Abstract
Intrauterine exposure to phthalates is known to cause disorders of male reproductive function including androgen insufficiency, decreased fertility, and germ cell defects in rodents. In this study, we set out to investigate the effects of intrauterine exposure to di-(2-ethylhexyl)-phthalate (DEHP) on fetal development of the B6:129S4 mouse strain. Time-mated pregnant C57BL/6 dams were exposed to 0, 5, 250, or 500 mg/kg DEHP with corn oil as the vehicle via oral gavage from embryonic days (E)7 to 16. Survival and gross morphology of the pups were analyzed one day after the last treatment. Anogenital distance (AGD) and testicular cell functions were examined in male embryos to confirm the known effects of phthalate exposure. DEHP exposure significantly reduced the survival rate of fetuses in the 250 and 500 mg/kg dosage groups compared with the control and 5 mg/kg groups. Exposure to 250 and 500 mg/kg DEHP was teratogenic and induced exencephaly and limb malformations such as polydactyly in the B6:126S4 embryos. No gross malformations were observed in control or 5 mg/kg DEHP groups. In male embryos, exposure to both 5 and 250 mg/kg DEHP in utero was sufficient to induce the formation of multinucleated germ cells in the testes and widespread changes in mRNA expression of germ cell, interstitium and Sertoli cell-associated genes. These findings reveal that intrauterine DEHP exposure has a strong teratogenic, and lethal impact on the fetuses of B6:129S4 mouse strain.
Keywords: phthalate, endocrine disruptor, polydactyly, testis, germ cells.
Phthalate esters are used in the production of plastics, especially polyvinyl chloride (PVC), to ensure malleability of the material. Phthalates and their derivatives are found in a variety of consumer products such as clothing, food packaging and toys, as well as in medical devices including tubing and blood transfusion packaging (Kavlock et al., 2006). The exposure level of phthalates in a preterm newborn in intensive care can reach up to 6000 µg/kg of body weight; 2000 times the normal exposure of adults (1–30 µg/kg body weight) (Kavlock et al., 2006). One of the most commonly used phthalate esters is di(2-ethylhexyl) phthalate (DEHP), which accounts for one-third of the phthalates produced in the European Union and 80% of phthalate production in China (Gao and Wen, 2016).
Exposure to high doses (410–2200 mg/kg) of DEHP in utero is teratogenic and a cause of embryonic death in different mouse strains (Schmidt et al., 2012; Shiota and Mima, 1985; Shiota and Nishimura, 1982). In addition, phthalates are known as an endocrine disruptor in the rat model, where prenatal exposure to different phthalates during the male programming window induces hypospadias and cryptorchidism, and decreases germ cell numbers and testosterone production (Akingbemi et al., 2001; Gray et al., 2000; Welsh et al., 2008; Zhu et al., 2009). In developing mouse fetuses, exposure to phthalates and their active metabolites alters the expression of Sertoli and germ cell-associated genes and induces multinucleation of germ cells (Gaido et al., 2007; Saffarini et al., 2012; van den Driesche et al., 2015; Wang et al., 2016). Contrary to the rat model, the fetal testosterone production and reproductive tract development remain largely normal in the mouse model (Gaido et al., 2007; van den Driesche et al., 2015), indicating a species-specific effect of phthalate exposure.
In this study, the effect of a range of DEHP doses (5 mg/kg, corresponding to the exposure level of preterm newborns in intensive care), moderate dose (250 mg/kg), and a high dose [500 mg/kg, commonly used in toxicological studies on fetal mouse development (Do et al., 2012; Schmidt et al., 2012; Shiota and Mima, 1985; Shiota and Nishimura, 1982)] was analyzed in mouse embryos. The animals were exposed during the postimplantation period (embryonic day or E7–16) to target the main events of organogenesis, but avoid the possible confounding effect of DEHP on implantation efficiency (Li et al., 2012). The pregnancy outcome and the gross morphology of the fetuses were analyzed right before birth at E18. The effect of DEHP on the fetal testicular development was analyzed at morphological and transcriptional levels.
MATERIALS AND METHODS
Animals and Treatments
Female C57BL/6 mice at the age of 8–10 weeks (Jackson Laboratories, Bar Harbor, ME) were time-mated with B6; 129S4-Pou5f1tm2Jae/J) males (Jackson Laboratories, stock no. 008214). The B6; 129S4-Pou5f1tm2Jae/J reporter strain was utilized to label germ cells for use in parallel experiments. The C57BL/6 strain was chosen to aid with genomic analyses for the reason that the mouse reference genome is established from the C57BL/6 strain. The fetuses resulting from the mating also have a component of 129S4 in their genetic background. Noon of the day that the vaginal plug was detected was considered as embryonic day 0.5 or E0.5 and the pregnant females were housed individually in disposable Polyethylene terephthalate cages (Allentown Inc., Allentown, NJ) using Sani Chips bedding (P.J. Murphy Forest Products Corp., Montville, NJ) and Enviro-Dri nesting material (Shephard Specialty Papers, Watertown, TN). Pregnant females were provided at libitum with NIH-31 chow and water processed through a reverse osmosis deionized system. Animals were maintained in a temperature and humidity-controlled environment with a 12:12-h light/dark cycle. At E7, pregnant females were randomly assigned to one of the following groups (8–18 pregnant females per group): (1) control, corn oil (Sigma Aldrich, St. Louis, MO); (2) 5 mg/kg/day DEHP, Cat. no. D201154; Sigma Aldrich, St. Louis, MO); (3) 250 mg/kg/day DEHP; or (4) 500 mg/kg/day DEHP diluted in corn oil. Dams were weighed and dosed daily by oral gavage. The dams were monitored for clinical signs of distress throughout the study period. Dosing stock solutions were prepared every 3–4 days and were stored in HDPE plastic at room temperature. The treatment window was from E7 to E16, spanning organogenesis, gonadal formation, and sex determination of the gonads. Euthanasia was performed by CO2 inhalation followed by cervical dislocation. To normalize maternal weight gain throughout pregnancy to the number of fetuses, the following equation was used: dam weight (E18.5) – weight (E13.5)/number of fetuses delivered by cesarean section +1. The dosing with 500 mg/kg DEHP was capped at eight dams, because of the severe embryonic lethality. All animal procedures were approved by the National Institutes of Health Animals Care and Use Committee and were performed in accordance with an approved National Institute of Environmental Health Sciences animal study proposal. All animals were treated humanely with regard to alleviation of suffering.
Morphological Measurements and Sample Collection
Pregnant females were euthanized by CO2 inhalation and decapitation at either E14.5 or E18.5. Gross fetal morphology and anogenital distance (AGD: the length from the caudal base of the genital tubercle to the anterior aspect of the anus) were analyzed on E18.5 to allow a direct comparison with previous studies on DEHP that used the same time-point (Do et al., 2012; Shiota and Mima, 1985; Shiota and Nishimura, 1982). E14.5 was chosen for the gene expression analysis to capture germ cell status after the major developmental hallmarks (sex determination and epigenetic reprogramming) have been achieved but prior to the potential confounding effects of altered gonadal cellularity that were expected to occur with longer exposure. The fetuses were quickly delivered by cesarean section, weighed and euthanized by decapitation. At E18.5, the AGD of all fetuses was measured using digital microcalipers (Cat. no. 50013, Chicago Brand). The results are expressed as anogenital index (AGI), where the AGD is divided by the weight of the fetus to control for the effect of the size of the fetus to the AGD. Dead fetuses and uterine resorption sites were recorded at both the E14.5 and E18.5 time-points. All the live fetuses were examined for the presence of malformations using a dissecting microscope. For each male pup, the left testis was fixed in 4% paraformaldehyde for histological analysis and the right testis (mesonephros removed) was snap frozen for gene expression or testosterone analysis. Paws from E18.5 fetuses were fixed in 4% PFA overnight and processed for staining with alizarin red and alcian blue to visualize the skeletal structures.
Testicular Testosterone Assay
Testicular testosterone was measured in testes of E18 embryos that were collected from 0 mg/kg, 5 mg/kg and 250 mg/kg DEHP groups (n = 8/group). Each testis was obtained from an embryo of a different litter. The testes were mechanically homogenized and sonicated in PBS. The testosterone assay was carried out by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. Testosterone rat/mouse ELISA (Cat. no. IB79174, Tecan Group Ltd. Maennedorf, Switzerland) was used according to the instructions from manufacturer. The samples were diluted 10× and run in duplicate. The sensitivity of the assay is 0.066 ng/ml and intra-assay and interassay variations are 6.5–11 CV (%) and 9.3–11.3 CV (%), respectively. The testosterone concentration in the lysate was normalized to the DNA concentration. The DNA was isolated using the DNeasy Blood and Tissue Kit (Cat. no. 69504, Qiagen Gmbh, Hilden, Germany) and the concentration was measured using Qubit dsDNA HS Assay Kit (Cat. no. Q32851, Life technologies, Eugene, OR).
Histology and Immunohistochemistry
Testes were fixed in 4% PFA overnight at 4°C, then dehydrated through a sucrose gradient, embedded in O.C.T. (Tissue-Tek, Sakura Finetek USA Inc, Torrance, CA), and cryosectioned at 10 μm increments. After blocking in 5% normal donkey serum in PBS for 1 h, sections were incubated with either TRA98 (1: 1000, cat. no. RK-73-003, MBL International, Woburn, MA), AMH (1:500, cat. no. sc-6886, Santa Cruz Biotechnology, Dallas, TX), or 3βHSD (1:500, cat. no. K0607, CosmoBio USA, Carlsbad, CA) primary antibodies in PBS-(0.1%) Triton X-100 solution with 5% normal donkey serum at 4°C overnight. Tissue sections were washed and incubated in the appropriate secondary antibodies (1:500, Invitrogen/Thermo Fischer Scientific, Waltham, MA) for 1 hr at room temperature before final washing mounting in Vector Mount with 4,6-diaminidino-2-phenylindole (DAPI) (cat. no. H-1200, Vector laboratories, Burlingame, CA). Slides were imaged using a Leica DMI4000 confocal microscope. The number of TRA98 positive germ cell nuclei and the total testis area (by DAPI staining) of 2–6 representative testis tissue sections per pup were measured using Definiens Tissue Studio Software v3.0 (Definiens Inc., Cambridge, MA). Leydig cell area was quantified in the same sections by measuring the area of 3bHSD staining. Germ cell number was calculated as the total number of TRA98 positive cells per square mm of testis tissue, whereas Leydig cell area was quantified as the area of 3bHSD staining to the total DAPI positive area. Testes from five control, fourteen 5 mg/kg, and six 250 mg/kg dose animals were analyzed.
Nanostring Gene Expression Analysis
For each treatment group, four testes were obtained from four different animals from different litters for the gene expression assay. Total RNA was isolated from each testis using the Picopure RNA isolation kit (Ar/Thermo Fischer Scientific) according to the protocol of the manufacturer, with DNAse I treatment (Qiagen) performed on the columns following the first wash. Gene expression for a custom panel of testis and germ cell development genes was performed using NanoString nCounter system (NanoString Technologies, Inc., Seattle, WA, USA) and a custom codeset according to manufacturer’s instructions (Supplementary Table 1). The Nanostring assay quantifies mRNA numbers directly without an amplification step (Geiss et al., 2008); counts were normalized to positive and negative controls in the NanoString assay, and four housekeeping genes (Gapdh, Rpl17, Tubb5, Hprt) with NanoString nSolver software2.5. Partek Genomic Suite Software (Partek Inc., St. Louis, MO) was used for the statistical analysis.
Statistical Analysis
Data were analyzed using Prism (Version 6, GraphPad Software, La Jolla, CA). Values are presented as mean ± s.e.m. and a minimum of four biological replicates was used for each experiment. Repeated measures analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test was used to analyze dam weight gain over the course of the exposure period. Kruskal–Wallis ANOVA was used to compare maternal weight gain adjusted for litter size, fetal survival rates, and litter-based malformation rates among the dose groups. Where a significant overall difference was found, one-sided Mann–Whitney test was used to compare each dosed group to the control group. One-way ANOVA was used to analyze the testicular testosterone measurements. For the AGI data, mixed effects analysis of variance was used to compare AGI across dose groups, whereas accounting for potential within litter correlations, followed by Dunnett’s test to identify dosed groups different from the control group. For the Nanostring gene expression assay, Partek Genomic Suite Software was used to perform Student’s t-test by comparing each dosed group to the control group.
RESULTS
In Utero Exposure to DEHP Increases the Incidence of Fetal Death and Malformations in B6:129S4 Mouse Embryos
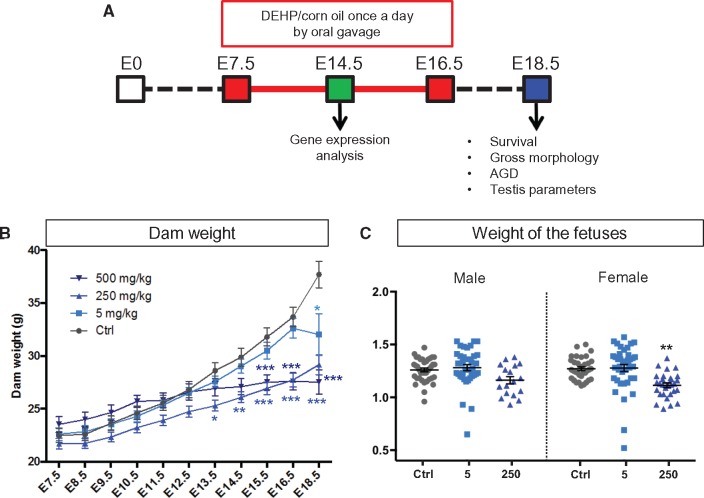
To investigate the effects of in utero exposure to DEHP on fetal development, time-mated C57BL/6J dams were exposed to 5, 250, and 500 mg/kg doses of DEHP in corn oil by oral gavage on E7 to E16 to target the window of organogenesis (Figure 1A). The 5 mg/kg dose was chosen to resemble the phthalate exposure level in preterm newborns in intensive care units (Kavlock et al., 2006). The higher 250 and 500 mg/kg doses were chosen to assess the teratogenic effects of DEHP in the B6:129S4 mice. DEHP is known to cross the mouse placenta and the active metabolite of DEHP, MEHP, can be detected in the sera and livers of the exposed fetuses (Calafat et al., 2006; Do et al., 2012; Hayashi et al., 2012). The dams were monitored for clinical signs of toxicity, such as hair loss, reduced activity or hunching, throughout the study. None of the dams (control and treatment groups) showed any clinical signs of toxicity. The body weight of the dams was measured daily during the exposure period and on the day of the collection. The control and the 5 mg/kg DEHP group demonstrated a similar weight gain until E16.5 (Figure 1B). The dams in the 250 mg/kg group showed a significantly lower bodyweight at each time-point from E13.5 onwards compared with the control group (Figure 1B). In the 500 mg/kg DEHP group, the bodyweight of the dams was significantly lower than control dams from E15.5 onwards (Figure 1B). By the time of cesarean section at E18.5, all the DEHP-treated groups showed a significantly lower bodyweight (Figure 1B). When repeated measures analysis of variance was used to analyze the trend of the maternal weight over the observation period, only 250 mg/kg DEHP group differed significantly from the control (P = 0.03). When the weight gain of the dam was adjusted with the number of the fetuses delivered by cesarean section, there were no significant differences in the weight gain between the control and treatment groups (Supplementary Figure 1), indicating that the decreased body weight of DEHP-treated pregnant dams was likely caused by a loss of the fetuses and did not represent a toxic effect of DEHP on the dam itself. Exposure to 250 mg/kg of DEHP resulted in general growth retardation; the bodyweights of female pups in the 250 mg/kg dosage group were significantly decreased at E18.5 (Figure 1C) and males followed a similar decreasing trend, albeit not significant. Exposure to 5 mg/kg DEHP did not affect the bodyweight of the fetuses (Figure 1C). Only one male and three female fetuses survived in the 500 mg/kg group and due to the insufficient sample size, they were not included in the weight analyses.
FIG. 1.
Effect of DEHP on the exposed dams. A, Time-mated C57Bl/6J dams were dosed via oral gavage once a day with 0, 5, 250, and 500 mg/kg of body weight of DEHP in corn oil from E7.5 to E16.5. Survival rate, gross morphology, anogenital distance (AGD), and testis parameters of the fetuses were examined on E18.5. Some dams were sacrificed at E14.5 for analyses of gene expression in the fetal testes. B, Weight (g) of the dams over the exposure period. One-way ANOVA followed by Dunnet’s multiple comparison test were used for statistical analyses. The weight of the treated dams was compared with the control group at each time point. *P < 0.05, ** P < 0.01, *** P < 0.001. C, Weight of the E18.5 fetuses at the time of collection. The weight of the DEHP exposed fetuses was compared with the control group for each sex. Statistical significance was analyzed using Dunnett’s test coupled with mixed effects analysis of variance to control for the litter effect. * P < 0.05, ** P < 0.01.
All the dams in the control and 5 mg/kg groups had live fetuses at collection on E18.5; however, in the 250 mg/kg and 500 mg/kg groups just 83% and 50% of the dams, respectively, produced live fetuses (Table 1). The absence of live fetuses in some litters of the 250 mg/kg and 500 mg/kg groups was a result of intrauterine death. The average number of resorbed fetuses per dam was four in the 250 mg/kg group and seven in the 500 mg/kg group, whereas less than one resorption per dam was observed in the control and 5 mg/kg groups (Table 1). A total of 56 resorptions were observed in the eight dams exposed to 500 mg/kg DEHP. In the 18 dams exposed to 250 mg/kg DEHP a total of 72 resorptions were observed. The control and the 5 mg/kg group had three and seven resorptions, respectively. The average litter size (the number of live fetuses per pregnancy) was significantly decreased in the 250 mg/kg group (3.2 fetuses) in comparison with the control group (8. fetuses). In the 500 mg/kg group, only four fetuses were recovered from eight pregnant dams at E18.5 (Table 1). The sex ratio (% of male fetuses per litter) was not significantly altered in the DEHP exposed groups compared with the control (48± 7.5% in control vs 41.2±7.9% in 5 mg/kg, 37.9± 9.7% in 250 mg/kg, and 36.7± 22.6% in 500 mg/kg DEHP group) but each group exhibited wide variation litter to litter. Taken together, in utero DEHP exposure decreased pup survival to less than 50% in the 250 mg/kg group and to 6.5% in the 500 mg/kg group at E18.5 (Table 1). The survival rate was decreased in the 250 mg/kg group already at E14.5 (Supplementary Table 2).
TABLE 1.
Effects of in utero exposure to DEHP on pregnancy and pup survival.
| DEHP dose (mg/kg/day) | No. of dams | Dams with live fetuses % | Dams with resorptions % | No. of live pups per dam (mean ± SEM) | No. of resorptions per dam (mean ± SEM) | Survival |
|---|---|---|---|---|---|---|
| 0 | 10 | 100% (10/10) | 10% (1/10) | 8.3 ± 0.8 | 0.1 ± 0.1 | 98.6% |
| 5 | 13 | 100% (13/13) | 8% (1/13) | 6.8 ± 0.7 | 0.4 ± 0.4 | 95.8% |
| 250 | 18 | 83% (15/18) | 88% (16/18) | 2.7 ± 0.6*** | 4.0 ± 0.7*** | 43.8%** |
| 500 | 8 | 50% (4/8) | 100% (8/8) | 0.5 ± 0.2**** | 7.0 ± 1.1**** | 6.5%** |
Parentheses: No. affected dams/Total no. of dams in group. Kruskal-Wallis (ANOVA) test was performed for statistical analyses, followed by one-sided Mann–Whitney test to compare the dosed groups with the control group.
P < 0.05,
P < 0.005,
P < 0.0005.
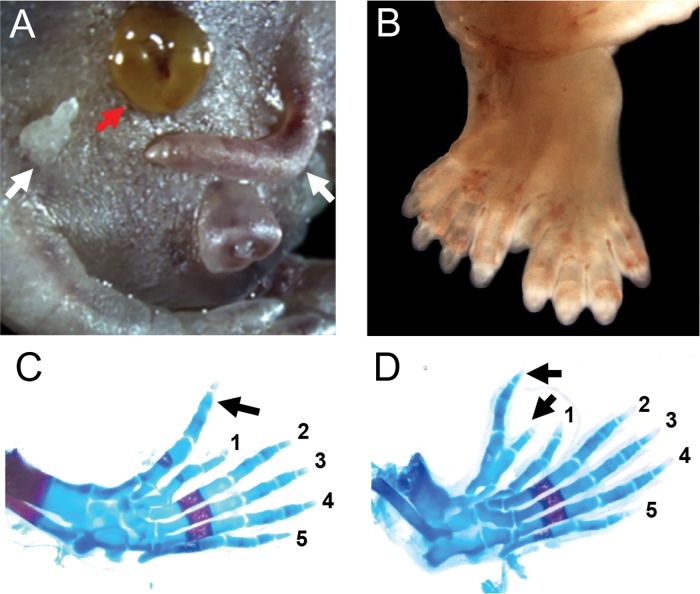
A significantly increased incidence of malformations was observed in live fetuses of the 250 mg/kg and 500 mg/kg groups (Table 2). The most common birth defect was limb malformations (Table 2). No limb defects were observed in the control and 5 mg/kg DEHP group, but 16.7% of the fetuses in the 250 mg/kg group showed some form of limb malformation (Table 2). The fetuses with limb defects were found in five of the 15 litters exposed to 250 mg/kg (Table 2). In the 500 mg/kg group, two out of the four surviving fetuses had limb malformations (Table 2). The limb defects were specific to hind limbs and represented a spectrum of polydactyly phenotypes. These include a mirror-image formation of extra digits (Figure 2B), to the formation of one or two anterior extradigits (Figure 2C and D, black arrows). We also observed femoral ectopic digit-like structures (Figure 2A, white arrow). The limb malformations affected only the autopods (carpal, metacarpal, and phalangeal bones) with no effects on the zeugopods (radius and ulna).
TABLE 2.
Malformation rate of E18.5 fetuses after in utero exposure to DEHP.
| DEHP (mg/kg/day) | Total no. of |
Limb malformation |
Exencephaly |
Other malformations |
Total malformations |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Litters | Fetuses | Fetuses % | Litters % | Fetuses % | Litters % | Fetuses % | Litters % | Fetuses % | Litters % | |
| 0 | 10 | 83 | 0 (0/83) | 0 (0/10) | 0 (0/83) | 0 (0/10) | 0 (0/83) | 0 (0/10) | 0 (0/83) | 0 (0/10) |
| 5 | 14 | 95 | 0 (0/95) | 0 (0/14) | 0 (0/95) | 0 (0/14) | 1.1 (1/95) | 7.1 (1/14) | 1.1 (1/95) | 7.1 (1/14) |
| 250 | 15 | 48 | 16.7 (8/48)* | 33.3 (5/15) | 16.7 (8/48)* | 40 (6/15) | 2/48 (4.2) | 2/15 (13.3) | 25 (12/48)** | 60 (9/15) |
| 500 | 4 | 4 | 50 (2/4)* | 50 (2/4) | 0 (0/4) | 0 (0/4) | 0 (0/4) | 0 (0/4) | 50 (2/4)* | 50 (2/4) |
Parentheses: Numbers of affected fetuses or litters/total number of fetuses or litters. Kruskal–Wallis (ANOVA) was used to compare the litter-based malformation rates between the groups.
Mean percent fetuses per litter differs from the control group at P < 0.05 by one-sided Mann–Whitney test.
P< 0.01. ‘Other malformations’ included cyclopia in the 5 mg/kg group and gastroschisis in the 250 mg/kg group.
FIG. 2.
In utero DEHP exposure induced malformations in the 250 mg/kg group. A, A pup with bilateral femoral extra digits (white arrow) and gastrochisis (red arrow). B, Complex polydactyly of a hind paw with a mirror image duplication of the digits. C, D, Alizarin red and alcian blue stained hindlimb autopod from 250mg/kg dosing group showing polydactyly with one (C) or two (D) extradigits (black arrows).
Another common malformation in the 250 mg/kg group was exencephaly, a neural tube closure defect where the skull cap fails to form (Table 2). Exencephaly affected a total of eight fetuses (16.7%) in six different litters in the 250 mg/kg group (Table 2). Open eyelids and gastroschisis (Figure 2A, red arrow) were among the other malformations observed in two out of 48 fetuses in the 250 mg/kg group.
In Utero DEHP Exposure Alters Testicular Somatic Cell Development
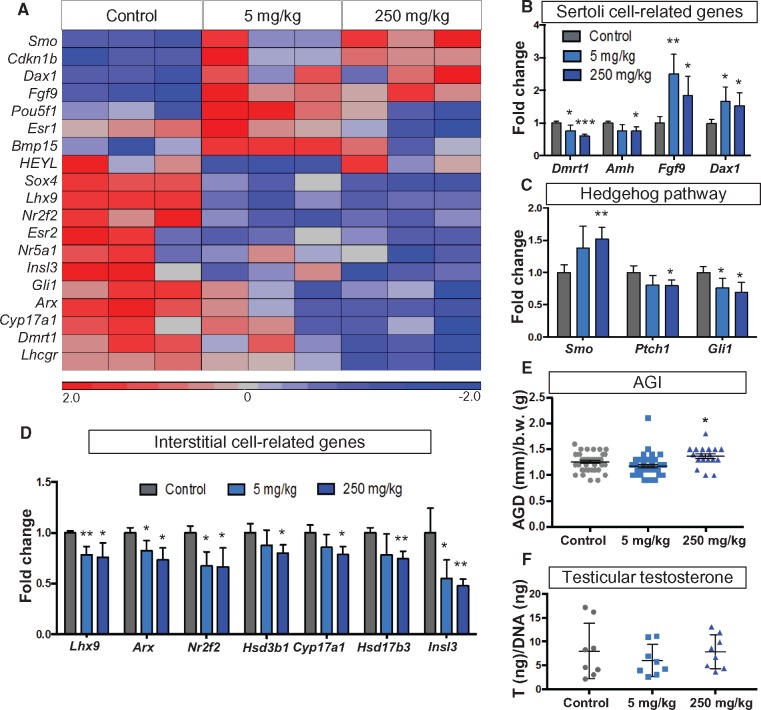
In utero exposure to phthalates is linked to altered Sertoli and Leydig cell function (Akingbemi et al., 2001; Kleymenova et al., 2005; Lin et al., 2008; Wang et al., 2016). To gain a broader view of the effect of DEHP on testicular development, we designed a Nanostring assay, which analyzed a customized list of 125 genes known to be involved in gonadal development (Supplementary Table 1). Due to the overt toxicity of DEHP at the 500 mg/kg dosage and the resulting low number of surviving fetuses, the subsequent analyses were performed only on the control, 5, and 250 mg/kg groups. We observed widespread changes in gene expression associated with the DEHP exposure. In the heatmap of the significantly altered genes in the assay, the 5 mg/kg and 250 mg/kg groups showed a similar expression pattern, different from the control group (Figure 3A). Several genes involved in Sertoli cell function were significantly altered after DEHP exposure (Figure 3B). Exposure to 5 mg/kg and 250 mg/kg DEHP resulted in an increase in the expression of Fgf9 (fibroblast growth factor 9), which is important for testis determination and male germ cell survival (Bowles et al., 2010; Colvin et al., 1996; DiNapoli et al., 2006), and Dax1 (nuclear receptor subfamily 0, group B, member 1 or Nr0b1), which is involved in testis cord formation during testicular development (Meeks et al., 2003). On the other hand, the expression of Dmrt1 (doublesex and mab-3 related transcription factor 1), which acts as a gatekeeper of Sertoli cell fate (Matson et al., 2011), and Amh (antiMüllerian hormone), which induces Müllerian duct regression, were significantly decreased in the 250 mg/kg group (Figure 3B).
FIG. 3.
Testicular gene expression patterns and anogenital distance after DEHP exposure. A, A heatmap of the differentially expressed genes in the Nanostring assay, where mRNAs from E14.5 testes were analyzed. B, Expression of Sertoli cell-related genes. C, Expression of Hedgehog signaling genes. D, Expression of interstitial cell-specific genes. Both groups were compared with the control group and statistical analysis was conducted using Student’s t-test. * P < 0.05, ** P < 0.005. E, Anogenital Index [AGI: AGD (mm)/body weight (g)] of the male fetuses was measured at the age of E18.5. Statistical significance was analyzed using Dunnett’s test coupled with mixed effects analysis of variance to control for the litter effect. * P < 0.05. F, Testicular testosterone was measured using ELISA from E18.5 testes. Eight samples per group were chosen from different litters. The testosterone content of the testis (ng) was normalized to the DNA content (ng) of the testis lysate. Statistical significance was analyzed using one-way ANOVA followed by Dunnett’s test.
Hedgehog signaling pathway is involved in the paracrine control of testicular somatic cells. Sertoli cells promote the formation of the fetal Leydig cell population by secreting Desert hedgehog (Yao et al., 2002). The hedgehog ligand receptor Ptch1 (patched1) and the downstream effector Gli1 (Kruppel family member GLI1) were decreased in the 250 mg/kg group (Figure 3C), whereas Smo (smoothened), an inhibitor of Gli-mediated transcription, was increased (Figure3C). In the 5 mg/kg group, only Gli1 was significantly altered (Figure 3C).
Exposure to both 5 and 250 mg/kg DEHP decreased the expression of several genes in steroidogenic cells (Figure 3D). The expression of Cyp17a1 and Hsb3b1, which encode for key enzymes of the steroidogenic pathway, was significantly decreased in the 250 mg/kg group (Figure 3D). Markers of the interstitial cell progenitor population: Arx, Nr2f2 (Coup-tfII), and Lhx9, were reduced in both the 5 mg/kg and 250 mg/kg groups compared with controls (Figure 3D). Expression of Insl3 (Insulin like 3), which controls testicular descent (Nef and Parada, 1999), was decreased in both DEHP-treated groups (Figure 3D). Anogenital index (AGI: anogenital distance/bodyweight), a readout of androgen action during fetal life in humans and rodents (Swan et al., 2005; Welsh et al., 2008), was measured for male fetuses at the age of E18.5 (Figure 3E). There was a modest yet significant increase in the AGI of the male 250 mg/kg group compared with the control group, but no difference was observed in the in the male 5 mg/kg group (Figure 3E). No significant changes in testicular testosterone levels at E18.5 were associated with exposure to 5 mg/kg and 250 mg/kg DEHP as compared with the control group (Figure 3F).
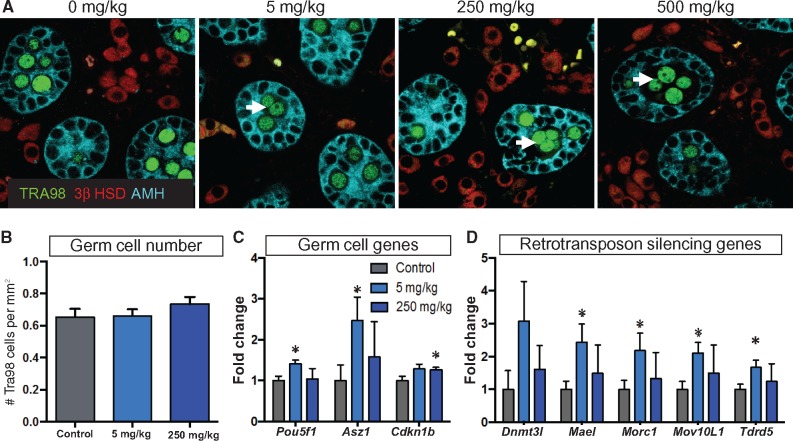
Exposure to DEHP, as Low as 5 mg/kg, Induces Multinucleation of Germ Cells and Alters the Expression of Genes Associated with Germ Cell Development
Male rodents are known to develop multinucleated gonocytes after exposure to 250–750 mg/kg of DBP or DEHP (Gaido et al., 2007; Gray et al., 2000; Saffarini et al., 2012). To investigate whether 5 mg/kg dose of DEHP-induced germ cell multinucleation, we labeled the germ cell nuclei with a TRA98 antibody at E18.5 (Figure 4A, green). Multinucleated germ cells were observed in all DEHP dosage groups (Figure 4A), but no significant differences in the overall number of germ cell nuclei were found (Figure 4B). Thus, the observed changes in expression of the different germ cell-associated genes were likely caused by altered germ cell function and not a consequence of an increased number of germ cells. We observed a widespread increase in the expression of germ cell-associated genes in the 5 mg/kg dosage group and a more restricted pattern of change in the 250 mg/kg dosage group (Figure 4C and D). Pou5f1 (or Oct4) and Asz1 (ankyrin repeat, SAM, and basic leucine zipper domain containing 1) showed an increased expression in the 5 mg/kg group (Figure4C). Cdkn1a (cyclin-dependent kinase inhibitor 1a or p21) expression was increased in the 250 mg/kg group (Figure 4C). Several genes related to retrotransposon silencing (Dnmt3l, Morc1, Mael, and Mov10L1) were significantly upregulated in the testes exposed to 5 mg/kg DEHP (Figure 4D).
FIG. 4.
Effects of DEHP exposure on germ cell development. A, Immunohistochemical detection of the different testicular cell populations in E18.5 testes. Germ cells: green, Tra98; supporting cells: turquoise, AMH; steroidogenic cells: 3βHSD, red; white arrow: multinucleated germ cells. B, Quantification of the germ cell number based on the counting of Tra98-positive germ cell nuclei/mm2. C, Expression of the germ-cell-associated genes analyzed using the Nanostring assay. D, Expression of several genes associated with retrotransposon silencing. Both groups were compared with the control group and statistical significance was analyzed using Student’s t-test. *P < 0.05, ** P < 0.005.
DISCUSSION
We studied the effects of a range of DEHP doses on fetal development in the B6:129S4 mouse strain by exposing pregnant dams from E7 to 16. In general, DEHP exposure had major effects on the survival and development of fetuses in the B6:129S4 strain. In addition to the known effects of phthalate on fetal testicular functions, we observed unexpected limb malformations and high rates of fetal death following exposure to 250 mg/kg DEHP.
The overall malformation rate of B6:129S4 fetuses exposed to 250 mg/kg DEHP was markedly higher in our study compared with previous studies performed using outbred mouse strains. Twenty five % of live B6:129S4 fetuses exposed to 250 mg/kg DEHP exhibited at least one malformation, whereas the malformation rate for ICR (CD-1) fetuses was only 4.3% after exposure to the same dose of DEHP on E7-9 (Shiota and Mima, 1985). When ICR mice were exposed to 190 mg/kg or 410 mg/kg DEHP throughout the entire pregnancy, the resulting malformation rates were 5.3% and 25.8% (Shiota and Nishimura, 1982). Limb malformations were the most prevalent defect observed in the 250 mg/kg group (16.7%) in our study. The DEHP-induced limb defects represented varying degrees of polydactyly from a single extra digit to a complete duplication of the hind paw. The only previous report linking DEHP to limb malformations was the study by Shiota and Nishimura, where 1 out of 14 fetuses had a club foot after a dietary exposure to 410 mg/kg DEHP through the entire pregnancy, but no polydactyly was reported (Shiota and Nishimura, 1982). Susceptibility to DEHP-induced limb malformations varies between different mouse strains: no polydactyly was observed in ICR mice exposed to 70, 190, 410, 830, and 2200 mg/kg DEHP throughout pregnancy (Shiota and Nishimura, 1982). When CD-1 mice were exposed to 500 mg/kg of mono(2-ethylhexyl) phthalate (MEHP), an active metabolite of DEHP, on E11-16, the survival of the fetuses was decreased, but no limb malformations were induced (Gaido et al., 2007). In both our studies and the Shiota and Nishimura studies, phthalate exposure spanned the early patterning phase of limb development (E9.5–11.5) (Yu and Ornitz, 2008), but polydactyly was observed only in the B6:129S4 strain. In some genetically modified mouse models, crossing the mice to C57BL/6 genetic background increases the penetrance of the polydactyly phenotype (Blanc et al., 2003; Dunn et al., 1997; Fawcett et al., 1995; Ikegawa et al., 2008). The genetic background of the fetuses in this study was predominantly C57BL/6, because their parents were B6 females mated with B6:129S4 males. Thus, it is likely that the genetic background, which is a combination of B6 and 129S4, increased the sensitivity of the developing limb to environmental insults. Polydactyly is the most prevalent human limb malformation and is estimated to occur in 0.3–3.6/1000 live-births (Malik, 2014). To date, there are no studies linking phthalate exposure and limb anomalies in humans. However, our study on mice implicates a potential link between limb defects and phthalate exposure and suggests a need to study whether such connection exists in humans.
The other major malformation induced by DEHP exposure in our study was exencephaly, which occurred in 16.7% of the live fetuses in the 250 mg/kg group. Exencephaly is a severe defect in the closure of the neural tube, where the skull cap fails to form and the brain is exposed to the environment (Greene and Copp, 2014). Exencephaly was also the most commonly observed malformation in ICR mice exposed to high doses (410–2000 mg/kg) of DEHP in utero (Shiota and Mima, 1985; Shiota and Nishimura, 1982). Despite the different nature of polydactyly and exencephaly, Hedgehog signaling might link both these phenotypes with the DEHP exposure. In the limb bud, ectopic Hedgehog signaling can cause limb patterning defects, such as polydactyly (Zeller et al., 2009). In the neural tube, ectopic hedgehog signaling disrupts the function of the primary cilia, which results in exencephaly (Hill, 2007; Murdoch and Copp, 2010). Whereas a link between DEHP exposure and the Hedgehog signaling pathway has yet to be established in the limb and brain, the potential effect of DEHP on the Hedgehog pathway was found in fetal mouse testes. The expression of both the Hedgehog receptor Ptch1 and the downstream effector of hedgehog-signaling Gli1 was decreased after exposure to 250 mg/kg DEHP. On the other hand, expression of Smo, a repressor of Hedgehog signaling, was increased following DEHP exposure, implying a decreased Hedgehog-signaling in the testis. The Hedgehog ligands Shh, Dhh, and Ihh showed no changes in expression after the exposure to DEHP in the fetal testes. Decreased Hedgehog signaling after phthalate exposure was observed previously in the genital tubercle, where prenatal dibutyl phthalate (DBP) exposure had an adverse effect on genital tubercle growth (Zhu et al., 2009). These observations suggest that the Hedgehog-signaling pathway in embryos could be modified by phthalate exposure.
In our study, less than 50% of fetuses in the 250 mg/kg group and less than 10% of fetuses in the 500 mg/kg group were alive at collection on E18.5. Fetuses from inbred mouse strains are more sensitive to the lethal effects of DEHP and MEHP than outbred fetuses. An abortion rate of 100% was reported in the inbred C3H/N following an exposure of 500 mg/kg of bw/day through feed spanning the whole pregnancy (Schmidt et al., 2012). Also, when pregnant C57BL/6J mice were orally exposed to 500 mg/kg/day of the MEHP, a DEHP metabolite, on E11–16, 86.4% of the fetuses were resorbed (Gaido et al., 2007). On the other hand, in a study of CD-1 mice, using the same dose but a shorter exposure time-window (E7–9), 84% of the fetuses were alive at collection on E18 (Shiota and Mima, 1985). In summary, DEHP exposure has a similar effect on fetal survival in the B6:129S4 mice used in this study as in mice from purely inbred strains. In general, the strain of mice can drastically affect the outcome of toxicological studies (Bowen et al., 2010; Prows et al., 1997; Yan et al., 2011). For example, the C57BL/6 strain is more susceptible to the toxic effects of cadmium exposure, but appears to be more resistant to a low-level arsenic exposure than outbred strains (Hovland et al., 1999; Kimura et al., 2005; Machado et al., 1999; Robinson et al., 2010; Rodriguez et al., 2016). Naturally, the exposure time-window has a major impact on the survival rate. When C57Bl/6J mice were exposed to 500 mg/kg of MEHP, on E11–16, 86.4% of the fetuses died, but when the exposure was limited to E14–16 only 5.8% died (Gaido et al., 2007). DEHP compromises embryonic survival most severely when the animals are exposed between E9 and E14. To fully separate the effect of the mouse strain from the effects of the exposure time-window, dose, and dosing method, multiple mouse strains should be treated side-by-side using the same dosing scheme and housing conditions.
The decreased survival of embryos after DEHP exposure can be caused by a direct toxic effect on the embryos or a defect in the dams’ ability to support the pregnancy. Progesterone secreted from the corpus luteum is critical to maintain pregnancy. Mice are particularly sensitive to decreased progesterone levels in midpregnancy (E11–14) and decreased progesterone in this time-window typically leads to abortion (Milligan and Finn, 1997). DEHP has been shown to affect the maintenance of the corpora lutea in pregnant mice and result in decreased serum progesterone levels when the pregnant dams are exposed to 250 mg/kg on GD1-13 (Guo et al., 2015). In our study, the dams were exposed to 250 mg/kg from implantation over the progesterone-dependent midgestational period (E7–16), suggesting that a defect in the corpora lutea function could have contributed to the decreased fetal survival observed in dams exposed to 250 mg/kg DEHP. Moreover, a defect in placental function may have influenced the survival and growth of the fetuses.
DEHP has also been shown to affect placental fatty acid transport, reduce placental growth, and disrupt labyrinth vascularization of the placentae (Xu et al., 2008; Zong et al., 2015). A high dose of DEHP (1000 mg/kg) decreases endometrial receptivity and inhibits embryo implantation (Li et al., 2012). In our study, DEHP exposure was initiated after the implantation period (E4–6), therefore, if a placental defect contributed to the decreased litter size, it was likely due to a decreased placental function rather than an implantation defect.
The endocrine disrupting effects of phthalates are well established in the rat (Ferrara et al., 2006; Gray et al., 2000; Lin et al., 2008; Mylchreest et al., 1998; Mylchreest et al., 2002; Welsh et al., 2008). Mouse and rat fetal Leydig cells share many similarities, such as stimulation of steroidogenesis by corticotrophin-releasing hormone (CRH) (McDowell et al., 2012), but mouse fetal Leydig cells are more resistant to the endocrine disrupting effect of phthalates than rat fetal Leydig cells (Gaido et al., 2007; Heger et al., 2012; Mylchreest et al., 2002; van den Driesche et al., 2015). In the rat, intrauterine exposure to DBP decreases the expression of genes required for cholesterol biosynthesis and consequently androgen production, but this does not happen in the mouse (Johnson, McDowell et al., 2011; Liu et al., 2005; Ovacik et al., 2013; Thompson et al., 2004). This difference in phthalate-sensitivity between the mouse and rat is connected to the SREBP2-mediated (Sterol regulatory element-binding protein 2) control of genes required for cholesterol biosynthesis (Johnson et al., 2011). In our study, we observed a widespread decrease in the expression of interstitial cell and steroidogenesis-associated genes at E14.5 after in utero exposure to only 5 mg/kg and 250 mg/kg DEHP, which could reflect a temporary delay in the development of the interstitial cell populations, because there were no significant differences in the testicular testosterone levels after DEHP exposure in comparison with the control group at E18.5. Anogenital index is used as a biological readout of the fetal androgen exposure over the whole gestational period. Surprisingly, AGI was significantly increased in males in the 250 mg/kg group compared with the controls despite that testicular testosterone production was not changed. One possible explanation is that 250 mg/kg phthalate exposure could directly impact AGI or facilitate androgen action on AGI. Phthalates and testicular testosterone have a non-linear relationship and they can induce increased testicular testosterone levels in fetal testes at low doses (Do et al., 2012; Lin et al., 2008). In the rat, exposure to 10 mg/kg resulted in an increase in testicular testosterone but a treatment with 750 mg/kg decreased testicular testosterone and AGD (Lin et al., 2008). In CD-1 mice, there was a mild tendency for increased serum testosterone and AGD at very low DEHP levels (0.0005–0.5 mg/kg) but decreased serum testosterone and AGD in male fetuses treated with 500 mg/kg DEHP in utero (Do et al., 2012). A caveat in interpreting the higher AGI observed for fetuses in the 250 mg/kg group is that high-level DEHP exposure was associated with decreased fetal bodyweight. The mean AGD of male fetuses in the 250 mg/kg and control groups were nearly identical (1.58 ± 0.06 mm vs 1.54 ± 0.05 mm), but the trend towards lower bodyweight in 250 mg/kg male fetuses could lead to a bias of the AGI.
In addition to the alteration of interstitial cell-associated genes, we observed an effect of DEHP-exposure on genes that control Sertoli cell function. It was previously shown that exposure to over 2 mg/kg DEHP throughout pregnancy delays the expression of the testis-determining genes Sry and Sox9 on E11.5 and E12.5 (Wang et al., 2016). We examined gene expression in the testis on E14.5, 3 days after the onset of sex determination, and found changes in key genes that control Sertoli cell fate. Dmrt1, which is required to maintain Sertoli cell fate after sex determination (Matson et al., 2011), was down-regulated after exposure to DEHP. Conversely, Dax1, which promotes seminiferous tubule organization and establishment of the peritubular myoid cell population (Meeks et al., 2003), was upregulated following DEHP exposure. During sex determination, SRY triggers SOX9 expression, which in turn upregulates Fgf9 to form a feed forward loop with SOX9 (for review see Ungewitter and Yao, 2013). Fgf9 expression was upregulated following exposure to DEHP. The changes in expression of Sertoli cell fate-determining genes after exposure to DEHP suggests that the effects of DEHP on the testes are not limited to germ cells and steroidogenic cells.
The formation of multinucleated germ cells is another characteristic testicular defect after a fetal exposure to phthalates in mice (Gaido et al., 2007). In this study, exposure to 5 mg/kg DEHP spanning the germ cell development (E7–E16) was sufficient to induce the formation of multinucleated germ cell aggregates. Higher doses of phthalates (20–750 mg/kg) have been linked to this phenomenon in other rodent studies (Gray et al., 2000; Mylchreest et al., 2002; van den Driesche et al., 2015). The effect of phthalate exposure on the rodent germ cells depends on the exposure time-window (Ferrara et al., 2006; Jobling et al., 2011). When rats were exposed to a high dose of DBP during early testicular morphogenesis (E13.5–14.5), the predominant defects included reduced germ cell numbers and a delayed maturation and mitotic arrest, whereas exposure just prior to birth (E19.5–21.5) induced multinucleated germ cell aggregation (Ferrara et al., 2006; Jobling et al., 2011). We observed a combination of both phenomena in the DEHP exposed testes. There was a slight increase in the number of germ cell nuclei when they were quantified from tissue sections on E18 and multinucleated germ cells could be observed on the sections in all the groups. Oct4, a pluripotency factor which is required for PGC survival (Kehler et al., 2004), showed increased expression in the 5 mg/kg DEHP exposed testes at E14.5, suggesting that germ cells retain primitive or pluripotent traits longer following DEHP exposure. Also, the expression of Asz1 (Ankyrin repeat, SAM and basic leucine zipper domain containing 1) was increased in the 5 mg/kg treated testes. Asz1 promotes the formation of PGCs from embryonic stem cells (ESC) in an in vitro differentiation model, and conversely the loss of Asz1 diminishes the ability of the ESCs to form PGC clusters (Wang et al., 2013). Several genes associated (Mael, Morc1, Mov10L, Tdrd5) with retrotransposon silencing were upregulated in the testes after DEHP exposure. Active retrotransposons are mutagenic and several layers of silencing, including DNA methylation and RNA interference, operate in the germ cells to suppress their activity. DEHP has been shown to alter DNA methylation in germ cells (Prados et al., 2015). Thus, compromised retrotransposon silencing could be one of the mechanisms by which DEHP disturbs germ cell function.
Our study highlights the importance of the genetic background on the toxicological effects of phthalates on mice. The B6:129S4 mouse strain was sensitive to the lethal and teratogenic effects of DEHP. In addition, we observed an alarming association between DEHP exposure and limb malformations, which has not been reported in the other studies using different mouse strains. More studies are needed to identify the mechanism responsible for the DEHP-induced limb malformations and to determine the potential relevance of this finding with respect to humans.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Louise Harris of the NIEHS Comparative Medicine Branch for assistance with dosing the animals and the NIEHS Cellular and Molecular Pathology Branch and Molecular Genomics Center for their services. We are very thankful to Paula Brown for her invaluable help with the collection of the fetuses. We are grateful for Dr Paul Foster for reading the manuscript and providing helpful suggestions.
FUNDING
Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (ES102965 to H.H.-C.Y.) and National Institute of Arthritis, Musculoskeletal and Skin Diseases (AR064195 to Y.K.). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934.
REFERENCES
- Akingbemi B. T., Youker R. T., Sottas C. M., Ge R., Katz E., Klinefelter G. R., Zirkin B. R., Hardy M. P. (2001). Modulation of rat Leydig cell steroidogenic function by di(2-ethylhexyl)phthalate. Biol. Reprod. 65, 1252–1259. [DOI] [PubMed] [Google Scholar]
- Blanc I., Bach A., Lallemand Y., Perrin-Schmitt F., Guenet J. L., Robert B. (2003). A new mouse limb mutation identifies a Twist allele that requires interacting loci on chromosome 4 for its phenotypic expression. Mamm. Genome 14, 797–804. doi:10.1007/s00335-003-2284-x [DOI] [PubMed] [Google Scholar]
- Bowen S. E., Kimar S., Irtenkauf S. (2010). Comparison of toluene-induced locomotor activity in four mouse strains. Pharmacol. Biochem. Behav. 95, 249–257. doi:10.1016/j.pbb.2010.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J., Feng C. W., Spiller C., Davidson T. L., Jackson A., Koopman P. (2010). FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev. Cell 19, 440–449. doi:10.1016/j.devcel.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Brock J. W., Silva M. J., Gray L. E. Jr, Reidy J. A., Barr D. B., Needham L. L. (2006). Urinary and amniotic fluid levels of phthalate monoesters in rats after the oral administration of di(2-ethylhexyl) phthalate and di-n-butyl phthalate. Toxicology 217, 22–30. doi:10.1016/j.tox.2005.08.013 [DOI] [PubMed] [Google Scholar]
- Colvin J. S., Bohne B. A., Harding G. W., McEwen D. G., Ornitz D. M. (1996). Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 12, 390–397. doi:10.1038/ng0496-390 [DOI] [PubMed] [Google Scholar]
- DiNapoli L., Batchvarov J., Capel B. (2006). FGF9 promotes survival of germ cells in the fetal testis. Development 133, 1519–1527. doi:10.1242/dev.02303 [DOI] [PubMed] [Google Scholar]
- Do R. P., Stahlhut R. W., Ponzi D., Vom Saal F. S., Taylor J. A. (2012). Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod. Toxicol. 34, 614–621. doi:10.1016/j.reprotox.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. R., Winnier G. E., Hargett L. K., Schrick J. J., Fogo A. B., Hogan B. L. (1997). Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev. Biol. 188, 235–247. doi:10.1006/dbio.1997.8664 [DOI] [PubMed] [Google Scholar]
- Fawcett D., Pasceri P., Fraser R., Colbert M., Rossant J., Giguere V. (1995). Postaxial polydactyly in forelimbs of CRABP-II mutant mice. Development 121, 671–679. [DOI] [PubMed] [Google Scholar]
- Ferrara D., Hallmark N., Scott H., Brown R., McKinnell C., Mahood I. K., Sharpe R. M. (2006). Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinology 147, 5352–5362. doi:10.1210/en.2006-0527 [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Hensley J. B., Liu D., Wallace D. G., Borghoff S., Johnson K. J., Hall S. J., Boekelheide K. (2007). Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol. Sci. 97, 491–503. doi:10.1093/toxsci/kfm049 [DOI] [PubMed] [Google Scholar]
- Gao D. W., Wen Z. D. (2016). Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 541, 986–1001. doi:10.1016/j.scitotenv.2015.09.148 [DOI] [PubMed] [Google Scholar]
- Geiss G. K., Bumgarner R. E., Birditt B., Dahl T., Dowidar N., Dunaway D. L., Fell H. P., Ferree S., George R. D., Grogan T., et al. (2008). Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat. Biotechnol. 26, 317–325. [DOI] [PubMed] [Google Scholar]
- Gray L. E. Jr, Ostby J., Furr J., Price M., Veeramachaneni D. N., Parks L. (2000). Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 58, 350–365. [DOI] [PubMed] [Google Scholar]
- Greene N. D., Copp A. J. (2014). Neural tube defects. Annu. Rev. Neurosci. 37, 221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Lai L., Zong T., Lin Y., Yang B., Zhang L., Li M., Kuang H. (2015). Exposure to di(2-ethylhexyl) phthalate inhibits luteal function via dysregulation of CD31 and prostaglandin F2alpha in pregnant mice. Reprod. Biol. Endocrinol. 13, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Ito Y., Yanagiba Y., Kamijima M., Naito H., Nakajima T. (2012). Differences in metabolite burden of di(2-ethylhexyl)phthalate in pregnant and postpartum dams and their offspring in relation to drug-metabolizing enzymes in mice. Arch. Toxicol. 86, 563–569. [DOI] [PubMed] [Google Scholar]
- Heger N. E., Hall S. J., Sandrof M. A., McDonnell E. V., Hensley J. B., McDowell E. N., Martin K. A., Gaido K. W., Johnson K.J., Boekelheide K. (2012). Human fetal testis xenografts are resistant to phthalate-induced endocrine disruption. Environ. Health Perspect. 120, 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. E. (2007). How to make a zone of polarizing activity: Insights into limb development via the abnormality preaxial polydactyly. Dev. Growth Differ. 49, 439–448. [DOI] [PubMed] [Google Scholar]
- Hovland D. N. Jr, Machado A. F., Scott W. J. Jr, Collins M. D. (1999). Differential sensitivity of the SWV and C57BL/6 mouse strains to the teratogenic action of single administrations of cadmium given throughout the period of anterior neuropore closure. Teratology 60, 13–21. [DOI] [PubMed] [Google Scholar]
- Ikegawa M., Han H., Okamoto A., Matsui R., Tanaka M., Omi N., Miyamae M., Toguchida J., Tashiro K. (2008). Syndactyly and preaxial synpolydactyly in the single Sfrp2 deleted mutant mice. Dev. Dyn. 237, 2506–2517. doi:10.1002/dvdy.21655 [DOI] [PubMed] [Google Scholar]
- Jobling M. S., Hutchison G. R., van den Driesche S., Sharpe R. M. (2011). Effects of di(n-butyl) phthalate exposure on foetal rat germ-cell number and differentiation: Identification of age-specific windows of vulnerability. Int. J. Androl. 34, e386–e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., McDowell E. N., Viereck M. P., Xia J. Q. (2011). Species-specific dibutyl phthalate fetal testis endocrine disruption correlates with inhibition of SREBP2-dependent gene expression pathways. Toxicol. Sci. 120, 460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R., Barr D., Boekelheide K., Breslin W., Breysse P., Chapin R., Gaido K., Hodgson E., Marcus M., Shea K.. et al. (2006). NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod. Toxicol. 22, 291–399. [DOI] [PubMed] [Google Scholar]
- Kehler J., Tolkunova E., Koschorz B., Pesce M., Gentile L., Boiani M., Lomelí H., Nagy A., McLaughlin K. J., Schöler H. R.. et al. (2004). Oct4 is required for primordial germ cell survival. EMBO Rep. 5, 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Ishida Y., Wada T., Yokoyama H., Mukaida N., Kondo T. (2005). MRP-1 expression levels determine strain-specific susceptibility to sodium arsenic-induced renal injury between C57BL/6 and BALB/c mice. Toxicol. Appl. Pharmacol. 203, 53–61. [DOI] [PubMed] [Google Scholar]
- Kleymenova E., Swanson C., Boekelheide K., Gaido K. W. (2005). Exposure in utero to di(n-butyl) phthalate alters the vimentin cytoskeleton of fetal rat Sertoli cells and disrupts Sertoli cell-gonocyte contact. Biol. Reprod. 73, 482–490. [DOI] [PubMed] [Google Scholar]
- Li R., Yu C., Gao R., Liu X., Lu J., Zhao L., Chen X., Ding Y., Wang Y., He J. (2012). Effects of DEHP on endometrial receptivity and embryo implantation in pregnant mice. J. Hazard. Mater. 241-242, 231–240. [DOI] [PubMed] [Google Scholar]
- Lin H., Ge R. S., Chen G. R., Hu G. X., Dong L., Lian Q. Q., Hardy D. O., Sottas C. M., Li X. K., Hardy M. P. (2008). Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc. Natl. Acad. Sci. *U S A 105, 7218–7222. doi:10.1073/pnas.0709260105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Lehmann K. P., Sar M., Young S. S., Gaido K. W. (2005). Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol. Reprod. 73, 180–192. [DOI] [PubMed] [Google Scholar]
- Machado A. F., Hovland D. N. Jr, Pilafas S., Collins M. D. (1999). Teratogenic response to arsenite during neurulation: Relative sensitivities of C57BL/6J and SWV/Fnn mice and impact of the splotch allele. Toxicol. Sci. 51, 98–107. [DOI] [PubMed] [Google Scholar]
- Malik S. (2014). Polydactyly: Phenotypes, genetics and classification. Clin. Genet. 85, 203–212. [DOI] [PubMed] [Google Scholar]
- Matson C. K., Murphy M. W., Sarver A. L., Griswold M. D., Bardwell V. J., Zarkower D. (2011). DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. N., Kisielewski A. E., Pike J. W., Franco H. L., Yao H. H., Johnson K. J. (2012). A transcriptome-wide screen for mRNAs enriched in fetal Leydig cells: CRHR1 agonism stimulates rat and mouse fetal testis steroidogenesis. PLoS One 7, e47359.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks J. J., Crawford S. E., Russell T. A., Morohashi K., Weiss J., Jameson J. L. (2003). Dax1 regulates testis cord organization during gonadal differentiation. Development 130, 1029–1036. [DOI] [PubMed] [Google Scholar]
- Milligan S. R., Finn C. A. (1997). Minimal progesterone support required for the maintenance of pregnancy in mice. Hum. Reprod. 12, 602–607. [DOI] [PubMed] [Google Scholar]
- Murdoch J. N., Copp A. J. (2010). The relationship between sonic Hedgehog signaling, cilia, and neural tube defects. Birth Defects Res a Clin. Mol. Teratol. 88, 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E., Cattley R. C., Foster P. M. (1998). Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicol. Sci. 43, 47–60. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Sar M., Wallace D. G., Foster P. M. (2002). Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod. Toxicol. 16, 19–28. [DOI] [PubMed] [Google Scholar]
- Nef S., Parada L. F. (1999). Cryptorchidism in mice mutant for Insl3. Nat. Genet. 22, 295–299. [DOI] [PubMed] [Google Scholar]
- Ovacik M. A., Sen B., Euling S. Y., Gaido K. W., Ierapetritou M. G., Androulakis I. P. (2013). Pathway modeling of microarray data: A case study of pathway activity changes in the testis following in utero exposure to dibutyl phthalate (DBP). Toxicol. Appl. Pharmacol. 271, 386–394. [DOI] [PubMed] [Google Scholar]
- Prados J., Stenz L., Somm E., Stouder C., Dayer A., Paoloni-Giacobino A. (2015). Prenatal exposure to DEHP affects spermatogenesis and sperm DNA methylation in a strain-dependent manner. PLoS One 10, e0132136.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prows D. R., Shertzer H. G., Daly M. J., Sidman C. L., Leikauf G. D. (1997). Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 17, 471–474. [DOI] [PubMed] [Google Scholar]
- Robinson J. F., Yu X., Hong S., Zhou C., Kim N., DeMasi D., Faustman E. M. (2010). Embryonic toxicokinetic and dynamic differences underlying strain sensitivity to cadmium during neurulation. Reprod. Toxicol. 29, 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez K. F., Ungewitter E. K., Crespo-Mejias Y., Liu C., Nicol B., Kissling G. E., Yao H. H. (2016). Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ. Health Perspect*, 124 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffarini C. M., Heger N. E., Yamasaki H., Liu T., Hall S. J., Boekelheide K. (2012). Induction and persistence of abnormal testicular germ cells following gestational exposure to di-(n-butyl) phthalate in p53-null mice. J. Androl. 33, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. S., Schaedlich K., Fiandanese N., Pocar P., Fischer B. (2012). Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ. Health Perspect. 120, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota K., Mima S. (1985). Assessment of the teratogenicity of di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate in mice. Arch. Toxicol. 56, 263–266. [DOI] [PubMed] [Google Scholar]
- Shiota K., Nishimura H. (1982). Teratogenicity of di(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) in mice. Environ. Health Perspect. 45, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H., Main K. M., Liu F., Stewart S. L., Kruse R. L., Calafat A. M., Mao C. S., Redmon J. B., Ternand C. L., Sullivan S., et al. Study for Future Families Research, T. (2005). Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 113, 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ross S. M., Gaido K. W. (2004). Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology 145, 1227–1237. [DOI] [PubMed] [Google Scholar]
- Ungewitter E. K., Yao H. H. (2013). How to make a gonad: Cellular mechanisms governing formation of the testes and ovaries. Sex. Dev. 7, 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S., McKinnell C., Calarrao A., Kennedy L., Hutchison G. R., Hrabalkova L., Jobling M. S., Macpherson S., Anderson R. A., Sharpe R. M., et al. (2015). Comparative effects of di(n-butyl) phthalate exposure on fetal germ cell development in the rat and in human fetal testis xenografts. Environ. Health Perspect. 123, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Liu X., Tang N., Archambeault D. R., Li J., Song H., Tang C., B He, Matzuk M. M., Wang Y. (2013). GASZ promotes germ cell derivation from embryonic stem cells. Stem Cell Res. 11, 845–860. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yang Q., Liu W., Yu M., Zhang Z., Cui X. (2016). Di(2-ethylhexyl) phthalate exposure in utero damages Sertoli cell differentiation via disturbance of sex determination pathway in fetal and postnatal mice. Toxicol Sci doi:10.1093/toxsci/kfw063 [DOI] [PubMed] [Google Scholar]
- Welsh M., Saunders P. T., Fisken M., Scott H. M., Hutchison G. R., Smith L. B., Sharpe R. M. (2008). Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Invest. 118, 1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Agrawal S., Cook T. J., Knipp G. T. (2008). Maternal di-(2-ethylhexyl)-phthalate exposure influences essential fatty acid homeostasis in rat placenta. Placenta 29, 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Willett T. L., Gu X. M., Martinez-Mier E. A., Sardone L., McShane L., Grynpas M., Everett E. T. (2011). Phenotypic variation of fluoride responses between inbred strains of mice. Cells Tissues Organs 194, 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H. H., Whoriskey W., Capel B. (2002). Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 16, 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Ornitz D. M. (2008). FGF signaling regulates mesenchymal differentiation and skeletal patterning along the limb bud proximodistal axis. Development 135, 483–491. [DOI] [PubMed] [Google Scholar]
- Zeller R., Lopez-Rios J., Zuniga A. (2009). Vertebrate limb bud development: Moving towards integrative analysis of organogenesis. Nat. Rev. Genet. 10, 845–858. [DOI] [PubMed] [Google Scholar]
- Zhu Y. J., Jiang J. T., Ma L., Zhang J., Hong Y., Liao K., Liu Q., Liu G. H. (2009). Molecular and toxicologic research in newborn hypospadiac male rats following in utero exposure to di-n-butyl phthalate (DBP). Toxicology 260, 120–125. [DOI] [PubMed] [Google Scholar]
- Zong T., Lai L., Hu J., Guo M., Li M., Zhang L., Zhong C., Yang B., Wu L., Zhang D., et al. (2015). Maternal exposure to di-(2-ethylhexyl) phthalate disrupts placental growth and development in pregnant mice. J. Hazard. Mater. 297, 25–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.