Abstract
BACKGROUND AND OBJECTIVES
Infection due to Coxiella burnetii (C burnetii), the causative agent of Q fever is rarely sought for in clinical practice. This study was performed to detect C burnetii infection in patients with pyrexia of undetermined cause (PUC).
DESIGN AND SETTINGS
This is a prospective study conducted at King Khalid University Hospital, Riyadh between March 2011 and January 2013.
PATIENTS AND METHODS
A total of 3 mL venous blood was collected from 51 patients with PUC at King Khalid University Hospital, Riyadh. This group of patients included 30 males and 21 females (mean age 33.9 [21.3] years) with the history of febrile illness ranging between 4 and 8 weeks. A control group of 50 healthy individuals comprising 39 males and 11 females (mean age 27 [9] years) was also included in the study. Detection of phase II C burnetii–specific IgG antibodies was performed by immunofluorescence assay, and a titer of >1:64 was considered positive.
RESULTS
Phase II C burnetii–specific IgG antibodies were detected in 18 (35.2%) patients out of the total 51 tested. Two (4%) individuals out of 50 in the control group tested positive for anti–C burnetii IgG antibodies. The proportion of positive results among the patients was significantly higher than the controls (P<.0002, 95% CI, 15.09–46.25). The antibody titer range was between 1:128 and 1:1024 where 6 patients had titers of 1:256, 5 had 1:512, 4 had 1024, and 3 had 1:128.
CONCLUSION
The evidence of C burnetii infection in a sizable number of patients emphasizes the need for inclusion of serologic investigations for Q fever in patients with PUC.
Q fever (query fever) is a zoonosis caused by Coxiella burnetii (C burnetii) that can cause acute or chronic human disease with varying manifestations.1 Acute Q fever frequently presents as a self-limiting flu-like illness with sudden onset, high-grade fever, headache, weight loss, myalgia, cough, or atypical pneumonia or hepatitis.2 In the absence of appropriate treatment the fever usually lasts for 1 to 3 weeks, and in a relatively small proportion of patients fever may persist for a longer duration.3 Unlike acute, chronic Q fever can manifest within months or years after symptomatic or asymptomatic infection in the form of endocarditis, vascular infections, chronic hepatitis, osteoarthritis, ostemyelitis, or pulmonary infections.2 Endocarditis is a typical presentation in patients with pre-existing valvular or vascular defects.4 If left untreated, C burnetii endocarditis is always fatal compared to less than 2% mortality rate reported in acute Q fever.5
Because of the non-specific and diverse presenting symptoms of acute Q fever, the diagnosis is usually missed because the health care providers do not suspect the illness. This was evident in a seroepidemiological survey for C burnetii conducted on hospitalized US troops deployed in Iraq. A significant number of soldiers who were actually suffering from Q fever had an initial diagnosis of a febrile illness of unknown, pneumonia; organism not specified and unspecified viral infection were later found to be suffering from Q fever.6 Compared with the adults, children are less likely to have symptoms and usually suffer from a milder form of the illness. Although Q fever is a self-limiting disorder in a vast majority of children, it may follow a course of relapsing febrile illness lasting for several months in some children.7 Furthermore, Q fever in children may present with skin rash,8 gastrointestinal symptoms,9 or more sever manifestations such as hemolytic uremic syndrome,10 acute cholecystitis,11 or rhabdomyolysis.12 Similarly, Q fever infection shortly before conception or during pregnancy has been implicated in miscarriage, premature birth, intrauterine growth retardation, and stillbirths.13 Collectively, these observations highlight the importance of screening for C burnetii infection, particularly, in clinical situations posing a diagnostic challenge.
C burnetii exists in 2 antigenic phases referred to as phase I and phase II. Phase I is a highly virulent infectious form that transforms into an avirulent phase II after serial laboratory passages. Acute Q fever is characterized by a phase II antibody response that is higher than a phase I response associated with chronic infection.2 Serological diagnosis of Q fever is most commonly made by using commercially available immunofluorescence assay (IFA) in the US.14 The assay detects C burnetii phase II–specific IgG, and the demonstration of fourfold rise in the antibody titer between acute and convalescent phase sample taken 3 to 6 weeks apart is considered diagnostic. Using IFA, this study was performed at the Immunology unit of King Khalid University Hospital, Riyadh, Saudi Arabia, to detect the presence of C burnetii–specific IgG antibodies in sera samples from patients who were being investigated for pyrexia of undetermined cause (PUC).
PATIENTS AND METHODS
This prospective study was performed at the Immunology Unit of King Khalid University Hospital, Riyadh, between March 2011 and January 2013. After obtaining the informed consent, 3 mL of venous blood sample was collected from each patient and control. A total of 51 patients including 30 males and 21 females (mean age 33.9 [21.3], range 6–78 years) with the history of febrile illness of duration ranging between 4 and 8 weeks were included in the study. This group of patients included 6 patients who were either equal to or less than 12 years of age. These patients had already undergone exhaustive investigations for PUC. Apart from the routine laboratory investigations, all the patients had been tested negative for the presence of common causes for their febrile illness such as blood culture, antibody detection for brucellosis, typhoid fever, and serological tests for other bacterial and viral infections. A single blood specimen was collected from patients for screening purposes because all the patients were ill for more than 4 weeks and patient follow-up was not possible in the Immunology unit. Similarly, clinical details of the patients could not be retrieved because of the lack of access to clinical data. Relevant details regarding the serological workup of the patients were extracted from patient laboratory records available in the hospital information system. Blood samples were also collected from 50 healthy blood donors as a control group after having been declared fit to donate blood following routine screening for microbial infections. This group included 39 males and 11 females (mean age 27 [9], range 19–39 years).
Detection of anti-C burnetii antibodies
Phase II anti–C burnetii IgG antibodies were detected in sera samples using an IFA kit (Vircell, S. L. Pza. Dominguez Ortiz I. Poligono Industrial Dose de Octubre. 18320 Santa Fe, Granada, Spain). Each slide in the kit had 10 spots coated with C burnetii phase II, Nine Mile strain (ATCC 616-VR) grown in MRC-cells. The organisms were inactivated with formaldehyde and were fixed with acetone. The serum sample was initially diluted at 1:64, and then twofold dilutions were made for the titration of positive samples up to a maximum of 1:2048 when required. Diluted sera samples along with the positive and negative controls were overlaid onto the antigen spots and incubated at 37°C in a humidified chamber for 30 minutes. After the incubation, the slide was washed twice with phosphate-buffered saline and once with distilled water. After washing, the slide was air dried, and 20 μL anti-human IgG fluorescein isothiocyanate–conjugated antibodies were added to each antigen spot and then the slide was incubated for 30 minutes at 37°C in a humid chamber. Finally, the slide was washed as described previously and dried in air. Then a drop of mounting medium was added to the slide and it was examined immediately under 400× magnification using fluorescence microscope (Axioskop 2 plus; Zeiss, Gttingen, Germany). Apple green fluorescence of coco-bacillar morphology detected at the serum dilution of 1:64 or more was considered a positive test in accordance with the recommendations of the manufacturers.
RESULTS
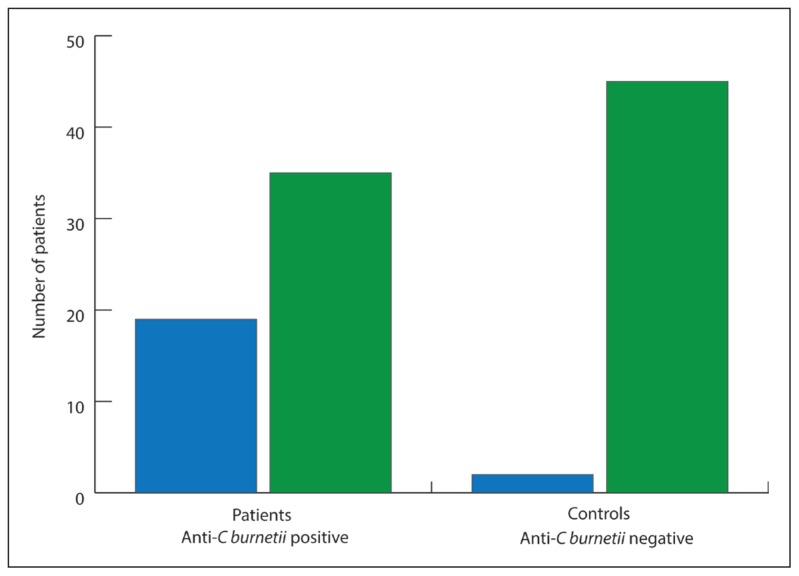
Figure 1 describes detection of C burnetii phase II antigen–specific IgG using IFA among the patients and the controls. Among the 51 patients investigated, 18 (35.2%) tested positive for the presence of C burnetii–specific IgG antibodies. Two (4%) individuals among the 50 normal healthy controls were found to harbor C burnetii–specific IgG antibodies in sufficient titers to yield a positive result. A comparative analysis of the proportions tested positive for C burnetii–specific IgG antibodies in 2 groups revealed that the reactivity was significantly higher among the patients when compared with the normal healthy controls (P<.0002, 95% CI, 15.09–46.25). Table 1 shows data for the distribution of detected titers of C burnetii–specific IgG antibodies among patients and controls. The titers among the patients varied between 1:128 and 1:1024 where 6 patients had titers of 1:256, 5 had 1:512, 4 had 1024, and 3 had 1:128. The titers in 2 normal healthy individuals who tested positive were 1:128 and 1:256.
Figure 1.
Detection of phase II C burnetii specific IgG antibodies in patients with pyrexia of undetermined cause (n=51) and healthy blood donors (n = 50).
Table 1.
Distribution of phase II Coxiella burnetii (C burnetii)–specific IgG antibody titers among patients with pyrexia of undetermined cause (51) and healthy blood donors (n=50).
| S # | Anti–C burnetii titer (patients) | Anti–C burnetii titer (controls) |
|---|---|---|
|
| ||
| 1. | 1:128 | 1:128 |
| 2. | 1:128 | 1:256 |
| 3. | 1:128 | - |
| 4. | 1:256 | - |
| 5. | 1:256 | - |
| 6. | 1:256 | - |
| 7. | 1:256 | - |
| 8. | 1:256 | - |
| 9. | 1:256 | - |
| 10. | 1:512 | - |
| 11. | 1:512 | - |
| 12. | 1:512 | - |
| 13. | 1:512 | - |
| 14. | 1:512 | - |
| 15. | 1:1024 | - |
| 16. | 1:1024 | - |
| 17. | 1:1024 | - |
| 18. | 1:1024 | - |
DISCUSSION
This study revealed that a significantly higher proportion of patients being investigated for PUC were harboring diagnostic titers of C burnetii phase II–specific IgG antibodies, indicating that they may have been suffering from acute Q fever. Interest in Q fever appears to be increasing worldwide as indicated by several publications on the subject in the past.15–17 This could be attributed to improvement in diagnostic tests contributing to the modification of clinical signs and symptoms of Q fever or to better awareness among treating physicians. Q fever continues to be a less sought for infection in Saudi Arabia. To best of our knowledge, the last 2 reports on Q fever in Saudi Arabia were published in the years I96618 and 1968.19 This was followed by a single case report of C burnetii endocarditis in 1997.20 The paucity of data from Saudi Arabia indicates the lack of awareness among the physicians and scientists. The findings of the present study—although in a relatively small number of patients—provides substantial evidence of the presence of Q fever in Saudi Arabia.
The control group in this study comprises healthy blood donors, and among them 4% were found to carry the evidence of C burnetii infection. A study conducted in France investigating seroprevalence of Q fever in humans has reported 3% reactivity among 620 blood donors tested for the presence of C burnetii infection, whereas in 785 individuals from general population when screened for C burnetii infection using IFA 5% tested positive.21 Similarly the national seroprevalence rate in the US is estimated to be 3.1% based on data from the National Health and Nutrition Examination Survey (2003–2004).22 In addition, asymptomatic C burnetii infection followed by seroconversion is a known phenomenon and has been reported in around 60% of cases identified during outbreak investigations.2,23 These asymptomatic infections may therefore contribute to the seroprevalence of Q fever among the general population. Although the seroprevalence of Q fever among the blood donors in the present study is no different than the previously reported figures, the number of the donors tested in the present study was however much smaller and may not represent the actual seroprevalence in the local population. Furthermore, the detection of Q fever among donors is a significant risk for the transmission of Q fever through blood transfusion. Large scale studies should be performed to determine the seroprevalence of Q fever in Saudi Arabia.
The seroprevalence of infection by C burnetii in humans as high as 36% has been reported from Canary Islands in an outbreak of Q fever.24 Similarly, being a zoonotic infection, 18% of farming population in Eastern Poland has been reported to harbor the evidence of C burnetii infection.25 Seroprevalence data for Q fever in Saudi Arabia are lacking. It is therefore difficult to compare the findings of the present study with the seroprevalence rates elsewhere because of the selection bias. Significantly higher percentage (35.2%) of patients with C burnetii–specific IgG antibodies found in the present study in a selected group of patients, however, emphasizes the need for the introduction of Q fever screening among the investigations routinely performed to investigate febrile illnesses. Similar recommendations have also been made in a study from Switzerland where 2 cases of PUC were found to be suffering from Q fever.26
The recommended practice of a fourfold increase in phase II C burnetii–specific IgG antibodies between the acute and convalescent samples taken 3 to 6 weeks apart makes this primarily a retrospective diagnosis. The testing of paired sera samples is also preferred due to the fact that seroconversion after symptomatic or asymptomatic infection, C burnetii antibodies may remain detectable for years or for life.27 In the early stage of the infection, specific antibodies are usually not detectable, and reliance on the PCR detection of C burnetii DNA for diagnosis may be a more useful approach. Because of the low level of specific antibodies in the early stages of the disease, IFA may yield a negative result in an acute phase sample of a patient suffering from Q fever. Despite these limitations, it is generally agreed that the detection of phase II IgG antibody titer either equal to or more than 1:128 in a single serum sample from a patient who is ill for more than 1 week, indicates acute C burnetii infection.14 Early detection of Q fever is of paramount importance because in approximately 2% of symptomatic patients with acute Q fever, the disease progresses to chronic Q fever that is associated high morbidity and mortality.28 The detection of C burnetii–specific IgM antibodies as a single diagnostic test for C burnetii has a limited clinical application. This is primarily due to the observations that C burnetii–specific IgM antibodies may persist for more than 1 year in some cases, IgM antibodies against C burnetii are less specific than IgG antibodies and exhibit a high cross-reactivity with Coxiella, Legionella, and Bartonella.29,30 Only 3 patients in the present study had a 1:128 titer of phase II C burnetii–specific IgG antibodies, whereas the rest of the patients had significantly higher titers of IgG. These findings therefore suggest that there is increased likelihood that all the patients who tested positive for the presence of phase II IgG antibodies were suffering from acute Q fever. This study was, however, limited because of the non-availability of paired sera samples, thus lacking the confirmation of diagnosis of Q fever on the basis of rising titers of C burnetii–specific IgG antibodies.
The evidence of Q fever prevailing in a substantial number of patients with PUC, however, indicates that the fever exists in the Kingdom. It also emphasizes the need for the introduction of appropriate test for Q fever on a regular basis while investigating PUC. The presence of Q fever among blood donors also poses a serious threat of blood-borne transmission of the disease and necessitates the need for the introduction of Q fever screening in transfusion services. Large scale studies are also recommended for the assessment of prevalence of Q fever in Saudi Arabia.
REFERENCES
- 1.Raoult D, Marrie T. Q fever. Clin Infect Dis. 1995;20:489. doi: 10.1093/clinids/20.3.489. [DOI] [PubMed] [Google Scholar]
- 2.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrie T. Acute Q fever. CRC press; Boca Raton: 1990. pp. 125–157. [Google Scholar]
- 4.Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis. 2001;33:312–6. doi: 10.1086/321889. [DOI] [PubMed] [Google Scholar]
- 5.Rolain JM, Boulos A, Mallet MN, Raoult D. Correlation between ratio of serum doxycycline concentration to MIC and rapid decline of antibody levels during treatment of Q fever endocarditis. Antimicrob Agents Chemother. 2005;49:2673–6. doi: 10.1128/AAC.49.7.2673-2676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson AD, Baker TR, Littrell AC, Mott RL, Niebuhr DW, Smoak BL. Seroepidemiologic survey for Coxiella burnetii among hospitalized U.S. troops deployed to Iraq. Zoonoses Public Health. 2011;58:276–83. doi: 10.1111/j.1863-2378.2010.01347.x. [DOI] [PubMed] [Google Scholar]
- 7.Richardus JH, Dumas A, Huisman J, Schapp G. Q fever in infancy: a review of 18 cases. Pediatr Infect Dis. 1985;4:369–73. doi: 10.1097/00006454-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Maltezou HC, Constantopoulou I, Kallergi C, Vlahou V, Georgakopoulos D, Kafetzis DA, et al. Q fever in children in Greece. Am J Trop Med Hyg. 2004;70(5):540–4. [PubMed] [Google Scholar]
- 9.Terheggen U, Leggat PA. Clinical manifestations of Q fever in adults and children. Travel Med Infect Dis. 2007;5:159–64. doi: 10.1016/j.tmaid.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Maltezou HC, Kallergi C, Kavazarakis E, Stabouli S, Kafetzis DA. Hemolytic-uremic syndrome associated with Coxiella burnetii infection. Pediatr Infect Dis J. 2001;20:811–3. doi: 10.1097/00006454-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Rolain JM, Lepidi H, Harlé JR, et al. Acute acalculous cholecystitis associated with Q fever: report of seven cases and review of the literature. Eur J Clin Microbiol Infect Dis. 2003;22:222–7. doi: 10.1007/s10096-003-0899-1. [DOI] [PubMed] [Google Scholar]
- 12.Carrascosa M, Pascual F, Borobio MV, González Z, Napal J. Rhabdomyolysis associated with acute Q fever. Clin Infect Dis. 1997;25:1243–4. doi: 10.1086/516064. [DOI] [PubMed] [Google Scholar]
- 13.Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A. Managing Q fever during pregnancy: the benefits of long-term cotrimoxazole therapy. Clin Infect Dis. 2007;45:548–55. doi: 10.1086/520661. [DOI] [PubMed] [Google Scholar]
- 14.Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, et al. Diagnosis and Management of Q Fever — United States, 2013: Recommendations from CDC and the Q Fever Working Group. Recommendations and Reports. 2013 Mar 29;62(RR03):1–23. [PubMed] [Google Scholar]
- 15.Kovacova E, Kazar J. Q fever–still a query and underestimated infectious disease. Acta Virol. 2002;46:193–210. [PubMed] [Google Scholar]
- 16.Madariaga MG, Rezai K, Trenholme GM, Weinstein RA. Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3:709–721. doi: 10.1016/s1473-3099(03)00804-1. [DOI] [PubMed] [Google Scholar]
- 17.Woldehiwet Z. Q fever (coxiellosis): epidemiology and pathogenesis. Res Vet Sci. 2004;77:93–100. doi: 10.1016/j.rvsc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Gelpi AP. Q fever in Saudi Arabia. Am J Trop Med Hyg. 1966;15:784–98. [PubMed] [Google Scholar]
- 19.Lippi M, Sebastiani A, el-Mutabakani H. [Detection of serum antibodies against Reoviruses, Adenoviruses and Coxiella burneti in a group of inhabitants of Riyad (Saudi Arabia)]. Arch Ital Sci Med Trop Parassitol. 1968;49:129–36. [Article in Italian] [PubMed] [Google Scholar]
- 20.al-Hajjar S, Hussain Qadri SM, al-Sabban E, Jäger C. Coxiella burnetii endocarditis in a child. Pediatr Infect Dis J. 1997;16:911–3. doi: 10.1097/00006454-199709000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Carrieri MP, Tissot-Dupont H, Rey D, Brousse P, Renard H, Obadia Y, et al. Investigation of a slaughterhouse-related outbreak of Q fever in the French Alps. Eur J Clin Microbiol Infect Dis. 2002;21:17–21. doi: 10.1007/s10096-001-0645-5. [DOI] [PubMed] [Google Scholar]
- 22.Anderson AD, Kruszon-Moran D, Loftis AD, et al. Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg. 2009;81:691–4. doi: 10.4269/ajtmh.2009.09-0168. [DOI] [PubMed] [Google Scholar]
- 23.Bamberg WM, Pape WJ, Beebe JL, et al. Outbreak of Q fever associated with a horse boarding ranch, Colorado, 2005. Vector Borne Zoonotic Dis. 2007;7:394–402. doi: 10.1089/vbz.2007.0104. [DOI] [PubMed] [Google Scholar]
- 24.Bolanos M, Santana OE, Angel-Moreno A, Perez-Arellano JL, Liminana JM, Serra-Majem L, et al. Seroprevalence of infection by Coxiella burnetii in Canary Islands (Spain) Eur J Epidemiol. 2003;18:259–262. doi: 10.1023/A:1023342624475. [DOI] [PubMed] [Google Scholar]
- 25.Cisak E, Chmielewska-Badora J, Mackiewicz B, Dutkiewicz J. Prevalence of antibodies to Coxiella burnetii among farming population in eastern Poland. Ann Agric Environ Med. 2003;10:265–267. [PubMed] [Google Scholar]
- 26.Fischer L, Garin N, Péter O, Praz G. [Q fever: a cause of fever of unknown origin in Switzerland]. Rev Med Suisse. 2012 Oct 10;8(357):1921–4. [Article in French] [PubMed] [Google Scholar]
- 27.Murphy AM, Field PR. The persistence of complement-fixing antibodies to Q-fever (Coxiella burnetii) after infection. Med J Aust. 1970;1:1148–50. doi: 10.5694/j.1326-5377.1970.tb84481.x. [DOI] [PubMed] [Google Scholar]
- 28.European Centre for Disease Prevention and Control. Risk Assessment on Q Fever. Stockholm: ECDC; 2010. [Accessed on 3 August 2012]. Available at: http://ecdc.europa.eu/en/publications/Publications/1005_TER_Risk_Assessment_Qfever.pdf. [Google Scholar]
- 29.La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol. 1996;34:2270–4. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musso D, Raoult D. Serological cross-reactions between Coxiella burnetii and Legionella micdadei. Clin Diagn Lab Immunol. 1997;4:208–12. doi: 10.1128/cdli.4.2.208-212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]