Abstract
Na,K-ATPase is a key enzyme that regulates a variety of transport functions in epithelial cells. In this study, we demonstrate a role for Na,K-ATPase in the formation of tight junctions, desmosomes, and epithelial polarity with the use of the calcium switch model in Madin-Darby canine kidney cells. Inhibition of Na,K-ATPase either by ouabain or potassium depletion prevented the formation of tight junctions and desmosomes and the cells remained nonpolarized. The formation of bundled stress fibers that appeared transiently in control cells was largely inhibited in ouabain-treated or potassium-depleted cells. Failure to form stress fibers correlated with a large reduction of RhoA GTPase activity in Na,K-ATPase-inhibited cells. In cells overexpressing wild-type RhoA GTPase, Na,K-ATPase inhibition did not affect the formation of stress fibers, tight junctions, or desmosomes, and epithelial polarity developed normally, suggesting that RhoA GTPase is an essential component downstream of Na,K-ATPase-mediated regulation of these junctions. The effects of Na,K-ATPase inhibition were mimicked by treatment with the sodium ionophore gramicidin and were correlated with the increased intracellular sodium levels. Furthermore, ouabain treatment under sodium-free condition did not affect the formation of junctions and epithelial polarity, suggesting that the intracellular Na+ homeostasis plays a crucial role in generation of the polarized phenotype of epithelial cells. These results thus demonstrate that the Na,K-ATPase activity plays an important role in regulating both the structure and function of polarized epithelial cells.
INTRODUCTION
Junctional complexes such as tight junctions, adherens junctions, and desmosomes play a fundamental role in maintaining the polarized phenotype and vectorial transport functions of epithelial cells. The tight junction (zonula occludens) forms a continuous belt at the boundary between the apical and lateral plasma membrane domains of neighboring epithelial cells (Farquhar and Palade, 1963). Structurally characterized by the fusion of the exoplasmic leaflets of contiguous plasma membranes, tight junctions selectively regulate the passage of molecules across the paracellular pathway (gate function) and passively separate molecules into the apical and basolateral plasma membrane domains (fence function). A functional tight junction is crucial to maintain the polarized phenotype of epithelial cells (Rodriguez-Boulan and Nelson, 1989; Mitic and Anderson, 1998; Stevenson and Keon, 1998). The adherens junction (zonula adherens) is localized below the tight junction and consists of cell adhesion and signaling molecules and may regulate events that mediate adhesion between epithelial cells (Yap et al., 1997). Desmosomes are focal points of intercellular contact at which neighboring cells are tightly bound to one another and provide resilience and tensile strength to the epithelial monolayer (Garrod et al., 1996). The mechanisms that regulate the formation and maintenance of these junctions in epithelial cells are not well understood.
The Na,K-ATPase is an oligomeric transmembrane protein consisting of two noncovalently linked α- and β-subunits. It catalyzes an ATP-dependent transport of three sodium ions out and two potassium ions into the cell per pump cycle, thereby generating a transmembrane sodium gradient across the plasma membrane. The sodium gradient generated by the enzyme provides the primary energy for uptake and extrusion of a variety of solutes by epithelial cells and is crucial for efficient functioning of other Na+-coupled transport systems (Katz, 1988; Lingrel et al., 1994). The Na,K-ATPase is localized to the basolateral plasma membrane in most epithelial cells and has been widely used as a marker for epithelial polarity (McNeil et al., 1990). However, a role for the Na,K-ATPase itself in the induction of epithelial polarity has not been shown.
Previous studies have implicated E-cadherin function in the formation and maintenance of junctional complexes and the polarized phenotype of epithelial cells (Imhof et al., 1983; Gumbiner et al., 1988; Watabe et al., 1994). E-Cadherin is a basolateral transmembrane protein that mediates cell-cell contact between epithelial cells by homophilic interaction (Nose et al., 1988). However, expression of a dominant negative mutant of E-cadherin in Madin-Darby canine kidney (MDCK) cells did not affect tight junctions or desmosomes (Troxell et al., 2000), indicating that other factors are involved in the maintenance of these junctions in MDCK cells. Recently, we have shown that in Moloney-sarcoma virus transformed MDCK (MSV-MDCK) cells expression of E-cadherin alone was not sufficient to induce tight junctions and desmosomes. Coexpression of Na,K-ATPase β-subunit and E-cadherin was required to induce these junctions and a polarized phenotype in MSV-MDCK cells (Rajasekaran et al., 2001). We also found that MSV-MDCK cells contain about threefold higher intracellular sodium levels ([Na+]i) compared with wild-type MDCK cells. Forced expression of both the Na,K-ATPase β-subunit and E-cadherin but not E-cadherin alone significantly reduced the [Na+]i (Rajasekaran et al., 2001). These studies suggested that [Na+]i regulated by the Na,K-ATPase may play a role in the formation of epithelial tight junctions and desmosomes and the induction of a polarized phenotype of epithelial cells.
RhoA GTPase, a Ras-related small GTP-binding protein, is involved in the formation of stress fibers in Swiss 3T3 cells (Ridley and Hall, 1992; Van Aelst and D'Souza-Schorey, 1997; Mackay and Hall, 1998). Recent studies have indicated a role for RhoA GTPase in the assembly and function of tight junctions in epithelial cells (Nusrat et al., 1995; Takaishi et al., 1997; Jou et al., 1998). However, mechanisms that regulate RhoA function in polarized epithelial cells are not known. In this study, we demonstrate that inhibition of Na,K-ATPase during epithelial polarization prevents the formation of tight junctions and desmosomes and suppresses the endogenous RhoA activity in MDCK cells. Na,K-ATPase inhibition in cells overexpressing wild-type RhoA GTPase does not affect the formation of tight junctions and desmosomes, indicating that RhoA GTPase is a downstream effector of Na,K-ATPase and is crucial for the formation of tight junctions and desmosomes in MDCK cells. Gramicidin, a sodium ionophore that specifically increases the intracellular sodium levels, mimicked the effects of Na,K-ATPase inhibition, suggesting that increased intracellular sodium levels may play a role in the inhibition of the formation of tight junctions, desmosomes, and polarized epithelia. Our results for the first time provide evidence that the Na,K-ATPase plays an important role in regulating both the structure and function of polarized epithelial cells.
MATERIALS AND METHODS
Ca2+-Switch Assay
Confluent MDCK monolayers (clone II, passage 4; kindly provided by Dr. Enrique Rodriguez-Boulan, Weill Medical College of Cornell, New York, NY) were subjected to calcium switch assay as described previously (Rajasekaran et al., 1996). Briefly, the cells were trypsinized until single cell suspensions were obtained and plated onto Costar Transwells with 0.4-μm pore size (Corning, Corning, NY) (3 × 106 cells/24 mm) or glass coverslips in a 12-well tissue culture plate (4 × 105 cells/well). The cells were allowed to attach in normal Ca2+-containing DMEM (1.8 mM Ca2+). Thereafter, the cells were rinsed gently in SMEM (minimum essential medium for suspension culture) (Invitrogen, Carlsbad, CA) containing <5 μM Ca2+ (low Ca2+ medium) and 5% dialyzed fetal bovine serum (FBS) and incubated for ∼16 h at 37°C. Before transfer of the cells to medium containing normal Ca2+ levels (1.8 mM), the MDCK monolayers were pretreated with either ouabain (50 μM; Sigma Chemical, St. Louis, MO), gramicidin (1 μM; Molecular Probes, Eugene, OR), or valinomycin (50 μM; Molecular Probes) for 1 h in low Ca2+ medium at 37°C. Dimethyl sulfoxide (DMSO), used as a solvent for these drugs, was added to control cells. For the switch, the low Ca2+ medium was replaced by normal culture medium containing the corresponding drugs and the cells were incubated at 37°C for indicated times. Sodium-free conditions were obtained as described in Fernandez and Malnic (1998). For this purpose single cell suspensions of MDCK cells were plated onto Transwells, allowed to attach, and incubated in low Ca2+ medium as described above. The switch was performed with the use of N-methyl-d-glucamine (NMDG) buffer in which NaCl was substituted with NMDG (Sigma Chemical) (5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 30 mM HEPES, 147 mM N-methyl-d-glucamine, 10 mM glucose, pH 7.4). Before the experiment the cells were rinsed twice with Ca2+-free NMDG buffer (NMDG buffer lacking CaCl2 and FBS) and then incubated in Ca2+-free NMDG buffer containing 5% dialyzed FBS and either DMSO or ouabain as described above. For the switch the Ca2+-free NMDG buffer was replaced with NMDG buffer containing DMSO or ouabain, respectively, and the cells were incubated at 37°C for up to 4 h. Potassium-free conditions were obtained as described in Le et al. (1999). For this purpose, the Ca2+-switch was performed with the use of a K+-free buffer (140 mM NaCl, 1.8 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, 10 mM glucose, pH 7.4), which contained 5% FBS dialyzed against the K+-free buffer. Before the experiment the cells were rinsed twice with Ca2+- and K+-free buffer (K+-free buffer lacking CaCl2) and preincubated in Ca2+/K+-free buffer/5% FBS for 1 h. For the K+ repletion the cells were incubated in normal medium at 37°C for 3–5 h. The Ca2+-switch assays for MDCK-RhoAwt cells were performed as described previously (Leung et al., 1999).
Immunofluorescence and Confocal Microscopy
Immunofluorescence was performed on cells fixed with methanol as described previously (Rajasekaran et al., 1996). Antibodies against ZO-1 and occludin (Zymed Laboratories, South San Francisco, CA), desmocollin (DC7G6; gift from Dr. Margaret Wheelock, University of Toledo, Toledo, Ohio), desmoplakin (gift from Dr. Manijeh Pasdar, University of Alberta, Alberta, Canada), and E-cadherin (DECMA; Sigma Chemical) were used. To detect filamentous actin the cells were fixed in paraformaldehyde and labeled with fluorescein isothiocyanate (FITC)-phalloidin (Sigma Chemical). Epifluorescence analysis was performed with the use of an Olympus AX 70 microscope. Confocal microscopy to monitor polarized distribution of domain-specific markers was performed with the use of a Fluoview laser scanning confocal microscope (Olympus America, Melville, NY). To detect FITC-labeled antigens, samples were excited at 488 nm with an Argon laser and the light emitted between 525 and 540 nm was recorded. Images were generated and analyzed with the use of the Fluoview image analysis software (version 2.1.39; Olympus America, Melville, NY).
Immunoblot Analysis
For immunoblot analysis, monolayers were lysed in a lysis buffer (95 mM NaCl, 25 mM Tris pH 7.4, 0.5 mM EDTA, 2% SDS, 1 mM phenylmethylsulfonyl fluoride, and 5 μg/ml each of antipain, leupeptin, and pepstatin). The lysates were briefly sonicated and centrifuged at 14,000 rpm in a Microfuge for 10 min. The supernatants were used for further analysis. Protein concentrations of the cell lysates were determined with the use of the Bio-Rad DC reagent (Bio-Rad Laboratories, Hercules, CA) according to manufacturer's instructions. Equal amounts of protein (100 μg) were separated by SDS-PAGE and transferred to nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The blots were blocked with 10% nonfat dry milk in phosphate-buffered saline (PBS) and then incubated for 2 h at room temperature with primary antibody diluted in 10% milk/PBS. After incubation the blots were washed three times with PBS/0.3% Tween 20 and then incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody (1:4000 in 10% milk). Bound antibody was detected by peroxidase-catalyzed enhanced chemiluminescence (PerkinElmer Life Sciences, Boston, MA). Densitometric analysis and quantification of the intensity of the individual bands was carried out with an ImageQuant software package (Molecular Dynamics, Sunnyvale, CA).
Transepithelial Electrical Resistance (TER) Measurements
To measure the transepithelial electrical resistance, the cells on Transwell filters were subjected to the Ca2+-switch assay as described above, and at the indicated time points the resistance of the monolayers was determined with the use of an EVOM epithelial voltohmeter (World Precision Instruments, Sarasota, FL). The values were normalized for the area of the filter after subtracting the background resistance of a filter without cells.
Electron Microscopy
Confluent monolayers grown on Transwells (see above) were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, for 2–4 h at room temperature and processed for transmission electron microscopy as described previously (Rajasekaran et al., 1996). Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined with a Joel (1200EX) electron microscope. The presence of tight junctions and desmosomes was quantified in ∼50 randomly selected cell-cell contact sites in each experiment. In MDCK cells adherens junctions were not easily discernible when tight junctions were present and therefore they were not quantified. However, when tight junctions were absent, like in ouabain-treated cells, adherens junctions were clearly observed and quantified. Representative results are shown.
Endogenous RhoA/Rac1 Activity Assay
The glutathione agarose-immobilized GST-PAK1, which contains the Rac1 interactive domain of human PAK1 (residues 51–135), and GST-Rhotekin, which contains the Rho binding domain of Rhotekin (residues 1–89), were expressed and purified in Escherichia coli by with the use of the pGEX-2T vector as described previously (Ren et al., 1999; Zhu et al., 2000). The cells were washed with ice-cold PBS buffer once before lysis in a buffer containing 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 1 mM dithiothreitol, 10 μg/ml each of leupeptin and aprotinin, and 1 mM phenylmethylsulfonyl fluoride at 4°C. Cell lysates were clarified by centrifugation at 13,000 × g at 4°C for 10 min. To load the endogenous small G proteins with GDP or guanosine-5′-O-(3-thio)triphosphate (GTPγS), aliquots of lysates were incubated for 10 min at ambient temperature in the presence of 10 mM EDTA and 0.5 mM GDP or GTPγS. The loading reactions were stopped by the addition of 20 mM MgCl2 and switching the temperature to 4°C. Equal volumes of lysates were incubated with GST-PAK1 or GST-Rhotekin (10 μg/lysate sample) immediately for 40 min at 4°C under constant agitation. The lysate-incubated beads were washed three times with the lysis buffer, and the bound RhoA and Rac1 were detected by anti-RhoA and anti-Rac1 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; Upstate Biotechnology, Lake Placid, NY) and visualized by enhanced chemiluminescence (PerkinElmer Life Sciences). Quantification of the immunoblots was performed with the use of AlphaImager system (Alpha Innotech, San Leandro, CA). For comparison of the level of the active RhoA and Rac1, the amount of GST-Rhotekin or PAK-bound small GTP-binding protein was normalized to the total amount of RhoA and Rac1 in cell lysates in each sample.
Atomic Emission Spectrometry
Subconfluent monolayers of MDCK cells treated without or with 50 μM ouabain, 1 μM gramicidin, or 50 μM valinomycin for 2 h or cells incubated in K+-free buffer for 2 h were rinsed three times with 10 ml each of 0.25 M sucrose. The cells of five 100-mm dishes each were pooled in 0.25 M sucrose, digested with HNO3 (Ultrex II; J.T. Baker, Phillipsburg, NJ) at a final concentration of 40% at 65°C for 15 h and diluted 1:2 with Millipore (Bedford, MA) Milli-Q UF plus filtered water. The ion concentrations were measured with the use of an inductively coupled plasma atomic emission spectrometer (Vista Axial 730; Varian, Walnut Creek, CA). The concentrations for Na+ (588.995 nm), K+ (766.941 nm), and Mg2+ (285.213 nm) were determined and the Na+ and K+ concentrations were normalized to the total Mg2+ content (internal control).
RESULTS
Na,K-ATPase Inhibition by Ouabain Prevents Formation of Functional Tight Junctions, Desmosomes, and Establishment of Epithelial Polarity
To understand the role of Na,K-ATPase in the formation of tight junctions and desmosomes we used a Ca2+-switch assay which is commonly used to monitor the development of junctional complexes in MDCK cells (Gonzalez-Mariscal et al., 1985). In the Ca2+-switch assay, MDCK monolayers formed in the presence of low calcium (<5 μM) medium, are transferred to medium containing normal calcium levels (1.8 mM), which increases cell-cell contact and initiates assembly of junctional complexes. To test whether Na,K-ATPase enzymatic activity is necessary for development of junctions, we pretreated MDCK monolayers maintained in low calcium medium with ouabain to specifically inhibit Na,K-ATPase for 1 h before transfer to normal calcium-containing medium. At selected times, cells were fixed and the presence of tight junctions was monitored by immunofluorescence localization of tight junction proteins, transmission electron microscopy (TEM), and by measuring the TER. Desmosomes were visualized by immunofluorescence of desmosomal proteins and TEM.
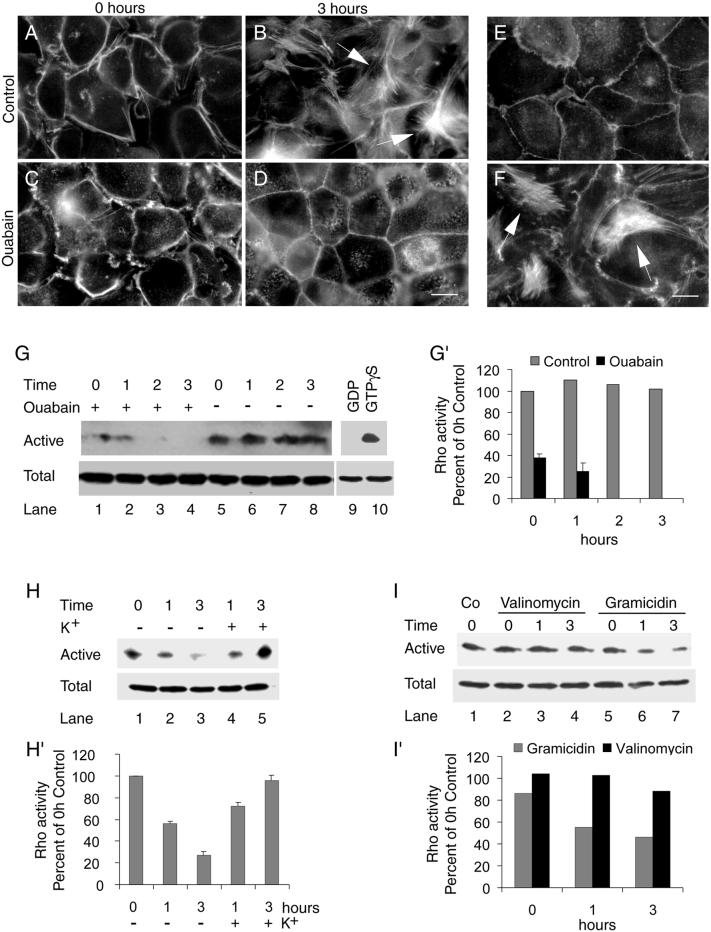
Before the Ca2+-switch (0 h), the tight junction protein ZO-1 showed cytoplasmic staining in both control and ouabain-treated cells (Figure 1, A and C). At 1 and 2 h after the Ca2+-switch, ZO-1 was localized to the plasma membrane with a discontinuous staining pattern. A visible difference in the ZO-1 staining pattern between control and ouabain-treated cells was not detected at these time points (our unpublished results). At 3 h, the ZO-1 staining in control cells showed a continuous staining pattern (Figure 1B), indicating that the tight junctions were established. In contrast, in ouabain-treated cells, the ZO-1 staining remained discontinuous with a beaded appearance and with regions clearly lacking ZO-1 (Figure 1D, arrows). Prolonged incubation did not change the discontinuous staining pattern of ZO-1. Instead, ZO-1 became even more intracellular. Other tight junction proteins such as occludin showed a similar staining pattern (our unpublished results). Ouabain treatment did not affect the protein levels of ZO-1 as revealed by immunoblot analysis (our unpublished results). These results showed that in the presence of ouabain tight junction proteins translocate to the plasma membrane but fail to form a complete ring-like pattern like in control cells.
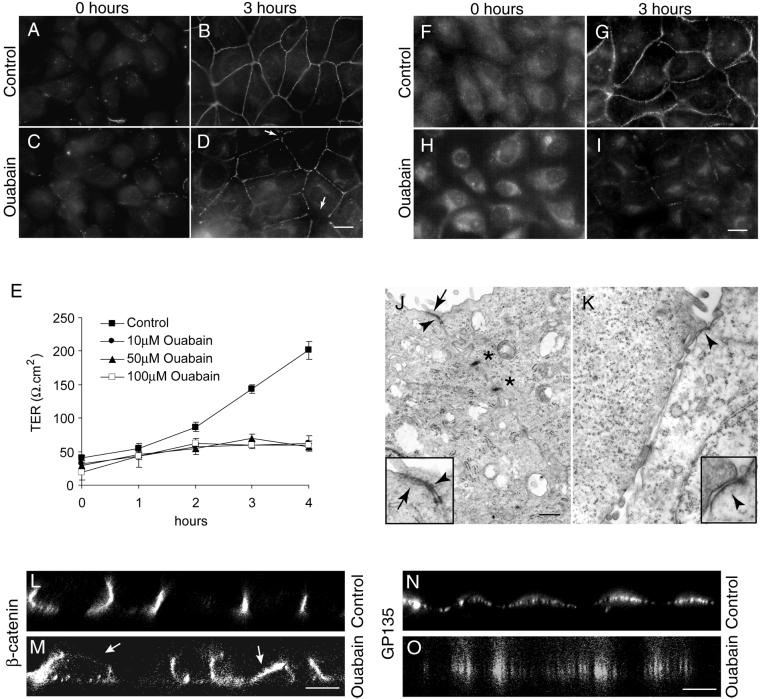
Figure 1.
Inhibition of Na,K-ATPase activity prevents the formation of tight junctions and desmosomes and the induction of polarity in MDCK cells. (A–D) Immunofluorescence localization of ZO-1. At 0 h in both control (A) and ouabain-treated cells (C), the ZO-1 staining is intracellular. At 3 h ouabain-treated cells show incomplete ZO-1 rings (arrows in D) compared with the complete ring-like staining revealed by control cells (B). (E) Transepithelial electrical resistance measurement. Ouabain-treated cells did not show an increase in the TER over time. (F–I) Immunofluorescence localization of desmocollin. Note that in ouabain-treated cells the desmocollin staining is predominantly intracellular (I) compared with control cells (G). At 0 h in both control (F) and ouabain-treated cells (H) the desmocollin staining is intracellular. (J and K) Transmission electron microscopy. Tight junctions (arrows), adherens junctions (arrowheads), and desmosomes (asterisks) were formed in control cells (J), whereas ouabain-treated cells (K) had no tight junctions and desmosomes. Inserts are the higher magnification of the tight junction regions in J and K. (L–O). Confocal microscope X-Z sections. Note the polarized localization of β-catenin (L) and GP135 (N) in control cells and nonpolarized distribution of these markers in ouabain-treated cells (M and O). Bars in A–D and F–I, 10 μm; J and K, 500 nm; and L–O, 5 μm.
A functional tight junction is required to maintain the polarized distribution of distinct apical and basolateral markers. In MDCK cells the TER measurement during the Ca2+-switch assay has been used to monitor formation of functional tight junctions (Gonzalez-Mariscal et al., 1985; Jou et al., 1998). Cells grown on Transwell filters were subjected to a Ca2+-switch assay and the development of TER was measured. The TER in control cells gradually increased after the Ca2+-switch and within 3 h reached ∼200 Ω.cm2, a value reported for MDCK clone II (Gonzalez-Mariscal et al., 1985; Jou et al., 1998) (Figure 1E). In contrast, the TER in ouabain-treated cells reached only ∼50 Ω.cm2, and this value did not increase over 4 h of ouabain treatment. Moreover, confocal microscope vertical (X-Z) sections revealed a polarized distribution of β-catenin (a basolateral marker) (Rajasekaran et al., 1996) (Figure 1L) and GP135 (an apical marker) (Ojakian and Schwimmer, 1988) (Figure 1N) in control cells, whereas in ouabain-treated cells these markers were distributed in a nonpolarized manner (Figure 1, M and O). These results indicate that ouabain-mediated inhibition of Na,K-ATPase activity prevents formation of functional tight junctions and the establishment of polarization in MDCK cells.
To monitor the assembly of desmosomes we used antibodies against desmoplakin, a peripheral membrane protein and desmocollin, a transmembrane protein localized to desmosomes (Garrod et al., 1996). Both markers showed identical patterns. The results obtained from the anti-desmocollin antibody staining are shown (Figure 1, F–I). In both control and ouabain-treated cells, at 0 h of the Ca2+-switch, desmocollin was distinctly localized intracellular with barely detectable levels on the plasma membrane (Figure 1, F and H). Desmocollin and desmoplakin were predominantly cytoplasmic at 1 and 2 h after the Ca2+-switch (our unpublished results). After 3 h of Ca2+-switch the desmocollin staining in control cells revealed a distinct dot-like pattern circumscribing the cells, typical of the desmosome staining pattern in MDCK cells (Figure 1G). In contrast, in ouabain-treated cells, after 3 h of Ca2+-switch, desmocollin was predominantly intracellular with barely detectable staining at the cell-cell contacts (Figure 1I). This pattern was maintained after prolonged incubation with ouabain. Immunoblot analysis of desmoglein (a membrane component of the desmosome) and desmoplakin did not show significant changes in the levels of these proteins in ouabain-treated cells (our unpublished results). These results demonstrate that inhibition of Na,K-ATPase activity largely affects translocation of desmosomal proteins to the plasma membrane, whereas it does not affect translocation of tight junction proteins to the plasma membrane.
To further confirm the effect of ouabain on the assembly of tight junctions and desmosomes we performed TEM of control and ouabain-treated cells after 3 h of Ca2+-switch. TEM revealed the presence of typical tight junctions, adherens junctions, and desmosomes in cell-cell contact sites in control cells (Figure 1J). In contrast, in ouabain-treated cells tight junctions and desmosomes were rarely detected, although adherens junction-like structures were clearly seen (Figure 1K). Quantitative analysis of the TEM data was performed by scoring the number of tight junctions and desmosomes in ∼50 cell-cell contact sites. Control cells revealed tight junctions and desmosomes in 96 and 100% of cell-cell contacts, respectively. In contrast, ouabain-treated cells showed putative tight junctions in only 14% and desmosome-like structures in 15% of the cell-cell contacts. Ouabain-treated cells showed typical adherens junctions in 54% of the cell-cell contacts. Taken together, these results demonstrate that inhibition of Na,K-ATPase by ouabain prevents formation of functional tight junctions and desmosomes and the polarized phenotype in MDCK cells.
Effect of Na,K-ATPase Inhibition on Formation of Tight Junctions, Desmosomes, and Establishment of Polarity Is Reversible
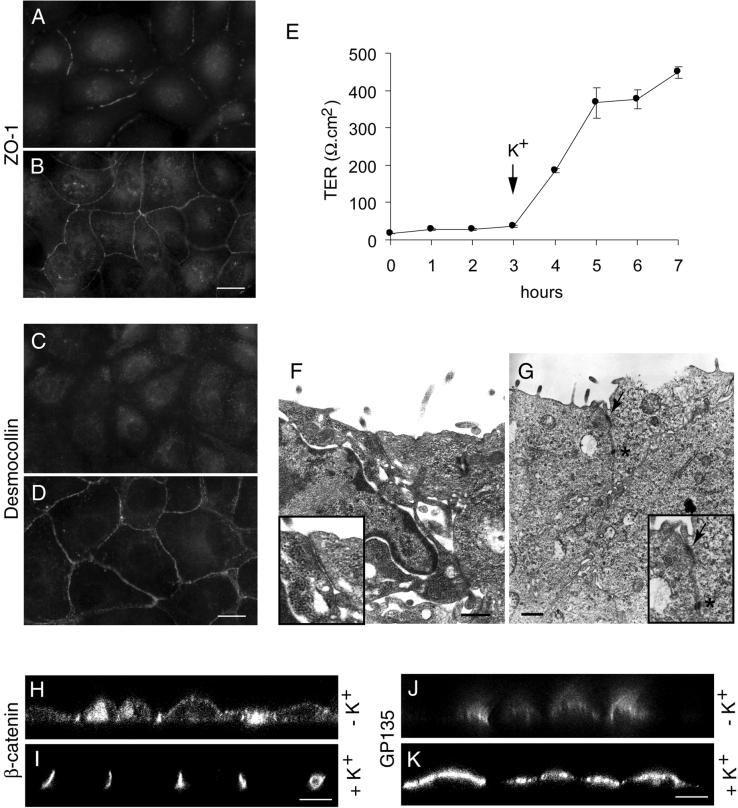
As an independent means to test the role of Na,K-ATPase in the formation of tight junctions and desmosomes we used K+ depletion to inhibit Na,K-ATPase (Pollack et al., 1981; Pressley 1988; Buffin-Meyer et al., 1996). In this assay, the pump function can be restored by the addition of K+ and thus the reversibility of the effect of Na,K-ATPase inhibition can be tested. MDCK cells maintained in low calcium medium were preincubated in K+- and Ca2+-free buffer for 1 h to inactivate the Na,K-ATPase and the Ca2+-switch assay was performed in K+-free medium. Immunofluorescence staining of ZO-1 after 3 h of switch to K+-free medium containing Ca2+ showed ZO-1 localized to the plasma membrane although without forming a continuous ring-like structure (Figure 2A). This pattern was similar to that of ouabain-treated cells (Figure 1D). Subsequent incubation of these cells for 5 h in K+-containing medium resulted in a complete ZO-1 ring circumscribing the cells (Figure 2B). The TER of MDCK cells in K+-free buffer did not increase up to 3 h after the Ca2+-switch (Figure 2E). However, upon transfer of the cells from K+-free to K+-containing culture medium, the TER increased dramatically and reached a value of ∼400 Ω.cm2 (Figure 2E) within 2 h, indicating formation of functional tight junctions. Confocal microscope vertical (X-Z) sections of cells maintained in K+-free medium revealed a nonpolarized distribution of β-catenin (Figure 2H) and GP135 (Figure 2J). Subsequent transfer of these cells to K+-containing culture medium resulted in the polarized distribution of these markers (Figure 2, I and K). The assembly of desmosomes in K+-free buffer was similar to ouabain-treated cells. In K+-free buffer the translocation of desmocollin to the plasma membrane was inhibited and the staining was predominantly intracellular (Figure 2C). Subsequent incubation of these cells in K+-containing culture medium for 5 h resulted in a dot-like desmocollin staining at the sites of cell-cell contact (Figure 2D), indicating formation of desmosomes. Electron microscopy studies confirmed the absence of tight junctions and desmosomes in MDCK cells maintained in K+-free buffer (Figure 2F), whereas a subsequent transfer to K+-containing culture medium resulted in the formation of tight junctions and desmosomes (Figure 2G). Quantitative TEM analysis of cells maintained in K+-free buffer showed the presence of tight junctions and desmosomes in only 2 and 3% of cell-cell contacts, respectively. After K+ restoration tight junctions were detected in 90% and desmosomes in 85% of cell-cell contacts. These results further demonstrate that Na,K-ATPase enzymatic activity is necessary for formation of tight junctions and desmosomes in MDCK cells.
Figure 2.
Na,K-ATPase-mediated inhibition of formation of tight junctions and desmosomes is reversible. Immunofluorescence localization of ZO-1 (A and B) and desmocollin (C and D). Cells incubated in K+-free medium show an incomplete ZO-1 ring at the plasma membrane region (A) and intracellular localization of desmocollin (C). Replenishment of K+ results in a complete ZO-1 ring (B) and plasma membrane localization of desmocollin (D). (E) Measurement of TER. Note an increase in the TER as soon as the cells are shifted to K+-containing medium. (F and G) Transmission electron microscopy. Note the absence of tight junctions (arrow) and desmosomes (asterisk) in K+-free medium (F) and their presence in K+-containing medium (G). Insets in F and G are the higher magnifications of tight junction regions. (H–K) Confocal microscope X-Z sections. Note the nonpolarized distribution of β-catenin (H) and GP135 (J) under K+-depleted condition and the polarized distribution of β-catenin (I) and GP135 (K) after K+ repletion. Bars in A–D, 10 μm; F and G, 500 nm; and H–K, 5 μm.
Inhibition of Na,K-ATPase in Na+-free Medium Does not Affect Formation of Tight Junctions, Desmosomes, and Epithelial Polarity
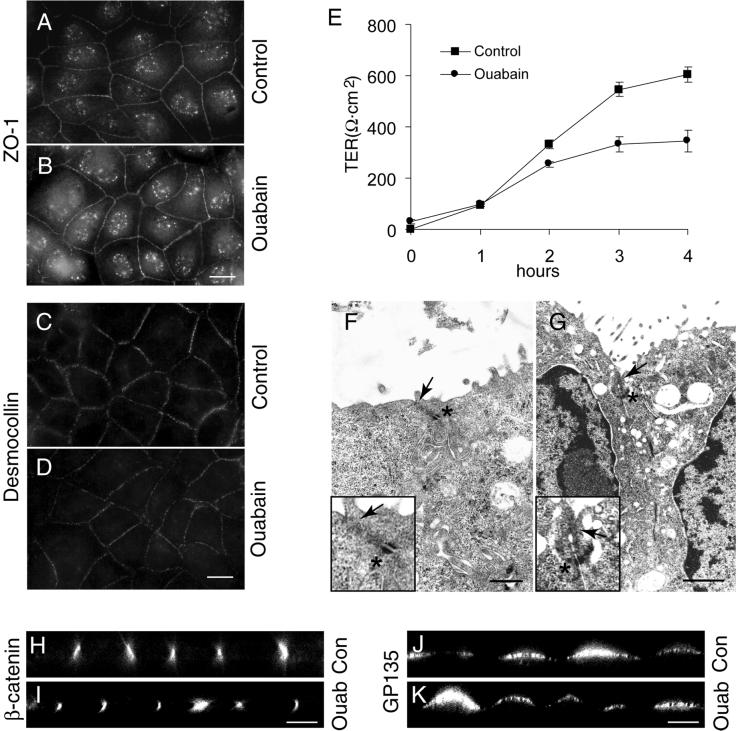
Inhibition of Na,K-ATPase results in an increased intracellular sodium concentration (Pollack et al., 1981; Rayson 1989; Yamamoto et al., 1993; Rajasekaran et al., 2001) and sodium measurements with the use of atomic emission spectrometry revealed that the intracellular sodium levels [Na+]i of MDCK cells (∼12 mM) (Rajasekaran et al., 2001) increased >10-fold upon ouabain treatment or sevenfold by K+ depletion. The intracellular K+ level of MDCK cells was 148 mM. Inhibition of sodium pump by ouabain or K+ depletion decreased the intracellular potassium concentration to 12 and 50% of the control, respectively. To test whether increased [Na+]i is involved in the inhibition of tight junction and desmosome formation we first treated MDCK cells with ouabain in Na+-free medium to prevent the increase of [Na+]i after ouabain treatment. Cells maintained in low calcium medium were subjected to a Ca2+-switch in a Na+-free medium with or without ouabain as described in MATERIALS AND METHODS. After 3 h of Ca2+-switch, the cells were fixed and stained for tight junction and desmosomal proteins. As shown in Figure 3, both control (Figure 3A) and ouabain-treated cells (Figure 3B) clearly showed a complete ZO-1 ring on the plasma membrane, indicating that tight junctions are formed in these cells. Immunofluorescence of desmocollin (Figure 3, C and D) and desmoplakin (our unpublished data) revealed a distinct dot-like staining pattern circumventing the cells in both control (Figure 3C) and ouabain-treated cells (Figure 3D). Control and ouabain-treated cells showed high TER values within 4 h of Ca2+-switch in Na+-free medium (Figure 3E). Under Na+-free conditions the control cells had a higher TER resistance compared with cells maintained in normal medium (compare Figures 1E and 3E), possibly due to decreased tight junction permeability. Confocal microscope vertical (X-Z) sections confirmed the polarized distribution of β-catenin and GP135 in both control and ouabain-treated cells (Figure 3, H–K). In contrast to the effect of ouabain on junction formation in Na+-containing medium ouabain treatment in Na+-free medium did not decrease the number of tight junctions or desmosomes in both control (Figure 3F) and ouabain-treated cells (Figure 3G). Cell-cell contacts of cells in Na+-free medium showed tight junctions and desmosomes similar to control cells in Na+-containing medium. Because in Na+-free medium ouabain treatment did not affect the formation of tight junctions, desmosomes and establishment of polarity these results are consistent with the idea that increased [Na+]i after inhibition of Na,K-ATPase may be involved in the inhibition of the formation of tight junctions and desmosomes in MDCK cells. Furthermore, these results also establish that the observed effect of ouabain is not due to a toxic side effect.
Figure 3.
Ouabain treatment under sodium-free conditions does not affect the formation of tight junctions and desmosomes. A and B and C and D are the immunofluorescence localization of ZO-1 and desmocollin, respectively. Note the comparable staining pattern in control (A and C) and ouabain-treated (B and D) cells. (E) Measurement of TER. (F and G) Transmission electron micrographs of control (F) and ouabain-treated (G) cells show tight junctions (arrow) and desmosomes (asterisk). Insets are the higher magnification of the tight junction region in F and G. (H–K) Confocal microscope X-Z sections. Note the polarized distribution of β-catenin and GP135 in control and ouabain-treated cells. Bars in A–D, 10 μm; F and G, 500 nm and 1 μm, respectively; and H–K, 5 μm.
Sodium Ionophore Gramicidin Treatment Affects Formation of Tight Junctions, Desmosomes, and Establishment of Polarity
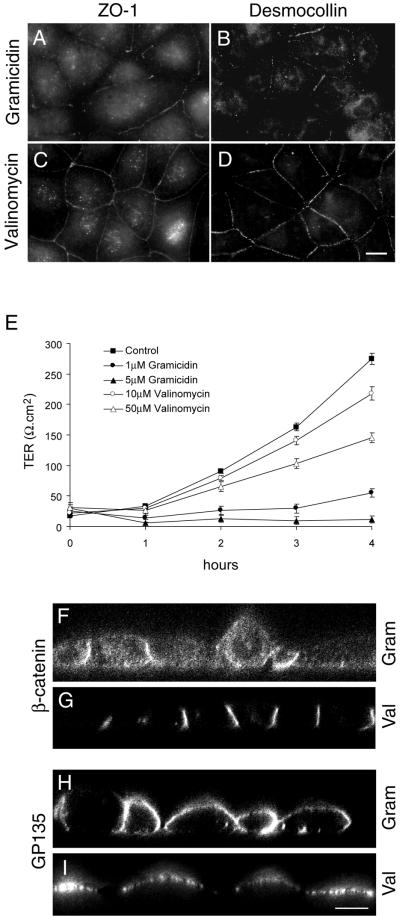
To further test whether increased [Na+]i affects formation of tight junctions and desmosomes we treated MDCK cells with the sodium ionophore gramicidin and a potassium ionophore, valinomycin. Sodium ionophores have been used widely to test the involvement of intracellular sodium on cell functions (Harber et al., 1987; Yamamoto et al., 1993; Liu et al., 2000). Gramicidin A is an ion channel-forming antibiotic that greatly facilitates Na+ entry into cells (Rothstein and Mack, 1990), and specifically increases the intracellular Na+ concentration. Valinomycin is a cyclic dodecadepeptide that transports K+ through the plasma membrane, inducing a potassium leak (Vaaraniemi et al., 1997). Intracellular sodium measurements revealed a >12-fold increase in [Na+]i in gramicidin-treated cells, whereas valinomycin-treated cells did not reveal a significant change in the intracellular sodium levels compared with untreated MDCK cells. Treatment of MDCK cells with gramicidin during the Ca2+-switch showed an effect similar to that of ouabain. Three hours after the Ca2+-switch, ZO-1 was clearly localized on the plasma membrane, although without a continuous ring pattern (Figure 4A). The immunofluorescence staining of occludin showed identical results (our unpublished results). In the presence of gramicidin, 3 h after the Ca2+-switch, immunofluorescence of desmocollin (Figure 4B) and desmoplakin (our unpublished results) showed a predominantly cytoplasmic staining pattern similar to ouabain-treated cells (compare Figures 4B and 1I). In contrast to gramicidin-treated cells, valinomycin-treated cells resembled control cells. Three hours after the Ca2+-switch ZO-1 formed a continuous ring at the plasma membrane (Figure 4C). Occludin showed a similar staining pattern (our unpublished results). Both desmocollin (Figure 4D) and desmoplakin (our unpublished results) showed a punctate staining pattern at cell-cell contact sites. Consistent with the localization of tight junction proteins, valinomycin-treated cells had TER values of ∼250Ω.cm2 within 4 hours. In contrast, gramicidin-treated cells did not develop TER with time (Figure 4E). Confocal microscope vertical (X-Z) sections of gramicidin-treated cells revealed nonpolarized distribution of β-catenin (Figure 4F) and GP135 (Figure 4H), whereas in valinomycin-treated cells these markers were distributed in a polarized manner (Figure 4, G and I). Furthermore, TEM revealed tight junctions and desmosomes in valinomycin-treated cells but showed a significant decrease in junctions in gramicidin-treated cells (our unpublished results). Tight junctions and desmosomes were present in 100% of the cell-cell contacts in valinomycin-treated cells, whereas gramicidin-treated cells showed putative tight junctions in only 23% and desmosome-like structures in 14% of the cell-cell contacts. Taken together, these results demonstrate that the intracellular Na+ homeostasis regulated by Na,K-ATPase is involved in the assembly of functional tight junctions, desmosomes, and the induction of polarity in MDCK cells.
Figure 4.
Treatment of MDCK cells with the sodium ionophore gramicidin inhibits the formation of tight junctions. (A and C) Immunofluorescence localization of ZO-1 and desmocollin (B and D), respectively, of gramicidin- (A and B) and valinomycin (C and D)-treated cells. (E). TER measurement of gramicidin- and valinomycin-treated cells. (F–I) Confocal microscope X-Z sections. Bars in A–D, = 10 μm; F–I, 5 μm.
Inhibition of Na,K-ATPase Prevents Formation of Bundled Stress Fibers
Recent studies have implicated a role for filamentous actin in regulation of tight junctions (Nusrat et al., 1995; Jou et al., 1998) and desmosomes (Vasioukhin et al., 2000). Therefore, we tested whether Na,K-ATPase plays a role in the organization of the actin cytoskeleton during the formation of tight junctions and desmosomes in MDCK cells. Organization of the actin cytoskeleton was monitored by FITC-phalloidin labeling and epifluorescence microscopy. In control cells, at 0 h stress fibers were not detected at the bottom of the cells (Figure 5A). After 1 h of Ca2+-switch stress fibers began to appear at the bottom of the cells (our unpublished results) and after 3 h bundles of stress fibers were apparent (Figure 5B). These bundles of stress fibers gradually disappeared and were not detected after 48 h of Ca2+-switch (our unpublished results). In contrast, in ouabain-treated cells the bundled stress fibers did not form (Figure 5, C and D). However, these cells showed a distinct cortical actin cytoskeleton more close to the bottom of the cells (Figure 5D). Like in ouabain-treated cells, K+ depletion also inhibited the formation of bundles of stress fibers and induced a distinct cortical actin closer to the base of the cells (Figure 5E). Adding K+ to the cells maintained in low K+ resulted in bundles of stress fibers as seen in control cells (compare Figure 5, B and F). Formation of bundled stress fibers was markedly inhibited by gramicidin but not by valinomycin (our unpublished results). These results indicate that the transient formation of bundled stress fibers correlates with the assembly of tight junctions and desmosomes and that inhibition of Na,K-ATPase prevents the formation of bundled stress fibers.
Figure 5.
Inhibition of Na,K-ATPase prevents the formation of bundled stress fibers and inhibits RhoA GTPase activity. (A–F) FITC-phalloidin staining of filamentous actin. At 0 h no stress fibers were detected in control cells (A) and cells treated with ouabain (C). Bundled stress fibers were present at 3 h in control cells (B, arrows) but were not detected in ouabain-treated cells (D). Phalloidin labeling of cells maintained under K+-free condition (E) and cells transferred to K+-containing medium (F) are shown. Note the presence of bundled stress fibers (arrows) after transfer to K+-containing medium. Bars in A–F, 10 μm. (G and G′) Effect of ouabain treatment on RhoA activity. (G) Immunoblot showing active and total RhoA in ouabain-treated and control cells. The results shown are the representative data obtained from three independent experiments. (G′) Quantification of the immunoblot data represents the average of three independent determinations done in duplicate. Bars indicate the SE. For control the error bars are so small that they are not seen in the figure. (H and H′) Effect of K+ depletion and repletion on the RhoA activity. Immunoblot of active and total RhoA (H) and quantification of the immunoblot data (H′) representing the mean of two independent determinations done in duplicate. (I and I′) Effect of gramicidin and valinomycin on RhoA activity. Immunoblot of total and active RhoA (I) and quantification of the immunoblot data done in duplicate (I′).
Endogenous RhoA Activity Is Highly Reduced in Na,K-ATPase Inhibited or Gramicidin-treated Cells
The RhoA GTPase has been implicated in regulation of actin stress fiber formation in fibroblasts and in epithelial cells (Ridley and Hall, 1992; Mackay and Hall 1998; Jou and Nelson, 1998; Jou et al., 1998). Moreover, both RhoA and Rac1, a closely related family member, are essential for the assembly and function of tight junctions in MDCK cells (Jou et al., 1998). Therefore, we tested the possible involvement of these GTPases in the Na,K-ATPase-mediated formation of junctional complexes. For this purpose, we determined the endogenous levels of active RhoA in control cells and ouabain-treated cells with the use of an in vitro biochemical assay (Ren et al., 1999; Zhu et al., 2000). In this assay, GST-Rhotekin, a GST-fusion protein containing the Rho binding domain (which binds active RhoA bound to GTP) is incubated with cell lysate and the bound, active RhoA is detected by immunoblot analysis. The specificity of this assay is demonstrated by loading the cell lysate with a nonhydrolyzable analog of GTP, GTPγS (total RhoA), and by replacing GTPγS with GDP that inactivates Rho and therefore does not bind to GST-Rhotekin (Figure 5G, compare lanes 9 and 10). Consistent with the formation of stress fibers, control cells showed high levels of active RhoA (Figure 5G, lanes 5–8, and G′) compared with ouabain-treated cells in which the level of active RhoA gradually decreased and became undetectable after 2 h of treatment (Figure 5G, lanes 1–4, and G′). The protein levels of RhoA remained unchanged in both control and ouabain-treated cells (Figure 5G, labeled as total, lanes 1–10). Like in ouabain-treated cells, depletion of K+ resulted in the inhibition of RhoA activity (Figure 5H, lanes 1–3, and H′) and addition of K+ resulted in the reactivation of RhoA activity (Figure 5H, lanes 4 and 5, and H′). These results indicate that the Na,K-ATPase activity has a direct impact on the activation state of RhoA. Gramicidin-treated cells showed reduced RhoA activity compared with valinomycin-treated cells (Figure 5I, compare lanes 2–4 and 5–7, and I′), indicating that increased intracellular sodium may either directly or indirectly inhibit the activity of RhoA. We also measured the endogenous Rac1 activity in response to modulation of Na,K-ATPase activity and found that neither the expression level nor the activation state of Rac1 was affected by ouabain or K+ depletion (our unpublished results). Thus, Na,K-ATPase appears to be a specific upstream regulator of RhoA GTPase.
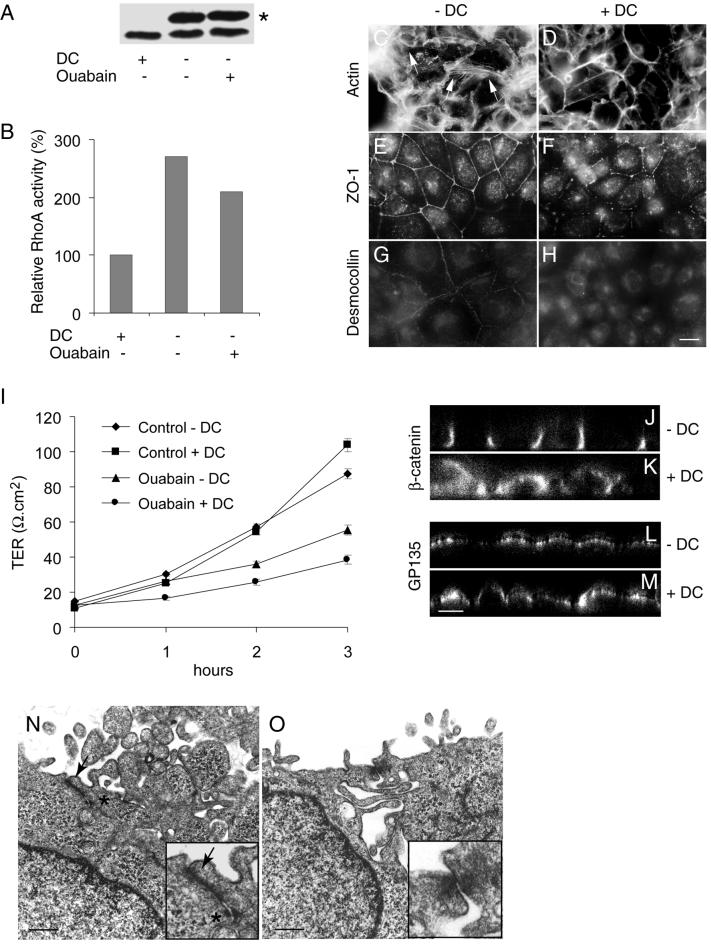
Overexpression of Wild-Type RhoA GTPase Abolishes Effects of Na,K-ATPase Inhibition on Junction Formation and Epithelial Polarity
Both the dominant negative and active forms of RhoA GTPase have profound effects on tight junctions in MDCK cells (Jou et al., 1998). Therefore, to further validate the specific role of RhoA in formation of tight junctions and desmosomes we used MDCK cells expressing wild-type RhoA GTPase (MDCK-RhoAwt) under control of the tetracycline repressible transactivator. In MDCK-RhoAwt cells, the levels of RhoA increased severalfold after withdrawal of the transcription repressor doxycycline (DC) (Figure 6A) (Leung et al., 1999). In induced cells (−DC) the level of active RhoA was 2.7-fold more than that of control cells maintained in the presence of doxycycline (+DC). Three hours after ouabain treatment, induced cells showed 2.1-fold more active RhoA GTPase than uninduced cells (Figure 6B). Consistent with the presence of active RhoA in induced cells, bundled stress fibers were distinctly seen after 3 h of ouabain treatment, whereas these fibers were scarcely present in uninduced cells (Figure 6, C and D). On ouabain treatment RhoA-induced cells revealed an uninterrupted ZO-1 staining pattern, whereas uninduced cells showed a disrupted staining pattern (Figure 6, E and F). The TER of ouabain-treated induced cells was significantly higher than that of uninduced cells (p < 0.01) (Figure 6I). Furthermore, confocal microscope vertical sections revealed a polarized distribution of β-catenin and GP135 in induced ouabain-treated cells (Figure 6, J and L). In contrast, in uninduced cells these markers were distributed in a nonpolarized manner (Figure 6, K and M). These results demonstrate that functional tight junctions are formed even in the presence of ouabain in RhoA-overexpressing cells. Desmocollin revealed a distinct plasma membrane localization in induced, ouabain-treated cells (Figure 6G), whereas uninduced cells showed an intracellular staining pattern (Figure 6H). TEM revealed tight junctions and desmosomes in 88% of the cell-cell contacts in induced, ouabain-treated cells, whereas uninduced cells showed putative tight junctions in only 12% and desmosome-like structures in 18% of the cell-cell contacts (Figure 6, N and O). These results are consistent with the idea that Na,K-ATPase mediates its action through RhoA and that RhoA function is essential for the formation of tight junctions and desmosomes and to maintain the polarized phenotype of MDCK cells.
Figure 6.
Formation of tight junctions, desmosomes and the induction of polarity in ouabain-treated MDCK-RhoAwt cells overexpressing RhoA. −DC and +DC represent ouabain-treated induced and noninduced MDCK-RhoAwt cells, respectively. (A) RhoA immunoblot showing induced high molecular mass Myc-tagged (∗) and endogenous RhoA after the withdrawal of DC. (B) Relative levels of active RhoA GTPase. The results shown are the representative data obtained from two independent experiments. (C and D) FITC-phalloidin staining of filamentous actin. Note the presence of bundled stress fibers in C and their absence in D. (E and F) Immunofluorescence of ZO-1. Ouabain-treated induced cells show a complete ring-like staining compared with the incomplete ZO-1 ring in noninduced cells. (G and H) Immunofluorescence of desmocollin. In ouabain-treated induced cells desmocollin is localized to the plasma membrane compared with the intracellular staining in noninduced cells (I). Measurement of TER (J–M). Confocal microscope X-Z sections. Note the polarized distribution of β-catenin and GP135 in ouabain-treated induced cells. (N and O) Transmission electron microscopy. Tight junctions (arrow) and desmosomes (asterisk) are present in ouabain-treated induced cells. Inserts are the higher magnification of the tight junction region in N and O. Bars in C–H, 10 μm; J–M, 5 μm; and N and O, 500 nm.
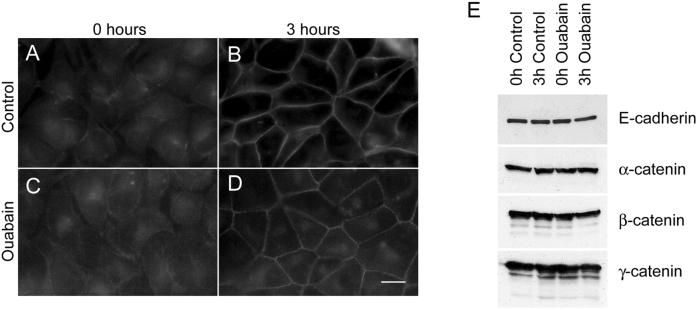
Levels and Localization of E-Cadherin and Its Associated α-,β-, and γ-Catenins Are not Affected in Na,K-ATPase-inhibited Cells
The cell adhesion molecule E-cadherin has been implicated in formation and maintenance of tight junctions and desmosomes (Gumbiner et al., 1988; Watabe et al., 1994). Therefore, we tested whether ouabain treatment of MDCK cells affects expression or localization of E-cadherin in these cells. In control cells, at 0 h E-cadherin showed a predominant intracellular staining. At this time point the plasma membrane localization of E-cadherin was barely detectable (Figure 7A). After 3 h of Ca2+-switch the intracellular staining of E-cadherin decreased dramatically, whereas the plasma membrane staining increased (Figure 7B). In ouabain-treated cells at 0 h E-cadherin was localized intracellularly similar to that of control cells (Figure 7C). At 3 h after Ca2+-switch, like in control cells, the intracellular E-cadherin staining decreased and the plasma membrane staining increased at cell-cell contact sites (Figure 7D). Immunoblot analysis of total cell lysates of control and ouabain-treated cells showed no differences in the levels of E-cadherin, α-, β-, and γ-catenin (Figure 7E). Coimmunoprecipitation showed no detectable differences in the levels of α-, β-, and γ-catenin associated with E-cadherin in control and ouabain-treated cells (our unpublished results). In addition, ouabain-treated cells revealed adherens junctions (Figure 1K), the formation of which requires functional E-cadherin (Gumbiner et al., 1988; Watabe et al., 1994; Yap et al., 1997). These results indicate that E-cadherin is functional in ouabain-treated cells and that inhibition of Na,K-ATPase function does not affect expression or localization to the plasma membrane of E-cadherin and catenins in MDCK cells.
Figure 7.
E-Cadherin and catenin expression in ouabain-treated MDCK cells. (A–D) Immunofluorescence of E-cadherin. At 0 h E-cadherin was localized intracellular in both control (A) and ouabain-treated (C) cells. At 3 h E-cadherin was localized on the plasma membrane in control cells (B) and in ouabain-treated cells (D). (E) Immunoblot analysis of E-cadherin, α-catenin, β-catenin, and γ-catenin. Ouabain treatment of MDCK cells during Ca2+-switch did not significantly alter the expression levels of E-cadherin, α-catenin, β-catenin, or γ-catenin. Bar, 10 μm.
DISCUSSION
Distinct Signaling Mechanisms May Regulate Formation of Tight Junctions and Desmosomes
We used two independent methods, ouabain treatment or K+ depletion, to show that inhibition of Na,K-ATPase prevents the formation of functional tight junctions and desmosomes and the induction of polarity in MDCK cells. Rapid restoration of tight junctions, desmosomes, and epithelial polarity upon K+ repletion demonstrated that the effects of Na,K-ATPase inhibition were reversible and that Na,K-ATPase function is involved in the formation of these junctions in MDCK cells. In a previous report we suggested that the tight junction formation may involve two independent events i.e., E-cadherin-dependent initial translocation of tight junction proteins to the plasma membrane and an E-cadherin-independent assembly of functional tight junctions (Rajasekaran et al., 2001). Na,K-ATPase function appears to be involved in the latter event because tight junction proteins were clearly seen on the plasma membrane in Na,K-ATPase inhibited cells yet these cells did not develop TER and had a highly reduced number of tight junctions compared with control cells. E-cadherin-dependent signaling events have been suggested to mediate the translocation of tight junction proteins from the cytoplasm to the plasma membrane (Balda et al., 1996; Rajasekaran et al., 1996). Because ouabain-treated MDCK cells express functional E-cadherin, the localization of tight junction proteins at the plasma membrane suggests that E-cadherin-mediated signaling was not affected in these cells. Therefore, functional E-cadherin might be essential for initial events that trigger the translocation of ZO-1 to the plasma membrane, whereas Na,K-ATPase function is crucial for events that regulate the formation of an undisrupted ZO-1 ring, functional tight junctions, and consequently, the polarized epithelial phenotype.
It has been suggested that tight junctions and desmosomes are formed in a coordinate manner after E-cadherin-mediated cell-cell contact in epithelial cells (Gumbiner et al., 1988). We found a distinct difference in the staining pattern of tight junction and desmosomal proteins in Na,K-ATPase inhibited cells. In contrast to tight junction proteins the localization of desmosomal proteins to the plasma membrane was substantially reduced and desmosomes were poorly assembled in the presence of ouabain and during K+ repletion. These results suggest that signaling events that mediate the translocation of desmosomal proteins to the plasma membrane and the formation of desmosomes require the function of Na,K-ATPase and might be regulated in part by E-cadherin-independent mechanisms.
Despite functional E-cadherin expression in ouabain-treated cells, the absence of functional tight junctions and desmosomes indicates that E-cadherin function is not sufficient for formation of these junctions in MDCK cells. Potempa and Ridley (1998) have shown that hepatocyte growth factor treatment of MDCK cells did not affect tight junctions and desmosomes but specifically affected adherens junctions the formation of which requires functional E-cadherin. Furthermore, we have shown that tight junction and desmosome formation is not stimulated by ectopic expression of E-cadherin alone in MSV-MDCK cells, but requires coexpression of the β-subunit of Na,K-ATPase (Rajasekaran et al., 2001). In view of these recent results, we suggest that E-cadherin-mediated cell-cell contacts have a role in the signaling events that mediate translocation of tight junction proteins to the plasma membrane, an essential early event required for the assembly of tight junctions in epithelial cells. Thus, E-cadherin function is necessary but may not be sufficient for formation of functional tight junctions and the induction of polarity in MDCK cells. Formation of functional tight junctions and desmosomes additionally requires E-cadherin-independent mechanisms that depend on normal functioning of Na,K-ATPase. Once these junctions are formed and polarity is established, epithelial cells should maintain these junctions to perpetuate their polarized phenotype. Expression of a dominant negative mutant of E-cadherin in polarized MDCK cells did not affect tight junctions or desmosomes, indicating that E-cadherin function may not be necessary to maintain tight junctions and desmosomes in polarized epithelial cells (Troxell et al., 2000). Contreras et al. (1999) suggested that prolonged treatment of MDCK monolayers with ouabain resulted in the loss of viability of ∼60% of cells and reduced cell-cell and cell-substratum contact and suggested the existence of a link between the pump and attachment. Recent studies in cardiac myocytes have implicated Na,K-ATPase as a signal transducer through protein–protein interactions (Liu et al., 2000). We conclude that signaling mechanisms mediated by both E-cadherin and Na,K-ATPase are likely involved in the formation of functional tight junctions and desmosomes in epithelial cells.
Regulation of RhoA GTPase Activity by Na,K-ATPase and Its Role in Tight Junction and Desmosome Formation
The large reduction of stress fibers in Na,K-ATPase-inhibited cells prompted us to test whether RhoA GTPase, which has been implicated in the formation of stress fibers (Ridley and Hall, 1992; Mackay and Hall, 1998), is affected by Na,K-ATPase inhibition. Ouabain treatment and K+ depletion consistently reduced levels of endogenous active RhoA in wild-type MDCK cells and of exogenously expressed RhoA in the MDCK T23 clone, indicating that inhibition of Na,K-ATPase specifically inhibits RhoA activity. Reactivation of the Na,K-ATPase by K+ repletion resulted in an increase in the levels of endogenous active RhoA GTPase and concomitant formation of bundled stress fibers, suggesting that Na,K-ATPase function reversibly regulates the activity of RhoA GTPase and formation of stress fibers. The levels of active Rac 1 remained the same in control and ouabain-treated cells (our unpublished results), indicating that Na,K-ATPase might mediate its action specifically through RhoA GTPase. Reduced RhoA activity correlated with highly reduced number of tight junctions, desmosomes, and lack of polarity in Na,K-ATPase-inhibited cells. Moreover, cells overexpressing wild-type RhoA GTPase can bypass the inhibitory effect of Na,K-ATPase on the formation of tight junctions, desmosomes, and induction of epithelial polarity, indicating that RhoA GTPase is an essential component downstream of Na,K-ATPase function linking Na,K-ATPase to the formation of functional tight junctions, desmosomes, and induction of polarity in MDCK cells. Whether the modulation of RhoA activity by Na,K-ATPase is through a direct or indirect effect on RhoA GTPase activity remains to be clarified.
Previous studies with the use of inhibitors and mutant forms of RhoA GTPase have implicated RhoA GTPase in the assembly and function of tight junctions. Nusrat et al. (1995) have shown that inhibition of Rho in T84 cells causes dispersion of ZO-1 to the cytoplasm and concomitant decrease in the TER. In MDCK cells expressing a dominant negative mutant of RhoA, ZO-1 was localized to the plasma membrane and the tight junction structure was preserved yet TER was low in these cells, indicating that altering RhoA activity affects the function of tight junctions (Jou et al., 1998). Na,K-ATPase inhibited cells did not develop TER, showed discontinuous ZO-1 staining on the plasma membrane, and revealed highly reduced numbers of tight junctions, indicating that Na,K-ATPase-mediated RhoA GTPase inhibition affects both the assembly and function of tight junctions in MDCK cells. However, inhibition of the Na,K-ATPase reduced TER even in cells overexpressing RhoA (Figure 6), suggesting that other factors are also important in the alteration in tight junctional permeability when intracellular Na+ is increased. Although the TER was consistently affected in all these reports (Nusrat et al., 1995; Jou et al., 1998; this study), the difference between our results and others regarding the localization of ZO-1 to the plasma membrane and tight junction assembly is not clear. We suggest that actin polymerization mediated by RhoA GTPase might be necessary for the molecular reorganization and association of tight junction proteins to the actin cytoskeleton to assemble tight junctions and to regulate their permeability function.
Plasma membrane localization of desmosomal proteins and the formation of desmosomes in ouabain-treated cells overexpressing wild-type RhoA demonstrated that Na,K-ATPase-regulated RhoA function is essential for both the translocation of desmosomal proteins to the plasma membrane and the formation of desmosomes in MDCK cells. These events may require active actin polymerization mediated by active RhoA GTPase. In fact, a recent study demonstrated a role for stress fibers in the assembly of desmosomes in keratinocytes (Vasioukhin et al., 2000).
The polarity of apical and basolateral markers was consistently altered in Na,K-ATPase-inhibited cells. Furthermore, polarized distribution of domain-specific markers in ouabain-treated wild-type RhoA overexpressing MDCK cells indicates that Na,K-ATPase-regulated RhoA GTPase function is essential to maintain the polarity in MDCK cells. Although the loss of polarity in Na,K-ATPase-inhibited cells largely appears to be due to the lack of tight junctions, we cannot rule out that Na,K-ATPase inhibition might also affect mechanisms involved in the polarized sorting of proteins in epithelial cells (Yeaman et al., 1999).
How Na,K-ATPase inhibition affects the formation of tight junctions, desmosomes, induction of polarity, and negatively regulates RhoA GTPase function is currently not known. The effect does not appear to be a simple degeneration of cellular function because it is readily reversible. Moreover, translocation of tight junction proteins to the plasma membrane under Na,K-ATPase-inhibited conditions suggests that other aspects of epithelial polarization are not impaired in these cells. The phenomena are better correlated with the absolute concentration of Na+ in the cell than with the Na+ gradient across the plasma membrane. Removal of extracellular Na+ will also collapse the gradient but did not prevent formation of tight junctions or induction of polarity. Thus, aspects of cell function such as cytoplasmic pH or Ca+2 concentration, which depend on the transmembrane Na+ gradient, are probably not involved. The simplest interpretation of the results is that the normal intracellular Na+ homeostasis primarily regulated by Na,K-ATPase is involved in the modulation of RhoA activity. It is also possible that the effects might be mediated by a decrease in cell K+ or depolarization of the plasma membrane potential rather than by Na+ itself. Finally, although there were no obvious differences in cell size between control and Na,K-ATPase-inhibited cells, we cannot rule out small cell volume changes that might have led to the observed phenotype. We recognize that alteration of the intracellular sodium homeostasis by the inhibition of Na,K-ATPase may induce multiple biochemical changes in cells. However, rapid reversibility of effects induced by Na,K-ATPase inhibition such as formation of tight junctions, desmosomes, bundled actin stress fibers, restoration of RhoA activity, and induction of polarity by K+ repletion strongly suggests that Na,K-ATPase function plays an important role in the assembly of junctions and induction of polarity in epithelial cells through a RhoA-mediated pathway.
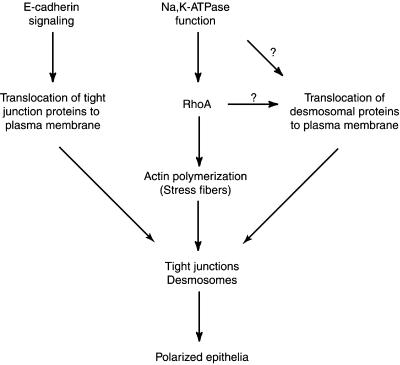
Model for Formation of Tight Junctions and Desmosomes in Polarized Epithelial Cells
Based on our results, we propose a model for formation of tight junctions and desmosomes in epithelial cells. According to this model tight junctions and desmosomes are formed in parallel by two independent pathways (Figure 8) yet linked by RhoA GTPase. E-Cadherin-mediated signaling events translocate tight junction proteins from the cytoplasm to the plasma membrane. Formation of functional tight junctions then requires active polymerization of actin mediated by RhoA. The desmosomal assembly is mediated by signaling events regulated by Na,K-ATPase. These signaling events might be directly involved in the translocation of desmosomal proteins to the plasma membrane. Alternatively, RhoA-mediated actin polymerization could be involved in the translocation of desmosomal proteins to the plasma membrane and the final assembly of desmosomes. Thus, we propose that Na,K-ATPase function, mediated at least in part by RhoA, plays an important role in formation of tight junctions and desmosomes and thus in the biogenesis of polarized epithelia.
Figure 8.
Schematic model of the formation of tight junctions and desmosomes in epithelial cells (see text for details).
ACKNOWLEDGMENTS
We thank Drs. Ernest Wright and Gregory Payne for critical reading of the manuscript. We thank Dr. Joel Pardee for encouragement and support. We thank Dr. Elliot Landaw for statistical analysis of the TER data. This work is primarily supported by a Grant-in-Aid award 1162-Gl1 from the American Heart Association (Western States Affiliate) (to A.K.R) and in part by Department of Defense grants PC-991140 and PC-970546 (to A.K.R), a Department of Defense Breast Cancer Program grant BC990290 (to Y.Z), and National Institutes of Health CA-67113 grant (to A.P.S). Metal analysis was supported by a National Science Foundation grant DBI-0077378 to J.F.H. S.A.R is supported by a Fellowship from Toohey Foundation. A.K.R is a member of the Jonsson Comprehensive Cancer Center.
REFERENCES
- Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffin-Meyer B, Marsy S, Barlet-Bas C, Cheval L, Younes-Ibrahim M, Rajerison R, Doucet A. Regulation of renal Na+,K+-ATPase in rat thick ascending limb during K+-depletion: evidence for modulation of Na+ affinity. J Physiol. 1996;490:623–632. doi: 10.1113/jphysiol.1996.sp021172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Cereijido M. Relationship between Na+,K+-ATPase and cell attachment. J Cell Sci. 1999;112:4223–4232. doi: 10.1242/jcs.112.23.4223. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez R, Malnic G. H+ ATPase and Cl− interaction in regulation of MDCK cell pH. J Membr Biol. 1998;163:137–145. doi: 10.1007/s002329900378. [DOI] [PubMed] [Google Scholar]
- Garrod D, Chidgey M, North A. Desmosomes: differentiation, development, dynamics, and disease. Curr Opin Cell Biol. 1996;5:670–678. doi: 10.1016/s0955-0674(96)80108-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK) J Membr Biol. 1985;86:113–125. doi: 10.1007/BF01870778. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber RS, Pressley TA, Loeb JN, Ismail-Beigi F. Ionic dependence of active Na-K transport: “clamping of cellular Na+ with monensin. Am J Physiol. 1987;253:F26–F33. doi: 10.1152/ajprenal.1987.253.1.F26. [DOI] [PubMed] [Google Scholar]
- Imhof BA, Vollmers HP, Goodman SL, Birchmeier W. Cell-cell interaction and polarity of epithelial cells: specific perturbation using a monoclonal antibody. Cell. 1983;35:667–675. doi: 10.1016/0092-8674(83)90099-5. [DOI] [PubMed] [Google Scholar]
- Jou T-S, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T-S, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz AI. Role of Na-K-ATPase in kidney function. In: Skou JC, Norby JG, Maunsbach AB, Esmann M, editors. The Na+,K+ Pump. Part B. Cellular Aspects. New York: Alan R. Liss; 1988. pp. 207–232. [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Leung S-M, Rojas R, Maples C, Flynn C, Ruiz WG, Jou T-S, Apodaca G. Modulation of endocytic traffic in polarized Madin-Darby canine kidney cells by the small GTPase RhoA. Mol Biol Cell. 1999;10:4369–4384. doi: 10.1091/mbc.10.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingrel JB, Van Huysse J, O'Brien W, Jewell-Motz E, Askew R, Schultheis P. Structure-function studies of the Na,K-ATPase. Kidney Int. 1994;45(suppl 44):S32–S39. [PubMed] [Google Scholar]
- Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na,K-A.T.Pase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- Mackay DJG, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- McNeil H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomurulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojakian GK, Schwimmer R. The polarized distribution of an apical cell surface glycoprotein is maintained by interactions with the cytoskeleton of Madin-Darby canine kidney cells. J Cell Biol. 1988;107:2377–2387. doi: 10.1083/jcb.107.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack LR, Tate EH, Cook JS. Na+,K+-ATPase in HeLa cells after prolonged growth in low K+ or ouabain. J Cell Physiol. 1981;106:85–97. doi: 10.1002/jcp.1041060110. [DOI] [PubMed] [Google Scholar]
- Potempa S, Ridley JA. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressley TA. Ion concentration-dependent regulation of Na,K-pump abundance. J Membr Biol. 1988;105:187–195. doi: 10.1007/BF01870996. [DOI] [PubMed] [Google Scholar]
- Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and Zonula Occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ, Jr, Bander NH, Peralta Soler A, Rajasekaran AK. Na,K-ATPase β-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell. 2001;12:279–295. doi: 10.1091/mbc.12.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayson BM. Rates of synthesis and degradation of Na+,K+-ATPase during chronic ouabain treatment. Am J Physiol. 1989;256:C75–C80. doi: 10.1152/ajpcell.1989.256.1.C75. [DOI] [PubMed] [Google Scholar]
- Ren X, Kiosses W, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rothstein A, Mack E. Volume-activated K+ and Cl− pathways of dissociated epithelial cells (MDCK): role of Ca2+ Am J Physiol. 1990;258:C827–C834. doi: 10.1152/ajpcell.1990.258.5.C827. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by Rac and Rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell ML, Gopalakrishnan S, McCormack J, Poteat BA, Pennington J, Garringer SM, Schneeberger EE, Nelson WJ, Marrs JA. Inhibiting cadherin function by dominant mutant E-cadherin expression increases the extent of tight junction assembly. J Cell Sci. 2000;113:985–996. doi: 10.1242/jcs.113.6.985. [DOI] [PubMed] [Google Scholar]
- Vaaraniemi J, Huotari V, Lehto VP, Eskelinen S. Effect of PMA on the integrity of the membrane skeleton and morphology of epithelial MDCK cells is dependent on the activity of amiloride-sensitive ion transporters and membrane potential. Eur J Cell Biol. 1997;74:262–272. [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma cell line. J Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, et al. Regulation of Na,K-adenosine triphosphatase gene expression by sodium ions in cultured neonatal rat cardiocytes. J Clin Invest. 1993;92:1889–1895. doi: 10.1172/JCI116781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Zhu K, Debrecini B, Li R, Zheng Y. Identification of Rho GTPase-dependent sites in the DH domain of oncogenic Dbl that are required for transformation. J Biol Chem. 2000;275:25993–26001. doi: 10.1074/jbc.M003780200. [DOI] [PubMed] [Google Scholar]