Abstract
BACKGROUND AND OBJECTIVES
Many studies were conducted to assess the relationship between Monoamine oxidase B (MAOB) A644G polymorphism and susceptibility to Parkinson disease (PD). However, the results were inconsistent and inconclusive.
DESIGN AND SETTINGS
A meta-analysis was conducted from all published studies on the associations between monoamine oxidase B (MAOB) A644G polymorphism and Parkinson disease.
METHODS
In this present study, the possible relationship between MAOB A644G polymorphism and PD risk was assessed by a meta-analysis. Eligible articles were identified for the period up to March 2013. Pooled odds ratios (OR) with 95% confidence intervals (CI) were appropriately derived from fixed-effects models.
RESULTS
Twenty case–control studies with a total of 2846 cases and 3508 controls were eligible. In a recessive model, MAOB A644G polymorphism was associated with PD risk (OR=1.32, 95% CI 1.18–1.47, P<.001). Subgroup analyses by ethnicity and gender also found significant relationships between this polymorphism and PD risk.
CONCLUSION
This meta-analysis suggested that MAOB A644G polymorphism may be associated with PD development.
Parkinson disease (PD) is a neurodegenerative disorder with unknown causes. More than 1 million people in the United States have PD, and PD affects approximately 1 in 100 Americans older than 60 years.1 Although PD typically presents in a sporadic fashion, between 10% and 15% of PD patients have a family history of the disease, indicating that there is a strong genetic basis for this disease.2
Monoamine oxidase B (MAOB) is one of the primary enzymes regulating metabolism of neurotransmitters such as dopamine. It catalyzes the production of hydrogen peroxide, and it activates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to MPP+ a toxic metabolite that can cause parkinsonism.3 Steventon et al. showed that patients with PD had higher platelet MAOB activity than control individuals. 4 In addition, MAOB activity increases with age as does predisposition toward PD, which has also been linked to increased oxidative stress.5 Furthermore, it is well documented that MAOB inhibition may prevent degeneration of the dopaminergic system in PD.6 Therefore, MAOB may play a critical role in the development of PD.
The MAOB gene is located on the X chromosome. It contains a single-stranded conformational polymorphism in intron 13, a transitional conversion of adenine (A) to guanine (G) at 36 bp upstream from the 5′ end of exon 14 (A644G, rs1799836). This polymorphism is associated with varying enzyme activity. The G allele of MAOB A644G polymorphism is associated with lower brain MAOB activity, and A allele is associated with higher mRNA levels of MAOB.7 A number of papers investigated the relationship between this polymorphism and PD risk. However, the results remained inconclusive.8–27 Meta-analysis is a useful method for investigating the relationship s between genetic factors and diseases, because a quantitative approach is used to combine the results from different studies on the same topic, thereby providing more reliable conclusions. Thus, we performed a meta-analysis to clarify the relationship of MAOB A644G polymorphism with PD risk. To our knowledge, this is the most comprehensive meta-analysis of the relationship between MAOB A644G polymorphism and PD risk.
METHODS
Search for publications
In our meta-analysis, we searched the articles using the search terms “MAOB,” “monoamine oxidase B,” “Parkinson disease,” and “polymorphism” in the PubMed, Embase, and CNKI databases, and the last search updated on March 2013. Additional studies were identified by a hand search of references of original studies or review articles on the relationship between MAOB A644G polymorphism and PD risk. No publication date or language restrictions were imposed.
Inclusion and exclusion criteria
The following inclusion criteria were used: (1) the study should have evaluated the relationship between MAOB A644G polymorphism and PD risk, (2) the study should have had a case–control design, and (3) sufficient data should have been provided to calculate odds ratios (OR) and 95% confidence intervals (CI). Studies were excluded if any of the following conditions applied: (1) irrelevant to PD, MAOB, or MAOB A644G polymorphism and PD risk, (2) abstract or review, (3) genotype frequencies not reported, (4) non-clinical study, and (5) studies repeated studies or overlapped publications.
Data extraction
Two investigators independently extracted data and reached a consensus on the following characteristics of the selected studies: the first author’s name, year of publication, country, ethnicity of the study population, gender, genotyping method, and numbers of cases and controls with various genotypes.
Statistical analysis
The strength of relationship between MAOB A644G polymorphism and PD risk was accessed by calculating ORs with 95% CIs. For this meta-analysis, we examined the recessive genetic model (AA [A] vs AG + GG [G]) because allele A is a risk allele for PD, and data were commonly presented either in this format or convertible to this format. The random-effects model (the DerSimonian and Laird method) was used. A chi-square test was used to determine if genotype distribution of the female control population reported conformed to Hardy-Weinberg equilibrium (HWE) (P<.05 was considered significant). Heterogeneity assumption was checked by the I2 statistic to quantify the proportion of the total variation towing to heterogeneity, and I2 value less than 50% indicates a lack of heterogeneity among studies. Subgroup analyses were performed by ethnicity, gender, and smoking status. Cumulative meta-analysis was done. Furthermore, sensitivity analysis was performed by sequential omission of individual studies. Galbraith plot was used to spot the outliers that were the sources of heterogeneity. Funnel plot and Egger test were used to detect publication bias.28 Analyses were performed using STATA 11.0 software (StataCorp LP, College Station, Texas, USA).
RESULTS
Characteristics of studies
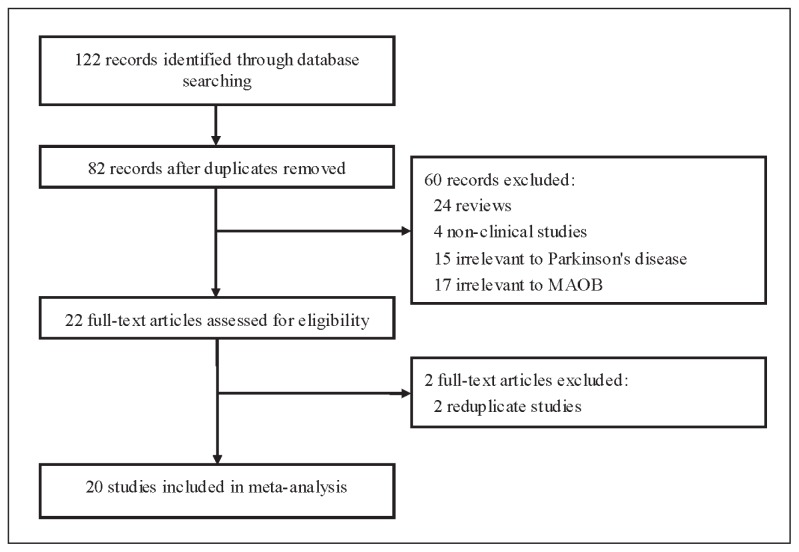
A total of 20 case–control studies (Figure 1) with 6354 subjects on the relationship between MAOB A644G polymorphism and PD risk were included for this meta-analysis.8–27 The study involved 8 studies of Caucasian population and 12 studies of Asian population. All studies indicated that the distribution of genotypes in the female controls was consistent with HWE. The characteristics of each case–control study and the genotype in each case–control study are presented in Tables 1 and 2.
Figure 1.
Flow of study identification, inclusion, and exclusion.
Table 1.
Characteristics of the case–control studies included in meta-analysis.
| First author | Year | Country | Ethnicity | Gender | Case | Control | Case | Control | Genotyping | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| number (n) | number (n) | AA (A) | AG + GG (G) | AA (A) | AG + GG (G) | Method | |||||
|
| |||||||||||
| Kurth | 1993 | USA | Caucasian | M, F | 64 | 177 | 25 | 39 | 36 | 141 | PCR-SSCP |
| Morimoto | 1995 | Japan | Asian | M, F | 83 | 76 | 60 | 23 | 54 | 22 | PCR-SSCP |
| Costa | 1997 | USA | Caucasian | M, F | 62 | 79 | 26 | 36 | 23 | 56 | PCR-SSCP |
| Hwang | 1997 | China | Asian | M, F | 65 | 108 | 49 | 16 | 76 | 32 | PCR-SSCP |
| Checkoway | 1998 | USA | Caucasian | M, F | 82 | 118 | 52 | 30 | 58 | 60 | PCR-SSCP |
| Mellick | 1999 | Australia | Caucasian | M, F | 80 | 110 | 30 | 50 | 36 | 74 | PCR-SSCP |
| Shao | 2000 | China | Asian | M, F | 126 | 136 | 66 | 65 | 62 | 74 | PCR-SSCP |
| Wu | 2001 | China | Asian | M, F | 220 | 191 | 37 | 183 | 17 | 174 | PCR-SSCP |
| Hernán | 2002 | USA | Caucasian | M, F | 214 | 449 | 86 | 128 | 196 | 253 | Allele-specific PCR |
| Kelada | 2002 | USA | Caucasian | M, F | 186 | 296 | 106 | 80 | 138 | 158 | PCR-SSCP |
| Tan | 2003 | Singapore | Asian | M, F | 230 | 241 | 171 | 59 | 176 | 65 | PCR-SSCP |
| Jiang | 2004 | China | Asian | M, F | 266 | 154 | 207 | 59 | 114 | 40 | PCR-SSCP |
| Białecka | 2005 | Poland | Caucasian | M, F | 210 | 152 | 100 | 110 | 63 | 89 | PCR-SSCP |
| Singh | 2008 | India | Asian | M, F | 70 | 100 | 30 | 37 | 33 | 37 | PCR-SSCP |
| Gu | 2010 | China | Asian | M, F | 176 | 354 | 153 | 23 | 323 | 31 | DHPLC |
| Wang | 2010 | China | Asian | M, F | 125 | 66 | 88 | 37 | 34 | 32 | PCR-SSCP |
| Kiyohara | 2011 | Japan | Asian | M, F | 238 | 369 | 192 | 46 | 273 | 96 | PCR-SSCP |
| Li | 2011 | China | Asian | M, F | 166 | 170 | 111 | 55 | 103 | 67 | PCR-RFLP |
| Torkaman-Boutorabi | 2012 | Iran | Caucasian | M, F | 103 | 70 | 75 | 28 | 44 | 26 | PCR-RFLP |
| Zeng | 2012 | China | Asian | M, F | 95 | 104 | 67 | 28 | 71 | 33 | PCR-RFLP |
M: male, F: female, MF: male and female, PCR: polymerase chain reaction, SSCP: single-stranded conformational polymorphism, RFLP: restriction fragment length polymorphism, DHPLC: denaturing high-performance liquid chromatography.
Table 2.
Summary of different results of the association between MAOB A644G polymorphism and the risk of Parkinson disease.
| Comparison | Sample size | No. | Test of association | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Studies | OR (95% CI) | Z | P value | Model | c2 | P value | I2 (%) | ||
|
| |||||||||||
| AA (A) vs AG+GG (G) | Overall | 2846 | 3508 | 20 | 1.32(1.18–1.47) | 4.80 | <.001 | F | 24.45 | .18 | 22.0 |
| AA (A) vs AG+GG (G) | Asian | 1865 | 2069 | 12 | 1.28(1.10–1.49) | 3.22 | .001 | F | 13.25 | .28 | 17.0 |
| AA (A) vs AG+GG (G) | Caucasian | 981 | 1439 | 8 | 1.37(1.15–1.62) | 3.62 | <.001 | F | 10.80 | .15 | 35.0 |
| A vs G | Male | 1038 | 1143 | 12 | 1.49(1.22–1.81) | 3.97 | <.001 | F | 4.29 | .96 | 0 |
| AA vs AG+GG | Female | 764 | 970 | 12 | 1.32(1.06–1.64) | 2.45 | .01 | F | 6.99 | .80 | 0 |
F: Fixed-effects model.
Results of meta-analyses
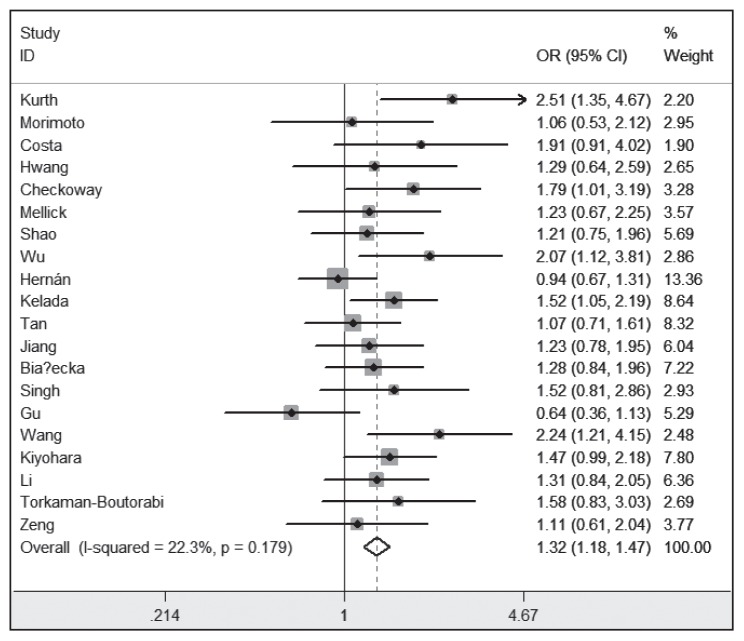
As shown in Figure 2, the overall OR was 1.32 (95% CI 1.18–1.47), and the Z-test value for the overall effect was 4.80 (P<.001) for the AA (A) vs AG + GG (G). Subgroup analysis by ethnicity was performed. For ethnicity, the populations were stratified into 2 groups: Asian (1865 cases and 2069 controls) and Caucasian (981 cases and 1439 controls). Significant relationships with PD risk in these populations were observed: Asian (OR=1.28, 95% CI 1.10–1.49, P=.001) and Caucasian (OR=1.37, 95% CI 1.15–1.62, P<.001). Besides, subgroup analyses by gender also found significant relationships between MAOB A644G polymorphism and PD risk in females and males (Table 2).
Figure 2.
Meta-analysis for the relationship between the MAOB A644G polymorphism and PD risk.
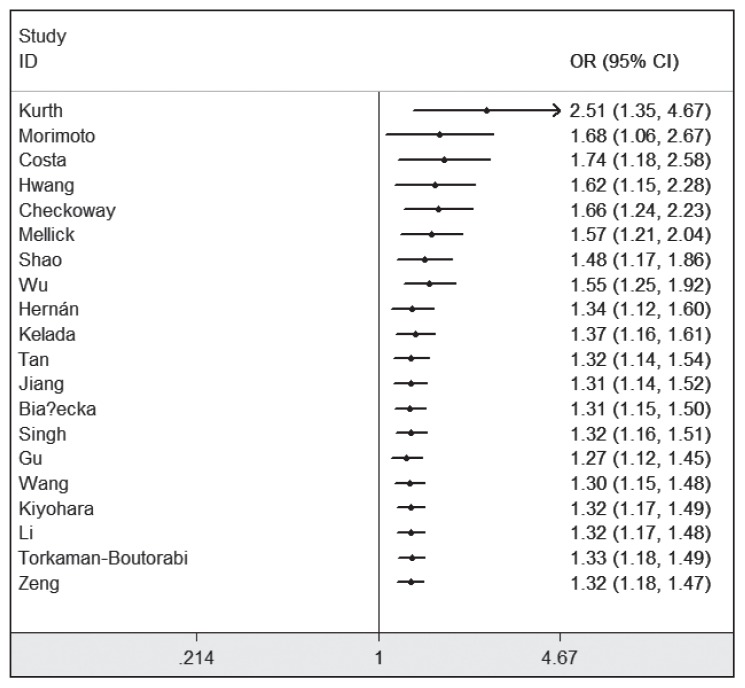
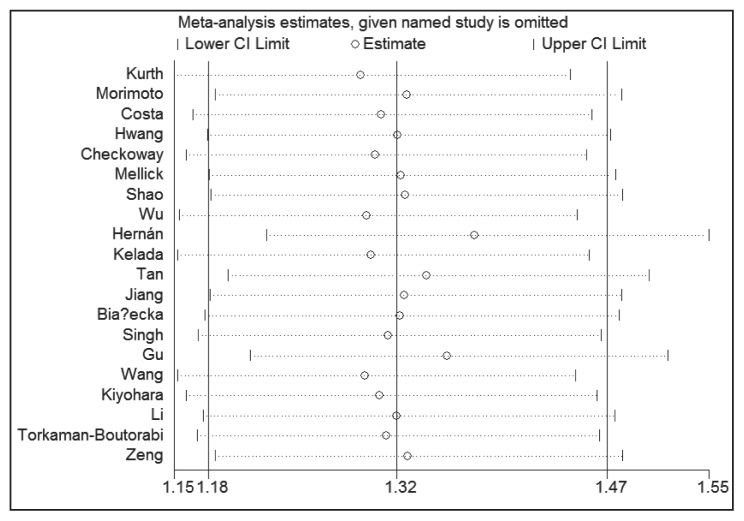
With regard to the cumulative meta-analysis, evidence was observed to support a significant relationship of MAOB A644G polymorphism with the susceptibility to PD (Figure 3). As shown in Figure 4, sensitivity analysis did not influence the result excessively by omitting any single study.
Figure 3.
Cumulative meta-analysis of relationship between the MAOB A644G polymorphism and PD risk.
Figure 4.
Sensitivity analysis for the MAOB A644G polymorphism and PD risk.
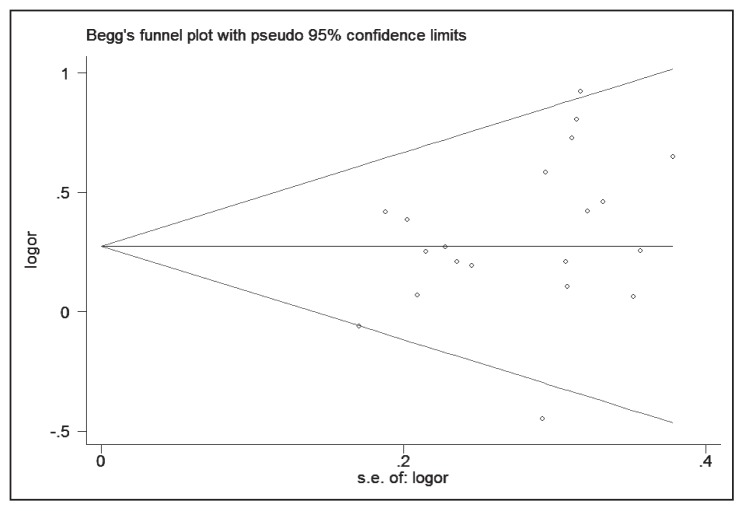
Funnel plot and Egger test were both performed to access the publication bias of this meta-analysis. The shape of the funnel plot seemed symmetrical, and P values of the Egger test was .116 (Figure 5), providing statistical evidence of the funnel plot symmetry.
Figure 5.
Funnel plot for publication bias test in the meta-analysis investigating the relationship between the MAOB A644G polymorphism and PD risk.
DISCUSSION
This present meta-analysis investigated the relationship between MAOB A644G polymorphism and PD risk. Twenty case–control studies with a total of 6354 subjects were eligible. At the overall analysis, MAOB A644G polymorphism seemed to be associated with PD risk. In addition, subgroup analyses by ethnicity and gender also found significant relationships. Moreover, to investigate the stability of the result, we performed sensitivity analyses. The removal of each study did not alter the result, suggesting the reliability of our result. The cumulative meta-analysis showed a trend of significant relationship between MAOB A644G polymorphism and the PD risk as data accumulated each year. Again, this procedure proved that our result was robust. Thus, results from this meta-analysis suggested that MAOB A644G polymorphism was significantly associated with PD risk.
MAOB plays an important role in the metabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues. Increased levels of MAOB mRNA and enzymatic activity have been reported in platelets from patients with PD.4 Additionally, Jakubauskiene et al. indicated that enhanced MAOB protein levels in platelets might be used as a disease marker of PD.29 Several DNA polymorphisms in the MAOB gene have been described. The only single-nucleotide polymorphism found in all human populations is the G/A dimorphism in the intron 13 sequence. The molecular analysis of MAOB polymorphism demonstrated that MAOB A644G polymorphism leads to an alterative MAOB activity.7 Thus, we hypothesized that MAOB A644G polymorphism could influence the susceptibility to PD. Our results supported a genetic relationship between this polymorphism and susceptibility to PD.
One of the major concerns in a sound meta-analysis is the degree of heterogeneity that exists between the component studies because non-homogeneous data are liable to result in misleading results. In the present study, the I2 statistics was carried out to test the significance of heterogeneity. Moderate heterogeneity between studies was observed in overall comparisons. In an attempt to find the sources of heterogeneity, subgroup analysis was performed. The heterogeneity was significantly reduced in the subgroup analysis by gender. Moreover, we re-analyzed the relationship in the female and male subgroups; the conclusions were consistent. Another important issue for any meta-analysis is publication bias owing to the selective publication of reports. In the current study, funnel plot and Egger test were performed to evaluate this problem. Both the shape of funnel plots and the statistical results did not show publication bias.
Some possible limitations in this meta-analysis should be acknowledged. First, only the published studies that were included in the selected electronic databases were identified; it is possible that some relevant published or unpublished studies may have been missed. Second, the effect of gene–gene and gene–environment interactions was not addressed in this meta-analysis because of the limited available data. Third, our meta-analysis was based on the unadjusted OR estimates because not all published studies presented adjusted ORs.
In conclusion, this meta-analysis suggests that MAOB A644G polymorphism may be associated with PD development. Further studies can assess the possible gene–environment and gene–gene interactions in the relationship between this polymorphism and PD risk.
REFERENCES
- 1.Fritsch T, Smyth KA, Wallendal MS, Hyde T, Leo G, Geldmacher DS. Parkinson Disease: Research Update and Clinical Management. South Med J. 2012;105:650–6. doi: 10.1097/SMJ.0b013e318273a60d. [DOI] [PubMed] [Google Scholar]
- 2.Zimprich A. Genetics of Parkinson’s disease and essential tremor. Curr Opin Neurol. 2011;24:318–23. doi: 10.1097/WCO.0b013e3283484b87. [DOI] [PubMed] [Google Scholar]
- 3.Langston JW. Epidemiology versus genetics in Parkinson’s disease: progress in resolving an age-old debate. Ann Neurol. 1998;44:S45–S52. doi: 10.1002/ana.410440707. [DOI] [PubMed] [Google Scholar]
- 4.Steventon G, Sturman S, Heafield M, Waring R, Napier J, Williams A. Platelet monoamine oxidase-B activity in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1989;1:255–61. doi: 10.1007/BF02263479. [DOI] [PubMed] [Google Scholar]
- 5.Kumar MJ, Andersen JK. Perspectives on MAO-B in aging and neurological disease: Where do we go from here? Mol Neurobiol. 2004;30:77–89. doi: 10.1385/MN:30:1:077. [DOI] [PubMed] [Google Scholar]
- 6.Strolin BM, Dostert P. Monoamine oxidase, brain ageing and degenerative diseases. Biochem Pharmacol. 1989;38:555. doi: 10.1016/0006-2952(89)90198-6. [DOI] [PubMed] [Google Scholar]
- 7.Balciuniene J, Emilsson L, Oreland L, Pettersson U, Jazin E. Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet. 2002;110:1–7. doi: 10.1007/s00439-001-0652-8. [DOI] [PubMed] [Google Scholar]
- 8.Kurth JH, Kurth MC, Poduslo SE, Schwankhaus JD. Association of a monoamine oxidase B allele with Parkinson’s disease. Ann Neurol. 1993;33:368–72. doi: 10.1002/ana.410330406. [DOI] [PubMed] [Google Scholar]
- 9.Morimoto Y, Murayama N, Kuwano A, Kondo I, Yamashita Y, Mizuno Y. Association analysis of a polymorphism of the monoamine oxidase B gene with Parkinson’s disease in a Japanese population. Am J Med Genet. 1995;60:570–2. doi: 10.1002/ajmg.1320600618. [DOI] [PubMed] [Google Scholar]
- 10.Costa P, Checkoway H, Levy D, Smith-Weller T, Franklin GM, Swanson PD, et al. Association of a polymorphism in intron 13 of the monoamine oxidase B gene with Parkinson disease. Am J Med Genet. 1997;74:154–6. doi: 10.1002/(sici)1096-8628(19970418)74:2<154::aid-ajmg7>3.3.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Hwang WJ, Lai ML, Tsai TT, Lai MD. Genetic polymorphism of monoamine oxidase B and susceptibility of Parkinson’s disease. Zhonghua Yi Xue Za Zhi (Taipei) 1997;60:137–41. [PubMed] [Google Scholar]
- 12.Checkoway H, Franklin GM, Costa-Mallen P, Smith-Weller T, Dilley J, Swanson PD, et al. A genetic polymorphism of MAO-B modifies the association of cigarette smoking and Parkinson’s disease. Neurology. 1998;50:1458–61. doi: 10.1212/wnl.50.5.1458. [DOI] [PubMed] [Google Scholar]
- 13.Mellick GD, Buchanan DD, McCann SJ, James KM, Johnson AG, Davis DR, et al. Variations in the monoamine oxidase B (MAOB) gene are associated with Parkinson’s disease. Mov Disord. 1999;14:219–24. doi: 10.1002/1531-8257(199903)14:2<219::aid-mds1003>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Shao M, Liu Z, Tao E, Chen B. Polymorphism of MAO-B gene and NAD(P)H: quinone oxidoreductase gene in Parkinson’s disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2001;18:122–4. [PubMed] [Google Scholar]
- 15.Wu RM, Cheng CW, Chen KH, Lu SL, Shan DE, Ho YF, et al. The COMT L allele modifies the association between MAOB polymorphism and PD in Taiwanese. Neurology. 2001;56:375–82. doi: 10.1212/wnl.56.3.375. [DOI] [PubMed] [Google Scholar]
- 16.Hernán MA, Checkoway H, O’Brien R, Costa-Mallen P, De Vivo I, Colditz GA, et al. MAOB intron 13 and COMT codon 158 polymorphisms, cigarette smoking, and the risk of PD. Neurology. 2002;58:1381–7. doi: 10.1212/wnl.58.9.1381. [DOI] [PubMed] [Google Scholar]
- 17.Kelada SN, Costa-Mallen P, Costa LG, Smith-Weller T, Franklin GM, Swanson PD, et al. Gender difference in the interaction of smoking and monoamine oxidase B intron 13 genotype in Parkinson’s disease. Neurotoxicology. 2002;23:515–9. doi: 10.1016/s0161-813x(02)00061-x. [DOI] [PubMed] [Google Scholar]
- 18.Tan EK, Chai A, Lum SY, Shen H, Tan C, Teoh ML, et al. Monoamine oxidase B polymorphism, cigarette smoking and risk of Parkinson’s disease: A study in an Asian population. Am J Med Genet B Neuropsychiatr Genet. 2003;120 B:58–62. doi: 10.1002/ajmg.b.20035. [DOI] [PubMed] [Google Scholar]
- 19.Jiang XH, Xu QY, Yang H, Chen B. Relationship between polymorphism of monoamine oxidase type B gene and Parkinson’s disease in chinese. Chin J Neurol. 2004;37:239–42. [PubMed] [Google Scholar]
- 20.Bialecka M, Drozdzik M, Honczarenko K, Gawronska-Szklarz B, Stankiewicz J, Dabrowska E, et al. Catechol-O-methyltransferase and monoamine oxidase B genes and susceptibility to sporadic Parkinson’s disease in a Polish population. Eur Neurol. 2005;53:68–73. doi: 10.1159/000084302. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Khan AJ, Shah PP, Shukla R, Khanna VK, Parmar D. Polymorphism in environment responsive genes and association with Parkinson disease. Mol Cell Biochem. 2008;312:131–8. doi: 10.1007/s11010-008-9728-2. [DOI] [PubMed] [Google Scholar]
- 22.Gu Z, Feng X, Dong X, Chan P. Smoking, genes encoding dopamine pathway and risk for Parkinson’s disease. Neurosci Lett. 2010;482:31–4. doi: 10.1016/j.neulet.2010.06.085. [DOI] [PubMed] [Google Scholar]
- 23.Wang XF, Zhang BS. Study of association between polymorphisms of monoamine oxidase B and early-onset Parkinson’ s disease. Chin J Neurol. 2010;43:388–93. [Google Scholar]
- 24.Kiyohara C, Miyake Y, Koyanagi M, Fujimoto T, Shirasawa S, Tanaka K, et al. Genetic polymorphisms involved in dopaminergic neurotransmission and risk for Parkinson’s disease in a Japanese population. BMC Neurol. 2011;11:89. doi: 10.1186/1471-2377-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W. Relationship between polymorphism of monoamine oxidase B gene intron 13 G/A and Parkinson disease. J Clin Neurol. 2011;24:261–3. [Google Scholar]
- 26.Torkaman-Boutorabi A, Ali Shahidi G, Choopani S, Reza Zarrindast M. Association of monoamine oxidase B and catechol-O-methyltransferase polymorphisms with sporadic Parkinson’s disease in an Iranian population. Folia Neuropathol. 2012;50:382–9. doi: 10.5114/fn.2012.32368. [DOI] [PubMed] [Google Scholar]
- 27.Zeng WJ, Xiao XY, Li YY, Liu Y, Li Y. Relationship between polymorphism of monoamine oxidaseB gene and Parkinson disease in Xinjiang. Clin Focus. 2012;27:764–6. [Google Scholar]
- 28.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakubauskiene E, Janaviciute V, Peciuliene I, Söderkvist P, Kanopka A. G/A polymorphism in intronic sequence affects the processing of MAO-B gene in patients with Parkinson disease. FEBS Lett. 2012;586:3698–704. doi: 10.1016/j.febslet.2012.08.028. [DOI] [PubMed] [Google Scholar]