Abstract
Pancreatic neuroendocrine neoplasms are relatively rare entities, representing approximately 1% to 2% of all pancreatic tumors. Owing to their rarity as well as their relatively indolent natural history, treatment approaches are not yet standardized. A formal pancreatic resection is usually mandatory for large and localized sporadic pancreatic tumors or in the presence of symptoms. However, in small and asymptomatic lesions, a conservative approach consisting in a careful wait-and-see policy is going to appear as more appropriate, particularly when, to remove the lesion, an aggressive surgical procedure is required, such as pancreaticoduodenectomy or distal splenopancreatectomy, depending on the localization of the tumor. Surgery has also a significant role in locally advanced and metastatic forms. In the setting of MEN 1 syndrome or Von-Hippel Lindau disease, the tumor size and the possible symptoms should be considered in the evaluation of a proper treatment.
Pancreatic neuroendocrine neoplasms (pNENs) are rare and clinically demanding diseases. pNENs are clinically defined as functioning (F-pNENs) or nonfunctioning (NF-pNENs) depending on the presence of a syndrome related to inappropriate hormone secretion and thus they are classified according to their hormonal secretion.1 A staging system based on tumor-node-metastasis (TNM) parameters and grading system based on the assessment of the proliferative activity of neoplastic cells have been proposed by the European Neuroendocrine Tumor Society (ENETS).2 The World Health Organization (WHO) classification of pNENs serves the primary purpose of assigning a diagnostic category with a clinical significance.3 The heterogeneous presentation and their different biological behavior pose a complicated set of questions about surgical management. The aim of the present review is to analyze the state of the art in the surgical management of pNEN.
Preoperative assessment
Preoperative imaging
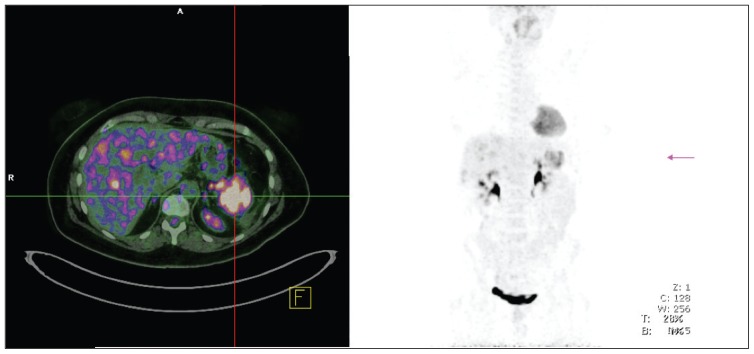
The imaging of the primary tumor location and extent of the disease is necessary for all phases in the management of patients with pNENs. Several imaging modalities have been widely used, including conventional imaging studies (computed tomography, magnetic resonance imaging, ultrasound, angiography), somatostatin receptor scintigraphy, endoscopic ultrasound, functional localization studies measuring hormonal gradients, and, recently, the use of positron emission tomography4 (Figure 1). During surgery, the routine use of intraoperative ultrasound (IOUS) is recommended, especially for insulinomas or whenever an enucleation is planned, and for small duodenal tumors (especially duodenal gastrinomas) endoscopic transillumination in addition to routine duodenotomy is recommended.5
Figure 1.
Gallium-68 somatostatin receptor positron emission tomography/computed tomography demonstrating a large neuroendocrine tumor in the pancreatic tail.
Tumor staging
The current WHO classification of NEN divided digestive tumors according to the grade of differentiation3 (Table 1). In 2006, a working group of the ENETS published a proposal for a TNM staging classification of pNENs.6 In this classification, pT1 tumors are <2 cm, pT2 are between 2 cm and 4 cm, and pT3 are >4 cm. In 2009, a TNM staging classification for pNET was suggested in the seventh edition of the AJCC/UICC TNM classification.2 The AJCC/UICC and ENETS classifications differ in the definitions of the T stages although a cutoff of 2 cm to distinguish pT1 from pT2 is used in both. On the contrary, the AJCC/UICC system requires recognition of peripancreatic soft tissue invasion to distinguish pT2 from pT3, whereas the ENETS classification based this distinction on tumor size using a cutoff of 4 cm. Both the ENETS and the AJCC classifications revealed to be accurate tools of prognostication for pNEN.7,8
Table 1.
Classification (WHO 2010) for neuroendocrine neoplasms of the digestive system.
| WHO 2010 |
|---|
|
|
G: Grade, NEC: neuroendocrine carcinoma, NET: neuroendocrine tumor.
Definition in parentheses as for the International Classification of Diseases for Oncology (ICD-O) coding.
“NET G3” has been used for this category but is not advised, since NETs are by definition well differentiated.
Surgical treatment of sporadic pNEN
Localized pNEN
The improvement and the widespread use of cross-sectional imaging technique significantly increased the detection of small NF-pNET.1 A recent study by Bettini et al9 demonstrated that the incidence of malignant forms was 6% among patients with incidentally discovered pNET <2 cm.9 The ENETS guidelines now recommend a wait-and-see policy for these small, incidentally discovered tumors, particularly when, to remove the lesion, an aggressive surgical procedure is required, such as pancreaticoduodenectomy or distal splenopancreatectomy, depending on the localization of the tumor.10 Although a follow-up protocol has not been investigated, a yearly imaging-based observation with a first control after 6 months from diagnosis seems to be reasonably safe. However, surgery still represents the treatment of choice for pNETs >2 cm and/or for symptomatic forms.
Radical surgery for pNET includes both formal and limited pancreatic resections. Formal resections differ according to the tumor site: lesions of the pancreatic head are treated with a pancreaticoduodenectomy (PD), whereas lesions of the body and tail are treated with a distal pancreatectomy (DP). Currently, when performed in high-volume centers, pancreatic formal resections have an acceptable mortality rate (less than 5%) although the percentage of complications is still significant ranging from 40% to 50%.11,12 Formal pancreatic resections are also associated with a high incidence of exocrine and endocrine insufficiency. The incidence of endocrine insufficiency ranges from 10% to 24% after a PD and from 8% to 60% after an LP, whereas the exocrine insufficiency ranges from 30% to 60% after a PD and from 0% to 40 % after an LP.13 The risk of a long-term pancreatic impairment has increased the use of parenchyma-sparing techniques or limited resections such as enucleation and middle pancreatectomy that consists of the resection of the central part of the gland. Currently, these procedures are limited only for small tumors (less than 2 cm) with a functional syndrome14 because nonfunctioning forms might be managed conservatively. The main advantage for limited resections is the association with a lower risk of a long-term endocrine/exocrine impairment when compared to standard resections.15,16 However, limited resections are associated with a high rate of pancreatic fistulas although they are mostly transient and with a low clinical impact.15
The role of lymphadenectomy for patients with pNEN is still unclear.17 Lymph node metastases occur only in the 30% of patients affected by pNET,17 but the association between node metastases and poorer survival is still debated.18 As a consequence, firm conclusions about the advantage of lymphadenectomy for pNEN are still difficult to be made. Cholecystectomy should not be performed anymore whenever a surgical procedure is required. Indeed, despite the association between long-term treatment with somatostatin analogs and the development of biliary symptoms and gallstones, episodes of cholecystitis are rare. Moreover, liver embolization when required is very selective, thus making cholecystitis by reflux of microspheres almost impossible.
Locally advanced pNEN
A large proportion of patients with pNEN have a locally advanced disease at diagnosis. The surgical removal of the tumor mass could mean a curative resection with improved survival for patients.19 Criteria for a surgical resection of pNEN do not exclude the presence of the nearby organ invasion (stomach, spleen, colon, kidney, adrenal gland) or the invasion of vascular structures. The treatment of choice is always a formal resection combined with lymphadenectomy and is associated, if necessary, to nearby organs resection. The presence of NF-pNENs should be assessed preoperatively excluding the surgical resection in the case of (1) circumferential invasion of the portal vein and 2) circumferential invasion of the superior mesenteric artery. The presence of celiac trunk invasion is not an absolute limitation for DP.10
Metastatic pNEN
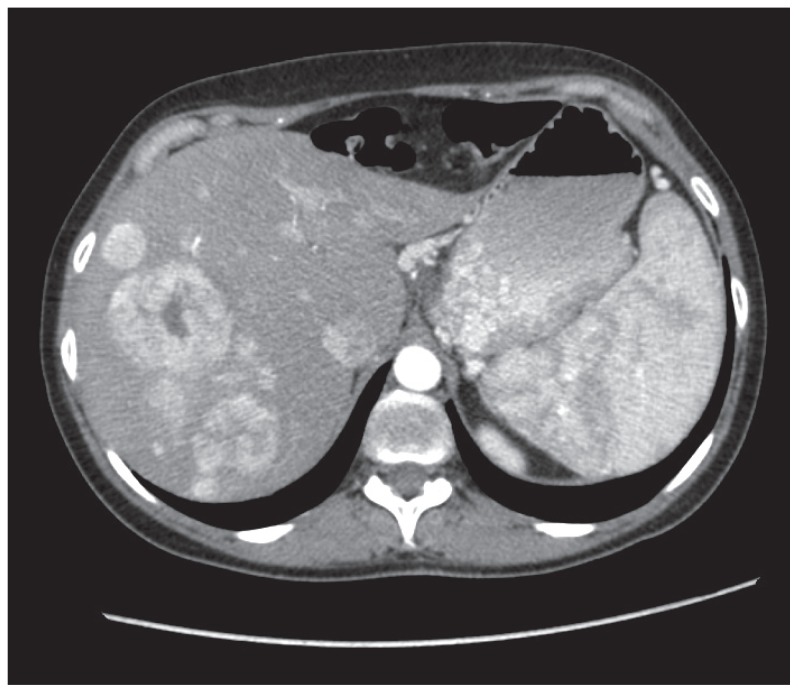
At diagnosis, 25% to 93% of patients with pNEN had synchronous neuroendocrine tumor liver metastases20 (Figure 2). A radical resection of the primary tumor, associated with complete, eventually multistep, resection of the liver metastases, is the treatment of choice.21,22 Surgery is effective at relieving symptoms and can be the only potential curative treatment when a complete resection (R0/R1 resection) of primary tumor and liver lesions is achieved. Nevertheless, a radical liver resection is only possible in less than 20% of patients due to the high incidence of multifocal and bilateral metastases. 23 The criteria that should be met before proposing surgery for metastatic PNET are as follows: (1) the absence of extra-abdominal disease, (2) the presence of low proliferative index (Ki-67) by fine-needle aspiration that must be preoperatively assessed, and (3) the existence of somatostatin receptors to deliver radiolabeled therapies because they resulted effective after cytoreductive surgery.24 A diagnosis of pNEC should exclude the patient from an upfront surgery. Moreover, in these patients with bilobar metastases or more than 75% of liver involvement, radical surgery can be rarely performed. In this light, medical, ablative and embolizing techniques can be provided to allow a radical resection.25 An alternative option for multifocal liver metastases consists of any integrated approach of partial hepatectomy combined with radiofrequency ablation. 26 The type of liver resection depends on the site and number of liver metastases, s and hepatic reserve itself. When surgery is indicated, it can range from simple enucleation to segmental resection or to hepatectomy. The survival rates of 3 and 5 years in patients who undergo radical liver surgery for neuroendocrine tumor metastases are 95% and 79%, respectively.27
Figure 2.
Computed tomography demonstrating multiple hypervascularized neuroendocrine liver metastases and a splenomegaly due to portal hypertension.
Liver transplantation has been proposed as a potentially curative treatment in patients free of extrahepatic metastases, for which standard surgical and medical therapies have failed.
As regard of primary tumor resection, cytoreductive surgery is helpful to control hormonal secretion–related symptoms in functioning metastatic endocrine carcinoma. In metastatic nonfunctioning pNEN, an advantage in terms of survival after primary tumor resection was not clearly demonstrated.28 In these nonfunctioning tumors, a pancreatic resection could be only justified to prevent life-threatening and obstructive complications, including bleeding or acute pancreatitis, jaundice, or gastric obstruction.28 However, a recent review29 demonstrated a trend toward a better survival for patients with metastatic pNEN who underwent a primary tumor resection. Nevertheless these results could have a major bias related to the likelihood that patients with earlier forms underwent a resection.
Surgical treatment of pNEN in inherited syndromes
Multiple endocrine neoplasia type I (MEN-I) syndrome
The surgical management of MEN-1–associated pNETs remains controversial because in this case they are almost always multifocal and because they are usually distributed through the pancreatic parenchyma.10 Up to 80% of patients affected by MEN-1 develop synchronous or metachronous islet cell pancreatic or duodenal tumors: gastrinomas (54%), insulinomas (18%), and nonfunctioning tumors (80%–100%).30 Currently, although surgery for pNET causing tumor mass–related symptoms or for functioning tumor-like insulinomas is mandatory; the role of surgical treatment in small (<2 cm) NF-pNET or gastrinomas is still unclear although most of the studies recommend a conservative management. 31 The indications for surgery are now limited only for tumors with metastases and/or tumors larger than 2 cm and/or with a yearly increased size >0.5 cm.32 If the lesion meets the aforementioned requirements, the surgery is the indicated treatment. In this case an IOUS is mandatory to assess the correct surgical procedure due to the high rate of multicentric lesions. The surgical procedure often results in a subtotal DP with enucleation of these tumors located in the head of pancreas or in the duodenal submucosa. This procedure, associated with an appropriate lymphadenectomy and duodenotomy for detecting small gastrinomas, is commonly called “Thompson procedure.”33 Although multifocal lesions are often demonstrated, a total pancreatectomy is not generally recommended, and it should be taken into consideration only in the cases in which the familial history evidences high mortality rates for the disease.30 When neoplasms involve the head and the tail of the pancreas leaving the body free of disease, a parenchyma preserving pancreatectomy is safe and feasible.34
Von Hippel-Lindau (VHL) disease
pNETs develop in 10% to 17% of patients affected by VHL disease and more than 98% are nonfunctioning forms.31 pNETs are generally an uncommon cause of death in VHL because most of these patients die due to metastatic renal cancer or complications of the cerebellar hemangiomas. As for MEN-I syndrome, surgery is mandatory for patients presenting mass-related symptoms, even if they occur in the minority of the cases (less than 5%).31 Regarding asymptomatic pNETs, the tumor size seems to be related to the presence of liver metastases, and it constitutes an indication for surgery. A study on 108 patients suffering from nonfunctioning pNET associated to VHL showed that lesions <3 cm, without mutation in exon 3 of the VHL gene or with a slow primary tumor doubling time (more than 500 days), were associated with a lower malignancy rate and were the possible candidates to a conservative observational approach. 35
Neurofibromatosis type I (NF-1)
In the 10% of cases, pNETs can be associated to NF-1. They are quite exclusively duodenal somatostatinomas that occur in periampullary region. Tumor size is not a good predictor in pNET associated to NF-1, and 30% of somatostatinomas are malignant. Due to these characteristics, the most commonly proposed procedures are standard resections also if satisfactory results with a local resection have been reported.36
Open or laparoscopic approach?
Laparoscopic procedures play an important role in the treatment of pNENs. It has been demonstrated that laparoscopic DP and enucleation are safe and feasible in patients with pancreatic endocrine tumors.37 Insulinomas are increasingly being treated by laparoscopic approach. In the 85% of patients, they are single tumors, almost always intrapancreatic, and if they can be localized preoperatively, they can be cured in the 70% to 100% of cases using a laparoscopic approach.5
As for insulinomas the laparoscopic approach is adequate, the role of laparoscopic surgery for gastrinomas appears to be limited.
The laparoscopic procedure needs to be integrated with the use of intraoperative ultrasonography to correctly define the area of pancreatic transection during DP. Moreover, laparoscopic ultrasonography provides information similar to that obtained by open IOUS; however, the success rate increases when the tumor mass is preoperatively detected.38 The advantages of minimally invasive surgery are a lower postoperative pain, better cosmesis outcomes, shorter length of hospital stay, and faster return to normal activity with a rate of pancreatic fistula comparable to that observed after open surgery.37 The value of laparoscopy for pancreatic malignancy lies mainly in its diagnostic and staging capabilities.39
In conclusion, surgical treatment of pNENs still remains a crucial point in the multimodal management of these tumors. Once considered mandatory for all localized forms of pNET, surgery tends to be more and more avoided in the case of asymptomatic pNET smaller than 2 cm in size, when a major formal resection is required. Nonetheless, a radical resection of aggressive forms is the only hope of cure despite recent advances in medical treatment of these tumors. Surgical procedures should always be adapted for each single patient both for the extension of resection and for the sequence in the multimodal treatment.
REFERENCES
- 1.Vagefi PA, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms. Arch Surg. 2007;142:347–354. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarpa A, et al. Pancreatic endocrine tumors: improved TNM staging and hystopathological grading permit a clinically efficient prognostic stratification of patients. Modern Pathology. 2010;23:824–833. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 3.Bosman. WHO Classification of Tumor of the Digestive System. Lyon: IARC Press; 2010. [Google Scholar]
- 4.Buchmann L, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 5.Kulke MH, et al. NANETS treatment guidelines. Well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rindi G, et al. TNM staging of foregut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449(4):395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rindi G, et al. TNM Staging of Neoplasms of the Endocrine Pancreas: Results From a Large International Cohort Study. J Natl Cancer Inst. 2012;104:1–14. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 8.Ellison TA. A Single Institution’s 26-Year Experience With Nonfunctional Pancreatic Neuroendocrine Tumors: A Validation of Current Staging Systems and a New Prognostic Nomogram. Ann Surg. 2013 doi: 10.1097/SLA.0b013e31828f3174. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettini R, et al. Tumor size correlates with malignancy in non-functioning pancreatic endocrine tumor. Surgery. 2011;150:75–82. doi: 10.1016/j.surg.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Falconi M, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–134. doi: 10.1159/000335587. [DOI] [PubMed] [Google Scholar]
- 11.Buchler MW, et al. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–4. doi: 10.1001/archsurg.138.12.1310. [DOI] [PubMed] [Google Scholar]
- 12.Yeo CJ, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226(3):248–57. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JK, et al. Complications after pancreatectomy for neuroendocrine tumors: a national study. J Surg Res. 2010;163:63–68. doi: 10.1016/j.jss.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Falconi M, et al. Parenchyma-preserving resections for small non functioning pancreatic endocrine tumors. Ann Surg Oncol. 2010;17(6):1621–7. doi: 10.1245/s10434-010-0949-8. [DOI] [PubMed] [Google Scholar]
- 15.Falconi M, et al. Pancreatic insufficiency after different resections for benign tumors. Br J Surg. 2008;95(1):85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 16.Aranha GV, Shoup M. Nonstandard pancreatic resections for unusual lesions. Am J Surg. 2005;189(2):223–8. doi: 10.1016/j.amjsurg.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Partelli S, et al. pattern and clinical predictor of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs) JAMA Surg. :2013. doi: 10.1001/jamasurg.2013.3376. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, et al. Prognostic Score Predicting Survival After Resection of Pancreatic Neuroendocrine Tumors. Analysis of 3851 Patients. Ann Surg. 2008;247:490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 19.Fischer L, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumors of the pancreas. Br J Surg. 2008;95(5):627–35. doi: 10.1002/bjs.6051. [DOI] [PubMed] [Google Scholar]
- 20.Saxena A, et al. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg. 2010;251(5):910–916. doi: 10.1097/SLA.0b013e3181d3d24a. [DOI] [PubMed] [Google Scholar]
- 21.Frilling A, et al. Treatment of liver metastases from neuroendocrine tumors in relation to the extent of hepatic disease. Br J Surg. 2009;96(2):175–184. doi: 10.1002/bjs.6468. [DOI] [PubMed] [Google Scholar]
- 22.Fendrich V, et al. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg. 2006;244(6):845–51. doi: 10.1097/01.sla.0000246951.21252.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmuller T, et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87(1):47–62. doi: 10.1159/000111037. [DOI] [PubMed] [Google Scholar]
- 24.Bloomston M, et al. Cytoreduction results in high perioperative mortality and decreased survival in patients undergoing pancreatectomy for neuroendocrine tumors of the pancreas. J Gastrointest Surg. 2006;10(10):1361–70. doi: 10.1016/j.gassur.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Touzios JG, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241(5):776–83. doi: 10.1097/01.sla.0000161981.58631.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias D, et al. Combined liver surgery and RFA for patients with gastroenteropancreatic endocrine tumors presenting with more than 15 metastases to the liver. Eur J Surg Oncol. 2009;35(10):1092–1097. doi: 10.1016/j.ejso.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Scigliano S, et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocr Relat Cancer. 2009;16(3):977–990. doi: 10.1677/ERC-08-0247. [DOI] [PubMed] [Google Scholar]
- 28.Bettini R, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumors. Ann Oncol. 2008;19(5):903–8. doi: 10.1093/annonc/mdm552. [DOI] [PubMed] [Google Scholar]
- 29.Capurso G, et al. Role of resection of the primary pancreatic neuroendocrine tumor only in patients with unresectable metastatic liver disease: a systematic review. Neuroendocrinology. 2011;93(4):223–9. doi: 10.1159/000324770. [DOI] [PubMed] [Google Scholar]
- 30.Triponez F, Cadiot G. Non-functioning tumors of the pancreas in MEN1 patients. J Gastrointestin Liver Dis. 2007;16(3):295–6. [PubMed] [Google Scholar]
- 31.Jensen RT, et al. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113(7 Suppl):1807–43. doi: 10.1002/cncr.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Triponez F, et al. Is surgery beneficial for MEN1 patients with small (< or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg. 2006;30(5):654–62. doi: 10.1007/s00268-005-0354-9. [DOI] [PubMed] [Google Scholar]
- 33.Fendrich V, et al. An Aggressive Surgical Approach Leads to Long-term Survival in Patients With Pancreatic Endocrine Tumors. Ann Surg. 2006;244:845–853. doi: 10.1097/01.sla.0000246951.21252.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Partelli S, et al. Middle-preserving pancreatectomy for multicentric body-sparing lesions of the pancreas. Am J Surg. 2009;198(3):e49–53. doi: 10.1016/j.amjsurg.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Blansfield JA, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pamcreatic neuroendocrine tumors (PNETs) Surgery. 2007;142(6):814–818. doi: 10.1016/j.surg.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartel M, et al. Carcinoid of the ampulla of Vater. J Gastroenterol Hepatol. 2005;20(5):676–81. doi: 10.1111/j.1440-1746.2005.03744.x. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Cruz L, et al. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumors? World J Surg. 2008;32(5):904–17. doi: 10.1007/s00268-008-9467-2. [DOI] [PubMed] [Google Scholar]
- 38.Iihara M, Obara T.Minimally invasive endocrine surgery: laparoscopic resection of insulinomas Biomed Pharmacother 2002. 56Suppl 1227s–230s. [DOI] [PubMed] [Google Scholar]
- 39.Angst E, et al. Laparoscopic surgery for cancer: a systematic review and a way forward. J Am Coll Surg. 2010;211(3):412–423. doi: 10.1016/j.jamcollsurg.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]