maintenance dialysis therapy frequently require erythropoietin (EPO) therapy for CKD-related anemia. Some studies have shown that anemia control with EPO is obtained more easily in PD patients than in HD patients.6 To test this theory in diabetic and non-diabetic Saudi patients, we compared the anemia profile and EPO requirements among PD and HD patients at King Khalid University Hospital, King Saud University in Riyadh, Saudi Arabia.
Anemia is an independent risk factor for cardiac disease and mortality in patients on dialysis. We need to evaluate if the control of anemia is affected by dialysis modality.
Our objective was to evaluate the differences in EPO requirements in matched groups of diabetic and non-diabetic patients who receive maintenance HD compared to diabetic and non-diabetic patients who receive PD.
METHODS
In this retrospective study conducted between January 2010 and December 2011, we included 47 patients from the PD unit and 57 patients from the HD unit in King Khalid University Hospital. A total of 24 (51%) of the 47 PD patients suffered from diabetes, whereas 30 (52.6%) of the 57 HD patients suffered from diabetes. All patients were older than 15 years of age, and they were clinically stable with their current dialysis regimen. We recorded the demographic data, levels of Hb, hematocrit (Hct), serum ferritin, transferrin saturation index (TSAT), serum C-reactive protein (CRP) and parathyroid hormone (PTH) levels, as well as total EPO dose that each patient received on a weekly basis. The target Hb level was set as 100–120 g/L in accordance with K/DOQI Guidelines. Iron replacement was given as oral ferrous fumerate or intravenous iron saccharate, depending on the patients’ iron profile and hematological indices (for instance, Hb).
We collected a 24-hour urine output for all patients to measure their residual renal function (RRF). A urine output of more than 100 mL/d was considered significant, and glomerular filtration (mL/min) was calculated for these patients using the Cockcroft–Gault formula. A glomerular filtration rate of 5 mL/min or more was considered to be significant RRF.
All patients with polycystic kidney disease, hematological or bone marrow disease (e.g., sickle cell anemia), active malignancy, active gastrointestinal bleeding, or patients resistant to EPO (requiring more than 25 000 units per week) were excluded from our study.
Data analysis
We analyzed the data using IBM SPSS for Windows, version 18.0 (Chicago, SPSS Inc., New York, United States). Descriptive data are expressed as the mean (standard deviation) and percentage. We used Student t test to compare the variables and a P<.05 was considered significant.
RESULTS
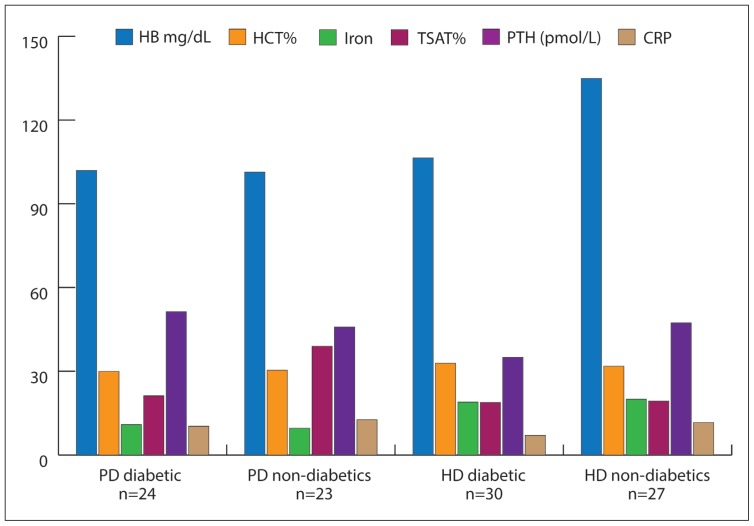
The demographic parameters for diabetic and non-diabetic patients in both groups are outlined in Table 1. We analyzed the data for various factors that affect the Hb and EPO requirements, including blood counts, serum iron levels, TSAT, PTH, ferritin, serum CRP, and use of medications, including statins and ACE inhibitors. The mean values for these biochemical and hematological parameters are shown in Figure 1.
Table 1.
Demographic parameters.
| PD | HD | |||
|---|---|---|---|---|
| Diabetic patients (n=24) | Nondiabetic patients (n=23) | Diabetic patients (n=30) | Nondiabetic patients (n=27) | |
|
| ||||
| Age (y) | 56.92 (19.4) | 37.83 (38.21) | 54.75 (24.587) | 59.4 (33.45) |
| M:F (no. of patients) | 13/11 | 11/12 | 9/21 | 10/16 |
| Time on dialysis (mo) | 69.1 (23.98) | 30.826(28.24) | 33.3 (25.8) | 80.7 (93.33) |
| CAPD/CCPD | 5/19 | 7/16 | ||
| Route of EPO | Subcutaneous | Subcutaneous | Intravenous | Intravenous |
| Using ACEi or ARBs | 17 (70.83%) | 15 (65.2%) | 10 (33.3%) | 5 (18.51%) |
| Using statins | 14 (58.33%) | 10 (43.4%) | 22 (73.3%) | 14 (51.85%) |
PD: Peritoneal dialysis, HD: hemodialysis, M:F: male/female ratio, CAPD continuous ambulatory peritoneal dialysis, CCPD: continuous cycler peritoneal dialysis, EPO: erythropoietin, ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor blocker.
Figure 1.
Biochemical parameter in both PD and HD patients.
In the HD patients, the mean Hct was 32.9 (3.3%) in diabetic patients and 31.8 (6.%) in non-diabetic patients. The mean serum iron was 10.9 (3.8) μmol/L in diabetic PD patients and 9.6 (7.2) μmol/L in non-diabetic PD patients. In the HD patients, serum iron was 18.9 (5.1) μmol/L in diabetic patients and 20.0 (8.6) μmol/L in non-diabetic patients. The mean TSAT was 21.2 (8.1%) in diabetic PD patients and 39 (32.4%) in non-diabetic PD patients. In the HD patients, the mean TSAT was 18.9 (11.7%) in diabetic patients and 19.3 (5.5%) in non-diabetic patients. The mean PTH level was 37.4 (33.8) pmol/L in diabetic PD patients and 62.7 (51.1) pmol/L in non-diabetic patients. In the HD patients, the mean PTH level was 37.2 (28.9) pmol/L in diabetic patients and 65.1 (45.4) pmol/L in non-diabetic patients. The mean CRP level was 10.3 (8.5) mg/L in diabetic PD patients and 13.0 (11.5) mg/L in non-diabetic PD patients. In the HD patients, the mean CRP level was 7.1 (6.8) mg/L in diabetic patients and 11.6 (12.6) mg/L in non-diabetic patients.
The mean Hb was 102.0 (28.9) g/dL in diabetic PD patients and 101.4 (22.4) g/dL in non-diabetic PD patients. In the HD patients, the mean Hb was 106.4 (10.6) g/dL in diabetic patients and 134.9 (156.5) g/dL in non-diabetic patients (post-dialysis). The mean Hct was 30.0 (8.7%) in diabetic PD patients and 30.4 (6.2%) in non-diabetic PD patients. The distribution of Hb levels according to target levels is summarized in Table 2, which shows that PD patients had slightly better overall Hb control (more patients within the target range) than had HD patients. However, the difference was not found to be statistically significant.
Table 2.
Distribution of hemoglobin levels in both groups.
| Dialysis modality | Hemoglobin level | n | PTH (pmol/L) | Using statins (%) | Using ACEi/ARB (%) |
|---|---|---|---|---|---|
|
| |||||
| PD diabetic patients (n=24) | Hb<100 g/L | 4 (16.6%) | 22.2 | 12.5 | 45.5 |
| Hb 100–120 g/L | 17 (70.8%) | 37.5 | 69.2 | 67.4 | |
| Hb>120 g/L | 2 (8.3%) | 51.5 | 50 | 100 | |
| PD nondiabetic (n=23) patients | Hb<100 g/L | 7 (30.4%) | 44.9 | 55.5 | 82.8 |
| Hb 100–120 g/L | 14 (60.8%) | 93.15 | 57.1 | 76.4 | |
| Hb>120 g/L | 2 (8.69%) | 72.46 | 50 | 75 | |
| HD diabetic (n=30) patients | Hb<100 g/L | 8 (26.7%) | 51.2 | 85.7 | 46.7 |
| Hb 100–120 g/L | 18 (60%) | 31.6 | 88.8 | 73.5 | |
| Hb>120 g/L | 4 (13.3%) | 34.46 | 25 | 50 | |
| HD nondiabetic (n= 27) patients | Hb<100 g/L | 9 (33.3%) | 40.1 | 20 | 82.3 |
| Hb 100–120 g/L | 13 (48.8%) | 82.1 | 91.7 | 74.5 | |
| Hb>120 g/L | 5 (18.5%) | 58.7 | 83.3 | 60 | |
PD: Peritoneal dialysis, HD: hemodialysis, PTH, parathyroid hormone, ACEi: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor blocker.
In PD patients, 33 (70.2%) of the total 47 received oral iron replacement during or in the 6 months before the study period (62.5% of diabetic PD patients and 78.6% of non-diabetic PD patients). Of the total 47 PD patients, 20 (42.5%) patients received intravenous iron replacement during or in the 6 months before the study period; 37.5% of diabetic PD patients and 47.8% of non-diabetic PD patients received intravenous iron saccharate infusions.
In HD patients, 40 (70.2 %) of the total 57 received oral iron replacement during or in the 6 months before the study period (63.3% of diabetic PD patients and 77.78 % of non-diabetic PD patients). Of the total 57 PD patients, 19 (33.3%) patients received intravenous iron replacement during or in the 6 months before the study period; 30.1 % of diabetic PD patients and 40.7% of non-diabetic PD patients received intravenous iron saccharate infusions.
In all groups, we calculated an average dose for EPO based on the weekly dose during the previous 4 months. We adjusted the dose on a monthly basis to achieve Hb values within the target range of 100–120 g/L (10–12 g/dL). The mean doses of EPO for each group are outlined in Table 3.
Table 3.
Erythropoietin doses in PD and HD (diabetic and nondiabetic) patients.
| PD | EPO dose (units/wk) | HD | EPO dose (units/wk) | P |
|---|---|---|---|---|
|
| ||||
| Diabetic patients (n=24) | 3818.2 (4489.52) | Diabetic patients (n=30) | 8814.8 (5121.9) | .001 |
| Nondiabetic (n=23) | 6545.5 (3863.47) | Nondiabetic patients (n= 27) | 12 222 (6210) | .022 |
| Total (diabetic + nondiabetic) patients | 5391.4 (4692.3) | Total (diabetic + nondiabetic) patients | 9869.6 (5631.7) | .001 |
| Total (HD + PD) diabetic patients | 5576 (5190) | Total (HD + PD) nondiabetic patients | 6877 (5968) | .048 |
PD: Peritoneal dialysis, HD: hemodialysis, EPO: erythropoietin.
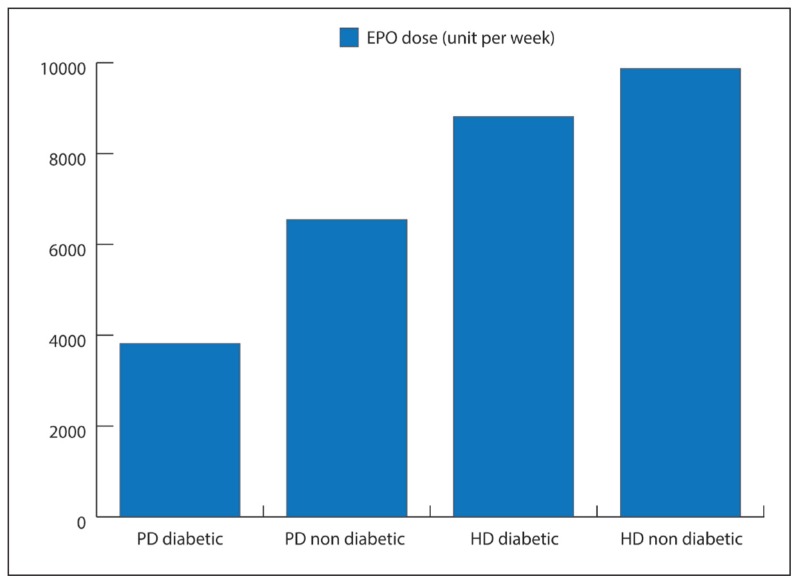
The difference between the mean dose of EPO in PD and HD patients was analyzed by Student t test. The mean overall dose in PD (diabetic and non-diabetic) patients was 5391.3 (4692.7) units, compared to an overall dose of 9869.6 (5631.7) units per week in HD (diabetic and non-diabetic) patients. The mean difference was 4478.3 (6615) units (P=.001). The mean dose in diabetic PD patients was lower, 3818.2 (4489.5) units per week, compared to a dose of 8814.8 (5121.9) units per week in diabetic HD patients (P=.001). The mean dose in non-diabetic PD patients was lower, 6545.4 (3863.5) units per week, compared to a dose of 12 222 (6210) units per week in non-diabetic HD patients (P=.022). Within each group, the diabetic PD patients, on average, required a lower dose of EPO compared to the non-diabetic PD patients (3818.2 [4489.5] units vs 6545.4 [3863.5] units per week) (P=.036). Similarly, in HD patients, the mean dose of EPO was lower in diabetic patients compared to non-diabetic patients (8814.8 [5121.9] units vs 12 222 [6210 units per week) (P=.043) (Figure 2).
Figure 2.
Erythropoietin doses in PD and HD patients.
We found serum PTH levels to be lower in diabetic patients compared to non-diabetic patients. In PD patients the mean serum PTH level was 37.45 (33.8) pmol/L vs 62.7 (51.1) pmol/L (P=.30) and in HD patients (37.2 [28.9] vs 65.1 [45.4] pmol/L, P=.37). Most of the PD patients were using ACE inhibitors, including diabetic (70.8% patients in PD vs 33.3% patients in HD, P=.004) and non-diabetic patients (65.2% patients in PD vs 18.5% patients in HD, P=.013). More diabetic patients were using statins compared to non-diabetic patients, who received PD and HD, due to increased incidence of dyslipidemia and hypertriglyceridemia in diabetes.
We found serum ferritin levels to be lower in non-diabetic patients compared to diabetic patients. In PD, we found the following: 403.3 (420.2) μg/L in non-diabetic patients vs 581.0 (524.2) μg/L, in diabetic patients, P=.19; and in HD patients, we found 386.3.0 (343.7) μg/L in non-diabetic patients vs 421.0 (325.3) μg/L in diabetic patients, P=.13. We found serum ferritin levels to be lower in patients with Hb levels at or above the target range compared to patients with Hb levels below the target range (P=.04). Similarly, we found serum ferritin levels to be lower in patients with TSAT values above 30%. However, we found higher ferritin levels in patients with a high CRP (>10).
Of the 47 PD patients, 25 patients (53.2%) had RRF of 5 mL/min or more. We found that 14 (56%) patients were suffering from diabetes and 11 (44%) patients were not suffering from diabetes. We found the mean EPO dose to be lower (3802.1 [2638.5] units per week) in patients with RRF of 5 mL/min or more compared to a mean EPO dose of 5031.6 (3241.3) units per week in patients without significant RRF (<5 mL/min). Similar results were seen in diabetic PD patients (3137.2 [2389.5] units vs 4635.4 [3157.1] units per week, P=.038) and non-diabetic PD patients (4746.3 [4489.5] units vs 6422.4 [3863.5] units per week, P=.056, respectively).
In HD, 10 (17.5%) patients had significant RRF of 5 mL/min or more. We found that 6 (60%) patients were suffering from diabetes and 4 (40%) patients were not suffering from diabetes. We observed that the mean EPO dose was lower in the HD patients with significant RRF, compared to HD patients without significant RRF (6967.2 [4256.2] units vs 8426.4 [6732.6] units per week, P=.143). The difference was not found to be statistically significant.
DISCUSSION
Renal anemia is a major complication in patients with CKD, particularly dialysis patients; renal anemia remains a problem that clinicians must deal with daily as they treat CKD and dialysis patients.2 Hb concentrations also correlate with QOL.3 Some investigators felt that this problem might be solved by the development of recombinant human EPO, which became available for dialysis patients to use in the late 1980s.7 However, approximately 80% of the dialysis population used recombinant human EPO with unprecedented efficacy, which led to dramatic reduction in blood transfusions and more beneficial effects, including suppression of the progression of renal failure and an improvement in patient QOL.8 However, new problems were identified later with the target Hb levels in EPO therapy and also with the criteria for the concomitant use of iron preparations, including resistance to EPO.9 The guidelines used for the indication and administration of EPO for optimally managing anemia and minimizing the risk of complications comply with those used by the KDOQI, European Renal Best Practice (ERBP) and the Japanese Society for Dialysis Therapy.10 The current recommended level for correction of anemia due to ESRD is 10–12 g/dL (100–120 g/L).
Diabetes is known to be a risk factor for the severity of anemia in non-dialyzed patients with renal failure, as well as in patients who require maintenance dialysis. Few studies have evaluated the difference in response to EPO therapy in diabetic and non-diabetic patients.11,12 Other studies have shown that PD patients require lower doses of EPO compared with HD patients.13
The requirement for EPO and its response in PD and HD patients have not been widely reported for the Arab and especially Saudi CKD and dialysis populations. King Khalid University Hospital in Riyadh, Saudi Arabia, started their renal dialysis program almost 4 decades ago; PD was started in 1984. Currently, we have more than 50 active patients and more than 70 patients who receive regular HD in the PD program. In our study, we included 47 PD and 57 HD patients. A total of 24 of the 47 PD patients were suffering from diabetes, whereas 30 of the 57 HD patients were suffering from diabetes. Our data show that the dose of EPO required for the correction of anemia in PD patients was lower compared to what was required in HD patients. The total EPO dose for all PD (diabetic and non-diabetic) patients was lower in comparison to the mean dose in all (diabetic and non-diabetic) patients who received HD (Figure 2). Our finding shows that PD patients required a lower dose of EPO, compared to HD patients. This finding was consistent when we compared the diabetic patients in both groups with the non-diabetic patients in both groups. In 2001, Pagé and colleagues from Ontario, Canada, reported that in matched groups of diabetic and non-diabetic patients, the diabetic PD patients received an average 4497 units per week compared with 7593 units per week for non-diabetic PD patients.14 The difference (approximately 3000 units per week) was found to be statistically significant. Our study results are comparable to the outcome reported by Pagé and colleagues.14 The diabetic patients in our study, from both groups, required less EPO dosage when compared to non-diabetic patients (PD as well as HD). On statistical analysis, we found the differences in the EPO requirements to be statistically significant among both groups, when compared for the diabetic PD, HD populations, the non-diabetic PD and HD groups, as well as between the overall PD and HD patients.
The requirement for EPO and its response in PD and HD can be affected by malnutrition, chronic inflammation, hyperparathyroidism, and iron deficiency, as well as comorbid conditions and other medications, including statins.15 Some reports have shown an improvement in the response to EPO with the use of ascorbic acid, as well as the concomitant use of statins.16
Serum PTH levels were found to be lower in diabetic patients compared to non-diabetic patients. This could be one of the reasons for the better Hb control seen in diabetic patients because several studies have shown that hyperparathyroidism is a risk factor for anemia in ESRD.15 The potential mechanisms include a direct effect of PTH on endogenous EPO synthesis, bone marrow erythroid progenitors, and red blood cell survival (accelerated hemolysis), as well as an indirect effect through the induction of bone marrow fibrosis.16
The use of ACE inhibitors was important in our study, as some studies have shown that ACE inhibitors can inhibit the response to EPO, particularly at high doses.17 However, other studies have shown no difference in EPO dose on patients using ACE inhibitors, compared to patients who were not using ACE inhibitors. 18 These drugs are more commonly used in PD patients, compared to HD patients, to preserve RRF. Most of the PD patients in our study were using ACE inhibitors, compared to HD patients. Furthermore, more diabetic patients were using statins compared to non-diabetic patients, who receive PD and HD, due to an increased incidence of dyslipidemia and hyper-triglyceridemia in diabetes. The use of statins has been linked to a better control of anemia and an improved response to EPO in some studies.19,20 We found the serum levels of CRP to be higher in PD patients compared to HD patients. We observed this difference in both diabetic and non-diabetic groups. Chronic inflammation has been implicated in the development of malnutrition and anemia in HD as well as PD patients.21,22 Some studies have shown that the majority of PD patients have increased levels of inflammatory markers.23
Serum ferritin levels are often increased in ESRD patients, especially in those patients who receive dialysis. The levels are related to TSAT, Hb, and Hct levels in those patients and can be affected by patient compliance to iron supplementation. Ferritin is also an inflammatory marker, and high levels are seen in many inflammatory conditions.24 PD patients have been shown to have a greater increase in inflammatory markers compared to HD patients.25 In our study, we found lower serum ferritin levels in non-diabetic patients compared to diabetic patients. PD patients also had higher serum ferritin levels compared to HD patients.
Our study has demonstrated better preservation of RRF in PD patients compared to HD patients. Additionally, a higher percentage of patients (both on HD and PD) had significant RRF, compared to non-diabetic patients. Increasing evidence suggests that the preservation of RRF is associated with several benefits such as improved QOL, reduced EPO requirements, and reduced treatment costs.26 Our study indicates that preservation of RRF might be a factor in reducing the EPO requirements in PD diabetic patients.
In conclusion, based on our study data, we can conclude that the dose of EPO required for the correction of anemia in PD patients was lower compared to HD patients. The diabetic patients in both groups (HD and PD) required a lower mean dose of EPO compared to the non-diabetic patients in both groups (HD and PD). Additionally, the diabetic patients who receive PD required a lower mean dose of EPO compared to the diabetic patients who receive HD.
Patients on PD (diabetic and non-diabetic patients) received a lower mean dose of EPO compared to the diabetic patients on HD. The differences in the EPO requirements were found to be statistically significant. However, there can be bias due to the small sample size as well as the difference in the method of administration of EPO injections. Additional studies are needed to understand the mechanisms involved to optimize the management of anemia in ESRD patients who require renal replacement therapy.
Acknowledgments
Professor Riaz Quraishi and Dr. Khaldoon Aljerian
REFERENCES
- 1.Keown PA, Churchill DN, Poulin-Costello Dialysis patients treated with Erythropoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodialysis International. 2010;14:168–173. doi: 10.1111/j.1542-4758.2009.00422.x. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Pisoni RL, Combe C, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004;19:121–132. doi: 10.1093/ndt/gfg458. [DOI] [PubMed] [Google Scholar]
- 3.Valderrabano F. Quality of life benefits of early anemia treatment. Nephrol Dial Transplant. 2000;15(Suppl 3):23–28. doi: 10.1093/oxfordjournals.ndt.a027972. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation. IV. NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: update 2006. Am J Kidney Diseases. 2001;37(1 Suppl 1):S182–S238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F, Aljama P, Barany P, et al. European Best Practice Guidelines Working Group. Revised European best practice guidelines for the management of anemia in patients with chronic renal failure. Nephrology DialysisTransplant. 2004;19(Suppl 2):ii1–ii47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 6.Coronel F, Herrero JA, Montenegro J. Erythropoietin requirements: a comparative multicenter study between peritoneal dialysis and Hemodialysis. J Nephrol. 2003 Sep-Oct;16(5):697–702. [PubMed] [Google Scholar]
- 7.Egrie Joan C, Eschbach Joseph W, Downing Michael R. TI - Correction of the Anemia of End-Stage Renal Disease with Recombinant Human Erythropoietin. New England Journal of Medicine. 1987;316(2):73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- 8.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16:2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 9.Priyadarshi A, Shapiro J. I Erythropoietin Resistance in the Treatment of the Anemia of Chronic Renal Failure, Issues In The Dialysis Patient. Hematology. 2006;19:273–278. doi: 10.1111/j.1525-139X.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsubakihara Y, Nishi S, Akiba T, Hirakata H. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Therapeutic Apheris and Dialysis. 2010 Jun;14(3):240–7. doi: 10.1111/j.1744-9987.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 11.Inaba Masaaki, Okuno Senji, Kumeda Yasuro, Yamada Shinsuke the Osaka CKD Expert Research Group. Glycated Albumin Is a Better Glycemic Indicator than Glycated Hemoglobin Values in Hemodialysis Patients with Diabetes: Effect of Anemia and Erythropoietin Injection. JASN. 2007 Mar;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Reyes MJ, Selgas R, Bajo MA, Jimenez C, Del Peso G, Sanchez MC, Dapena F, De Alvaro F. Increased response to subcutaneous erythropoietin on type I diabetic patients on CAPD: is there a synergistic effect with insulin? Perit Dial Int. 1995 Jul-Sep;15:231–235. [PubMed] [Google Scholar]
- 13.Snyder Jon J, Foley Robert N, Gilbertson David T, Vonesh Edward F, Collins Allan J. Hemoglobin Levels and Erythropoietin Doses in Hemodialysis and Peritoneal Dialysis Patients in the United States. JASN. 2004 Jan 1;15:174–179. doi: 10.1097/01.asn.0000102475.94185.54. [DOI] [PubMed] [Google Scholar]
- 14.Pagé DE, Cheung V, Poirier F. Diabetic patients on peritoneal dialysis need less erythropoietin to maintain adequate hemoglobin. Adv Perit Dial. 2001;17:130–1. [PubMed] [Google Scholar]
- 15.Grzegorzewska AE, Mariak I. Parathyroid hormone contributes to variations in blood morphology in diabetic and non diabetic patients treated with continuous ambulatory peritoneal dialysis. Adv Perit Dial. 2001;17:5–9. [PubMed] [Google Scholar]
- 16.Brancaccio Diego, Cozzolino Mario, Gallieni Maurizio. Hyperparathyroidism and Anemia in Uremic Subjects: A Combined Therapeutic Approach. JASN. 2004 Jan 1;15:S21–S24. doi: 10.1097/01.asn.0000093369.09194.12. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi Irfan Z, M.Phil, PhD, Abid Kausar. Angiotensin converting enzyme inhibitors impair recombinant human erythropoietin induced erythropoiesis in patients with chronic renal failure. Saudi Med J. 2007;28(2):193–196. [PubMed] [Google Scholar]
- 18.Abu-Alfa AK, Cruz D, Perazella MA, Mahnensmith RL, Simon D, Bia MJ. ACE inhibitors do not induce recombinant human erythropoietin resistance in hemodialysis patients. Am J Kidney Dis. 2000 Jun;35(6):1076–82. doi: 10.1016/s0272-6386(00)70043-6. [DOI] [PubMed] [Google Scholar]
- 19.Park Se Jin, MD, Shin Jae Il., MD The beneficial effect of statins on renal anemia in hemodialysis patients. Hemodialysis International. 2011 Nov;16:322–323. doi: 10.1111/j.1542-4758.2011.00631.x. [DOI] [PubMed] [Google Scholar]
- 20.Tangdhanakanond K, Raja R. Effect of statins on erythropoietin responsiveness in type 2-diabetic versus non-diabetic hemodialysis patients. Clin Nephrol. 2010 Jan;73(1):1–6. doi: 10.5414/cnp73001. [DOI] [PubMed] [Google Scholar]
- 21.Lowrie EG. Acute-phase inflammatory process contributes to malnutrition, anemia, and possibly other abnormalities in dialysis patients. American Journal of Kidney Diseases. 1998 Dec;32(6)(Supplement 4):S105–S112. doi: 10.1016/s0272-6386(98)70172-6. [DOI] [PubMed] [Google Scholar]
- 22.Pilkey RM, Morton AR, Iliescu EA. Inflammation, peritoneal transport, and response to erythropoietin in peritoneal dialysis patients. Adv Perit Dial. 2001;17:153–7. [PubMed] [Google Scholar]
- 23.Lai KN, Leung JCK. Inflammation in Peritoneal Dialysis. Nephron Clin Pract. 2010;116:c11–c18. doi: 10.1159/000314544. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh Kamyar, Kalantar-Zadeh Kourosh, Lee Grace H. The Fascinating but Deceptive Ferritin: To Measure It or Not to Measure It in Chronic Kidney Disease? CJASN. 2006 Sep 1;(Supplement 1):S9–S18. doi: 10.2215/CJN.01390406. [DOI] [PubMed] [Google Scholar]
- 25.Webb L, Gilg J, Wilkie M. Haemoglobin, Ferritin and Erythropoietin amongst UK Adult Dialysis Patients in 2010: National and Centre-Specific Analyses. Nephron Clinical Practice. 2012;120.1:c145–c174. doi: 10.1159/000342851. [DOI] [PubMed] [Google Scholar]
- 26.Li Philip KamTao, Cheng Yuk Lun. Therapeutic Options For Preservation Of Residual Renal Function In Patients On Peritoneal Dialysis. Perit Dial Int. 2007 Jun;27:S158–S163. [PubMed] [Google Scholar]